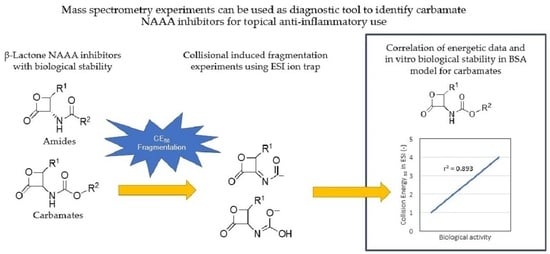
An Investigation on the Relationships between Mass Spectrometric Collisional Data and Biological Activity/Stability of Some N-Acylethanolamine Acid Amidase (NAAA) β-Lactone Inhibitors
Abstract
1. Introduction
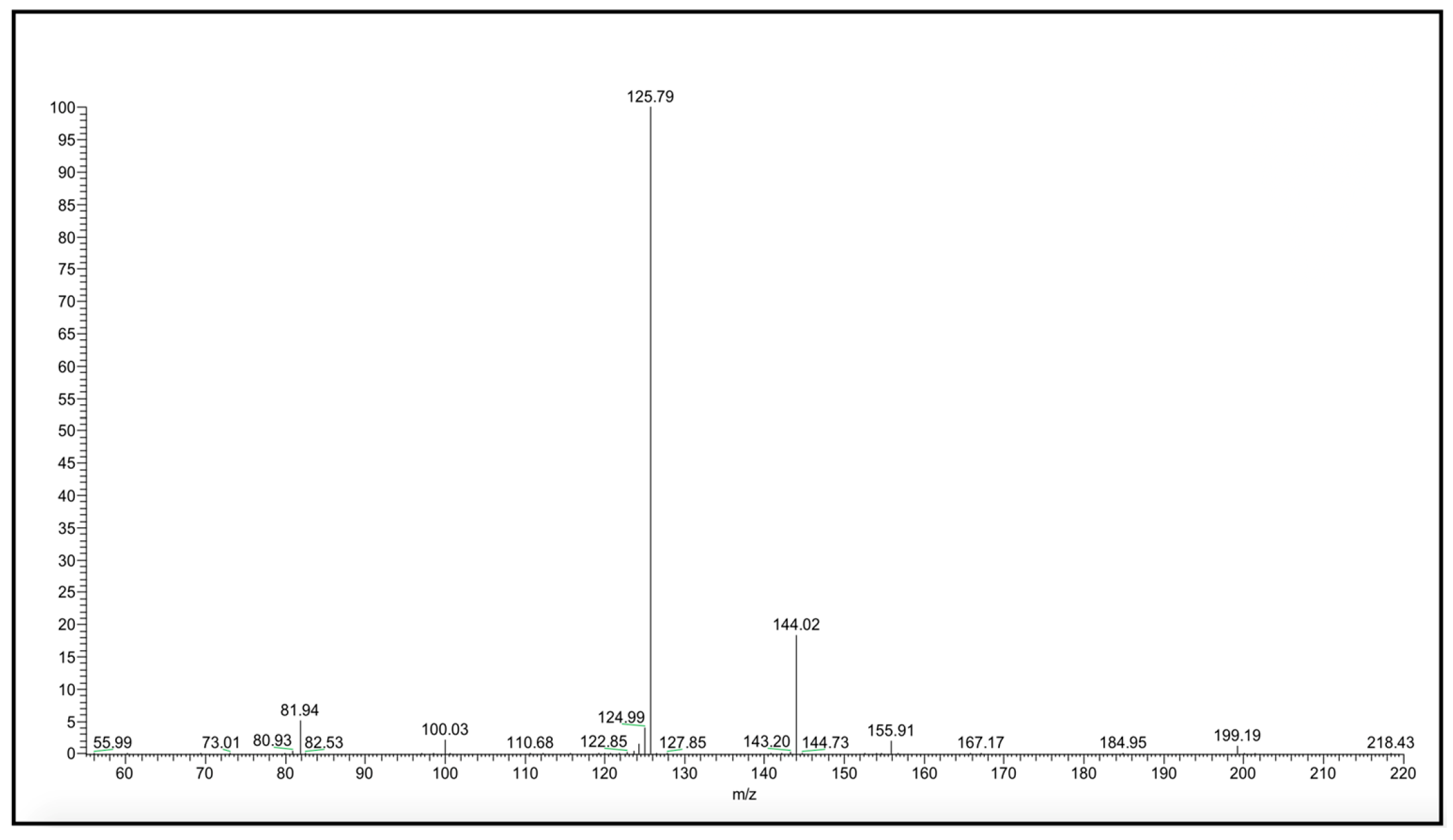
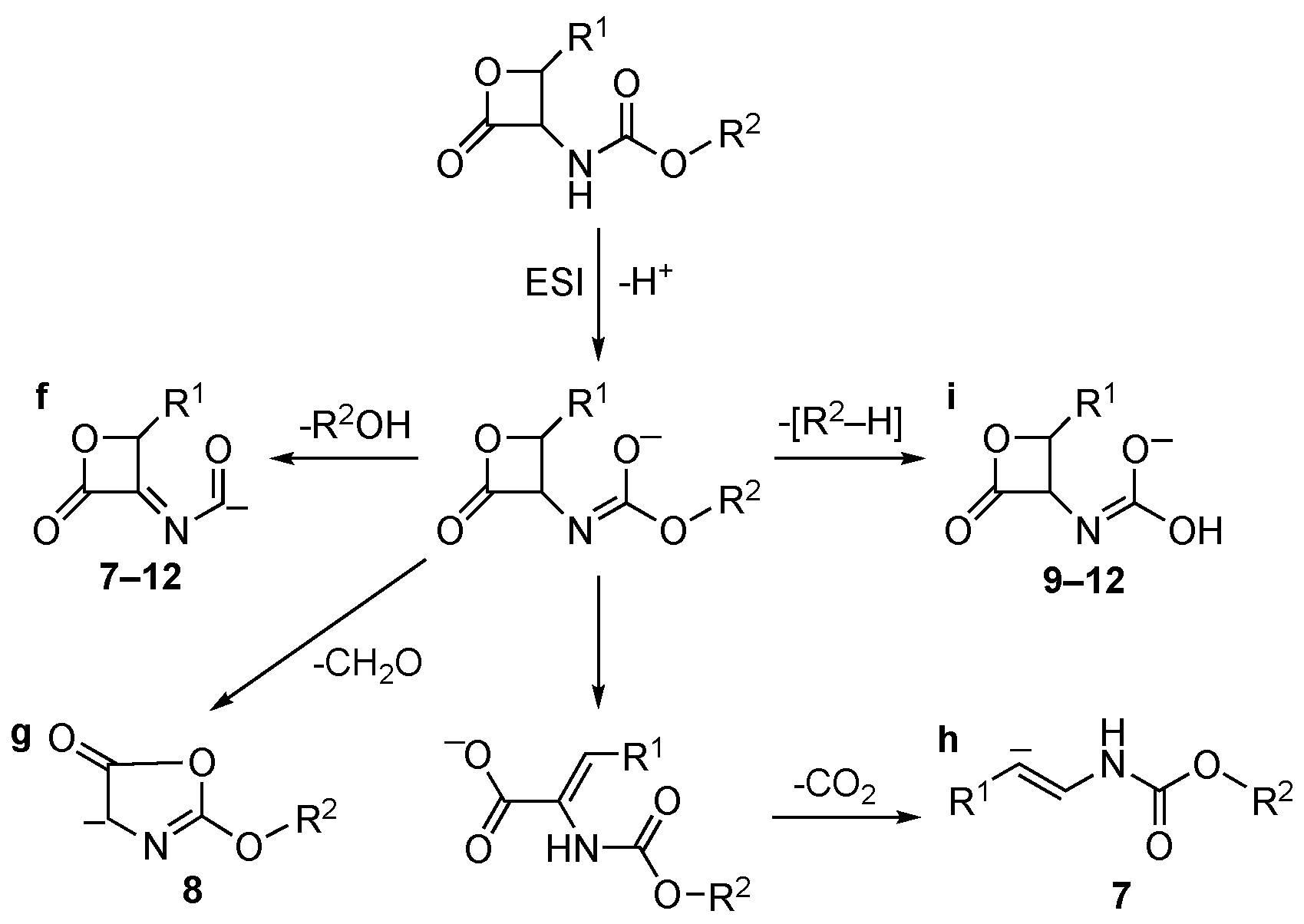
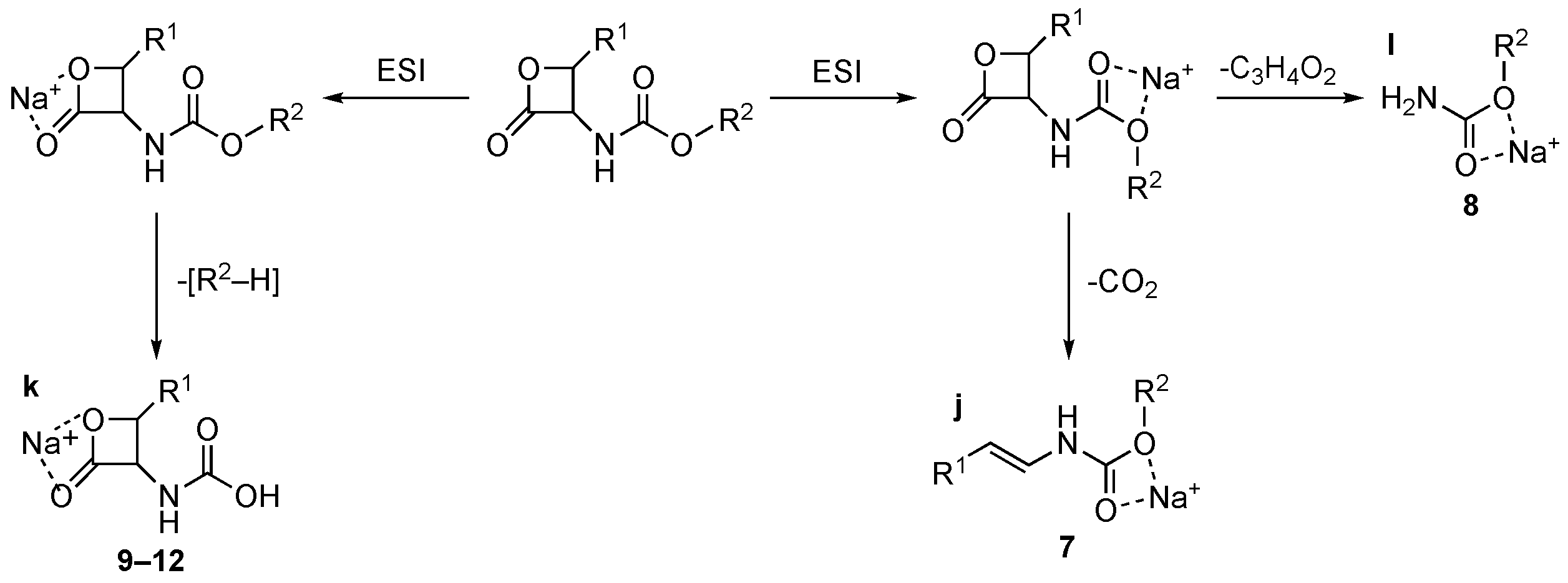
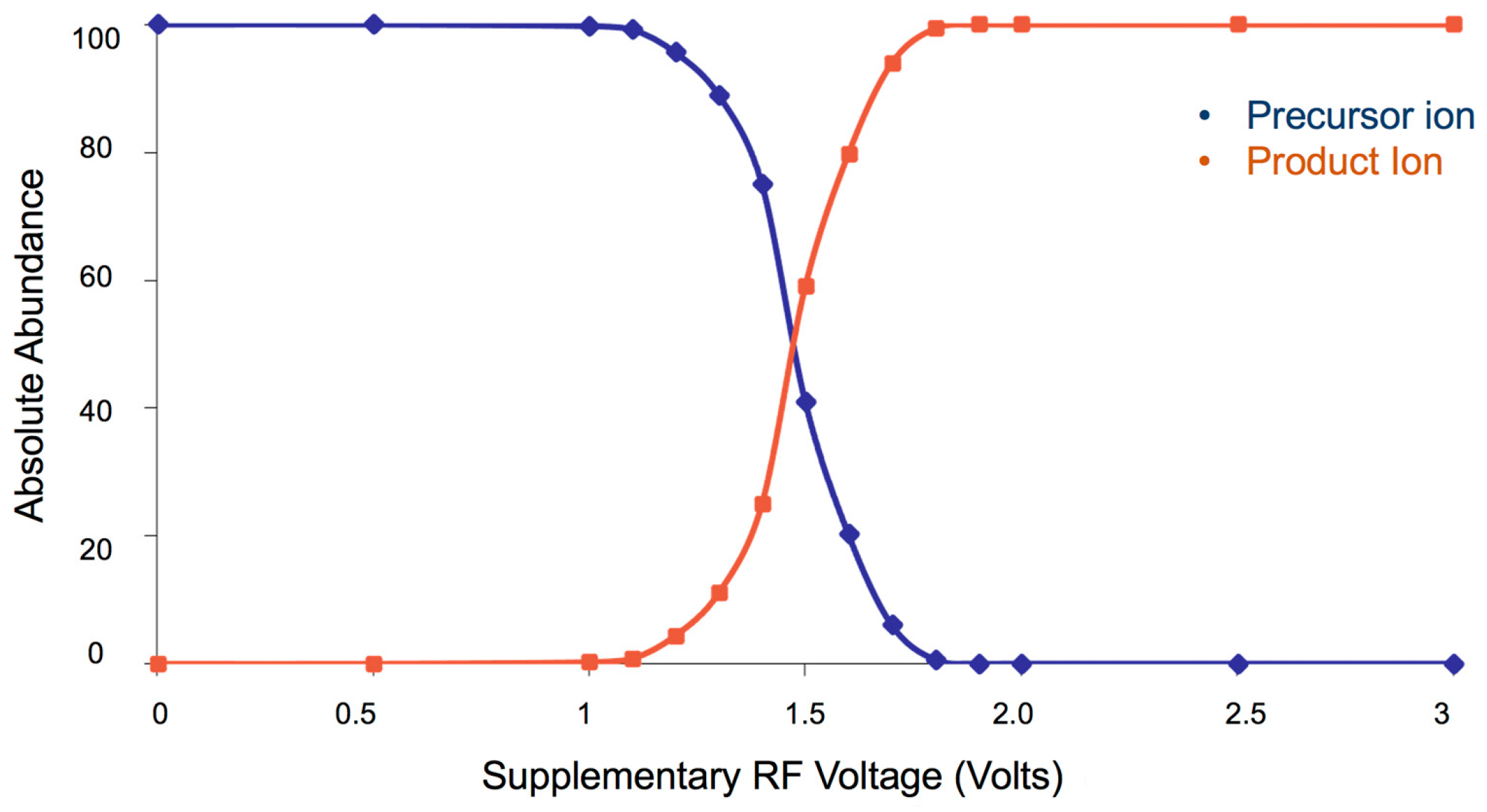
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instrumental Analysis
3.3. Biochemical Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsuboi, K.; Sun, Y.X.; Okamoto, Y.; Araki, N.; Tonai, T.; Ueda, N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J. Biol. Chem. 2005, 280, 11082–11092. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Tsuboi, K.; Uyama, T. N-acylethanolamine metabolism with special reference to N-acylethanolamine–hydrolyzing acid amidase (NAAA). Prog. Lipid Res. 2010, 49, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ying, H.; Yao, J.; Yang, L.; Ma, H.; Li, L.; Zhao, Y. Micronized palmitoylethanolamide ameliorates methionine and choline deficient diet-induced nonalcoholic steatohepatitis via inhibiting inflammation and restoring autophagy. Front. Pharmacol. 2021, 12, 744483. [Google Scholar] [CrossRef]
- Beggiato, S.; Tomasini, M.C.; Ferraro, L. Palmitoylethanolamide (PEA) as a potential therapeutic agent in Alzheimer’s disease. Front. Pharmacol. 2019, 10, 821. [Google Scholar] [CrossRef]
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides update: Palmitoylethanolamide and its formulations on management of peripheral neuropathic pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef]
- Clayton, P.; Hill, M.; Bogoda, N.; Subah, S.; Venkatesh, R. Palmitoylethanolamide: A natural compound for health management. Int. J. Mol. Sci. 2021, 22, 5305. [Google Scholar] [CrossRef]
- Fotio, Y.; Jung, K.-M.; Palese, F.; Obenaus, A.; Tagne, A.M.; Lin, L.; Rashid, T.I.; Pacheco, R.; Jullienne, A.; Ramirez, J. NAAA-regulated lipid signaling governs the transition from acute to chronic pain. Sci. Adv. 2021, 7, eabi8834. [Google Scholar] [CrossRef]
- Solorzano, C.; Zhu, C.; Battista, N.; Astarita, G.; Lodola, A.; Rivara, S.; Mor, M.; Russo, R.; Maccarrone, M.; Antonietti, F.; et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. USA 2009, 106, 20966–20971. [Google Scholar] [CrossRef]
- Bottemanne, P.; Muccioli, G.G.; Alhouayek, M. N-acylethanolamine hydrolyzing acid amidase inhibition: Tools and potential therapeutic opportunities. Drug Discov. Today 2018, 23, 1520–1529. [Google Scholar] [CrossRef]
- Piomelli, D.; Scalvini, L.; Fotio, Y.; Lodola, A.; Spadoni, G.; Tarzia, G.; Mor, M. N-acylethanolamine acid amidase (NAAA): Structure, function, and inhibition. J. Med. Chem. 2020, 63, 7475–7490. [Google Scholar] [CrossRef]
- West, J.M.; Zvonok, N.; Whitten, K.M.; Wood, J.T.; Makriyannis, A. Mass spectrometric characterization of human N-acylethanolamine-hydrolyzing acid amidase. J. Proteome Res. 2012, 11, 972–981. [Google Scholar] [CrossRef]
- West, J.M.; Zvonok, N.; Whitten, K.M.; Vadivel, S.K.; Bowman, A.L.; Makriyannis, A. Biochemical and mass spectrometric characterization of human N-acylethanolamine-hydrolyzing acid amidase inhibition. PLoS ONE 2012, 7, e43877. [Google Scholar] [CrossRef]
- Armirotti, A.; Romeo, E.; Ponzano, S.; Mengatto, L.; Dionisi, M.; Karacsonyi, C.; Bertozzi, F.; Garau, G.; Tarozzo, G.; Reggiani, A.; et al. β-Lactones inhibit N-acylethanolamine acid amidase by S-acylation of the catalytic N-terminal cysteine. ACS Med. Chem. Lett. 2012, 3, 422–426. [Google Scholar] [CrossRef]
- Duranti, A.; Tontini, A.; Antonietti, F.; Vacondio, F.; Fioni, A.; Silva, C.; Lodola, A.; Rivara, S.; Solorzano, C.; Piomelli, D. N-(2-Oxo-3-oxetanyl)carbamic acid esters as N-acylethanolamine acid amidase inhibitors: Synthesis and structure–activity and structure–property relationships. J. Med. Chem. 2012, 55, 4824–4836. [Google Scholar] [CrossRef]
- Sasso, O.; Moreno-Sanz, G.; Martucci, C.; Realini, N.; Dionisi, M.; Mengatto, L.; Duranti, A.; Tarozzo, G.; Tarzia, G.; Mor, M.; et al. Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models. Pain 2013, 154, 350–360. [Google Scholar] [CrossRef]
- Sasso, O.; Summa, M.; Armirotti, A.; Pontis, S.; De Mei, C.; Piomelli, D. The N-acylethanolamine acid amidase inhibitor ARN077 suppresses inflammation and pruritus in a mouse model of allergic dermatitis. J. Investig. Dermatol. 2018, 138, 562. [Google Scholar] [CrossRef]
- Basso, E.; Duranti, A.; Mor, M.; Piomelli, D.; Tontini, A.; Tarzia, G.; Traldi, P. Tandem mass spectrometric data–FAAH inhibitory activity relationships of some carbamic acid O-aryl esters. J. Mass Spectrom. 2004, 39, 1450. [Google Scholar] [CrossRef]
- Valitutti, G.; Duranti, A.; Lodola, A.; Mor, M.; Piersanti, G.; Piomelli, D.; Rivara, S.; Tontini, A.; Tarzia, G.; Traldi, P. Correlation between energetics of collisionally activated decompositions, interaction energy and biological potency of carbamate FAAH inhibitors. J. Mass Spectrom. 2007, 42, 1624. [Google Scholar] [CrossRef]
- Valitutti, G.; Duranti, A.; Mor, M.; Piersanti, G.; Piomelli, D.; Rivara, S.; Tontini, A.; Tarzia, G.; Traldi, P. The collisional behavior of ESI-generated protonated molecules of some carbamate FAAH inhibitors isosteres and its relationships with biological activity. J. Mass Spectrom. 2009, 44, 561. [Google Scholar] [CrossRef]
- Agostini, M.; Favretto, D.; Renzoni, C.; Vogliardi, S.; Duranti, A. Characterization of URB series synthetic cannabinoids by HRMS and UHPLC–MS/MS. Pharmaceuticals 2023, 16, 201. [Google Scholar] [CrossRef]
- Solorzano, C.; Antonietti, F.; Duranti, A.; Tontini, A.; Rivara, S.; Lodola, A.; Vacondio, F.; Tarzia, G.; Piomelli, D.; Mor, M. Synthesis and structure—Activity relationships of N-(2-oxo-3-oxetanyl)amides as N-acylethanolamine-hydrolyzing acid amidase inhibitors. J. Med. Chem. 2010, 53, 5770–5781. [Google Scholar] [CrossRef] [PubMed]
- Ponzano, S.; Bertozzi, F.; Mengatto, L.; Dionisi, M.; Armirotti, A.; Romeo, E.; Berteotti, A.; Fiorelli, C.; Tarozzo, G.; Reggiani, A.; et al. Synthesis and structure—Activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J. Med. Chem. 2013, 56, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Molinaro, R.; Fresta, M.; Duranti, A.; Cosco, D. α-Acylamino-β-lactone N-acylethanolamine-hydrolyzing acid amidase inhibitors encapsulated in PLGA nanoparticles: Improvement of the physical stability and protection of human cells from hydrogen peroxide-induced oxidative stress. Antioxidants 2022, 11, 686. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Ambrosio, N.; Fresta, M.; Duranti, A.; Cosco, D. Characterization and preliminary in vitro antioxidant activity of a new multidrug formulation based on the co-encapsulation of rutin and the α-Acylamino-β-lactone NAAA inhibitor URB894 within PLGA nanoparticles. Antioxidants 2023, 12, 305. [Google Scholar] [CrossRef]



| Compounds | Chemical Structure | MW | IC50 (μM ± S.E.M.) | BSA t1/2 (Min) | Ref. |
|---|---|---|---|---|---|
| 1 |  | 276.09 | 115 ± 13 | 3.3 ± 0.8 | [15,22] |
| 2 | 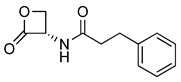 | 219.09 | 420 ± 20 | 7.0 ± 0.6 | [15,22] |
| 3 |  | 233.11 | >100 | 16.0 ± 1.2 | [15,22] |
| 4 |  | 297.10 | 90 ± 10 | 5.8 ± 1.0 | [15,22] |
| 5 | 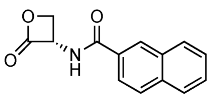 | 241.07 | 160 ± 40 | <1 | [15,22] |
| 6 |  | 313.10 | 300 ± 20 | 3.2 ± 0.7 | [15,22] |
| 7 |  | 235.08 | 1 ± 0.2 | 5.1 ± 0.8 | [15] |
| 8 | 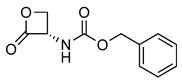 | 221.07 | 2.96 ± 0.30 | 1.2 ± 0.7 | [15] |
| 9 | 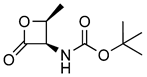 | 201.10 | 0.22 ± 0.03 | 129.1 ± 7.0 | [15] |
| 10 | 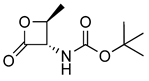 | 201.10 | 0.19 ± 0.04 | 87.7 ± 2.3 | [15] |
| 11 |  | 201.10 | >80 | 120.7 ± 1.1 | [15] |
| 12 | 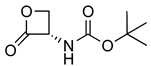 | 187.08 | 0.58 ± 0.20 | 19.6 ± 1.5 | [15] |
| Compounds | 1° Fragment Ion Abundance | 2° Fragment Ion Abundance | 1° Fragment Ion Abundance | 2° Fragment Ion Abundance |
|---|---|---|---|---|
| ESI (−) | ESI (+) | |||
| 1 | 100% a | – | 100% e | – |
| 2 | 100% a | 70% b | 100% e | – |
| 3 | 100% a | – | 100% d | 50% e |
| 4 | 100% a | – | 100% d | – |
| 5 | 100% a | – | 100% d | 60% e |
| 6 | 100% c | – | 100% e | – |
| 7 | 100% f | 50% h | 100% j | – |
| 8 | 70% f | 100% g | 100% l | – |
| 9 | 100% f | 20% i | 100% k | – |
| 10 | 100% f | 20% i | 100% k | – |
| 11 | 100% f | 20% i | 100% k | – |
| 12 | 100% f | 50% i | 100% k | – |
| Compounds | Crossing Point ESI (−) | Crossing Point ESI (+) |
|---|---|---|
| RF (Volts) | RF (Volts) | |
| 1 | 1.95 ± 0.00 | 1.64 ± 0.01 |
| 2 | 1.42 ± 0.01 | 1.51 ± 0.04 |
| 3 | 1.29 ± 0.01 | 1.44 ± 0.01 |
| 4 | 1.10 ± 0.00 | 1.67 ± 0.03 |
| 5 | 1.15 ± 0.05 | 1.28 ± 0.08 |
| 6 | – | 1.63 ± 0.01 |
| 7 | 0.93 ± 0.01 | 1.13 ± 0.00 |
| 8 | 0.95 ± 0.00 | 1.16 ± 0.01 |
| 9 | 1.04 ± 0.00 | 0.86 ± 0.01 |
| 10 | 1.05 ± 0.00 | 0.96 ± 0.01 |
| 11 | 1.05 ± 0.00 | 1.03 ± 0.01 |
| 12 | 1.06 ± 0.01 | 0.95 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isak, I.; Duranti, A.; Traldi, P. An Investigation on the Relationships between Mass Spectrometric Collisional Data and Biological Activity/Stability of Some N-Acylethanolamine Acid Amidase (NAAA) β-Lactone Inhibitors. Drugs Drug Candidates 2023, 2, 109-120. https://doi.org/10.3390/ddc2010007
Isak I, Duranti A, Traldi P. An Investigation on the Relationships between Mass Spectrometric Collisional Data and Biological Activity/Stability of Some N-Acylethanolamine Acid Amidase (NAAA) β-Lactone Inhibitors. Drugs and Drug Candidates. 2023; 2(1):109-120. https://doi.org/10.3390/ddc2010007
Chicago/Turabian StyleIsak, Ilena, Andrea Duranti, and Pietro Traldi. 2023. "An Investigation on the Relationships between Mass Spectrometric Collisional Data and Biological Activity/Stability of Some N-Acylethanolamine Acid Amidase (NAAA) β-Lactone Inhibitors" Drugs and Drug Candidates 2, no. 1: 109-120. https://doi.org/10.3390/ddc2010007
APA StyleIsak, I., Duranti, A., & Traldi, P. (2023). An Investigation on the Relationships between Mass Spectrometric Collisional Data and Biological Activity/Stability of Some N-Acylethanolamine Acid Amidase (NAAA) β-Lactone Inhibitors. Drugs and Drug Candidates, 2(1), 109-120. https://doi.org/10.3390/ddc2010007