Abstract
The uncontrolled increasing clinical resistance to the current anti-parasitic drugs towards important protozoan parasites (Plasmodium, Leishmania, Trypanosoma and Toxoplasma) has stimulated the search for novel and safe therapeutic agents at affordable prices for countries in which these parasites are endemic. For the past few decades, the criticality of the cationic lipid stearylamine (SA) in liposomes has been explored in these human parasites. Previously, SA was incorporated in the liposomal formulation to impart a net positive charge for enhanced cellular uptake. However, the discovery of SA in liposomes alone elicits a strong anti-parasitic activity with immunomodulatory potential. Additionally, the SA liposome possesses a significant inhibitory potential on multiple life stages of the parasite cycle and delivers an equal effect on both drug-sensitive and resistant parasites. Moreover, the delivery of standard anti-parasitic drugs using SA liposome vesicles has enhanced the efficacy of drugs due to the synergistic impacts without causing any apparent toxicity on the host cells. In addition, the delivery of antigens as vaccine candidates using SA liposomes elicits a pronounced immune response in clearing the infection compared to other cationic lipids and SA-free liposomes. Nonetheless, SA liposome mediates its anti-parasitic activity by targeting the negatively charged phosphatidylserine-exposed infected host cell surface or by interaction with negatively charged sialic acid of free-living parasites. Overall, SA liposome confers its protection by acting as a chemotherapeutic agent with immunomodulatory activity. Therefore, a broadly acting anti-parasitic agent (SA liposome) is promising in tackling the deadly parasitic infections in endemic regions and warrants further clinical investigations.
1. Introduction
Vector-borne parasitic disease is prevalent and life-threatening to humans. Plasmodium, Leishmania, Trypanosoma, and Toxoplasma cause serious health issues with eradication challenges even in the 21st century [1]. Malaria caused by Plasmodium species leads to severe health and economic burdens worldwide. Importantly, sub-Saharan Africa is the most affected area among Central and South America, tropical and subtropical regions of Africa, Asia, and Oceania [2]. According to the World Health Organization (WHO), 241 million cases are reported worldwide, and the mortality rate was about 627,000 in 2020. Children below the age of five and pregnant women are highly susceptible to Plasmodium infection [3,4]. In pregnant women, glycosaminoglycan chondroitin sulphate A receptor in the placenta sequesters the infected RBC through variant-specific antigens of the PfEMP1 family (pregnant-associated malaria) and aggravates Pf infection [5]. Pf reactivates the Epstein-Barr virus (EBV), and co-infection leads to severe cerebral malaria pathology [6]. Moreover, Pf co-infection with schistosome is most common in Uganda [7], whereas Pf co-infection with HIV is a significant health issue in Saharan Africa [8]. Another human parasite, Leishmania, transmitted by female phlebotomine sand flies, causes leishmaniasis [9]. Nearly 700,000 to 1 million cases are reported annually. Among three forms of leishmaniasis, the visceral form causes a severe illness with an estimated 50,000 to 90,000 cases worldwide [10,11] and co-infection with HIV has been reported in 45 countries. Cutaneous leishmania cases were reported in America, the Mediterranean basin, the Middle East, and Central Asia, accounting for around 95%. Mucocutaneous leishmaniasis affects more than 90% of the population in Bolivia, Brazil, Ethiopia, and Peru. Co-infection of L. infantum with EBV or L. donovani with tuberculosis considerably worsen the disease severity [12,13]. Chagas disease is caused by Trypanosoma, which is life-threatening and severely affects the heart and gastrointestinal tract [14,15]. In 2020, 663 cases were reported by WHO, and more than 95% of cases were caused by Trypanosoma brucei gambiense [16]. Co-infection with T. cruzi-HIV promotes the reactivation of Chagas disease by affecting the central nervous system manifesting a high mortality rate [17,18]. Another human parasite, Toxoplasma gondii causes toxoplasmosis; nearly 1 million cases are reported yearly to food contamination in the European region. Toxoplasmosis is more likely to affect individuals with a weakened immune system [19]. More than 60% of the world population is infected due to varied environmental conditions that facilitate the parasite’s survival. Co-infection between T. gondii and Clostridium perfringens raises uterine gas gangrene, a severe health complication in pregnant women [20]. Thus, co-infection further aggravates the disease’s severity, resulting in fewer treatment options. By an earlier report, all four human parasites rapidly attained clinical resistance to the most widely used anti-parasitic drugs, making them highly ineffective. The involvement of host–parasite-specific factors and parasite genotype in drug sensitivity plays a significant role in treatment failure and contributes to drug resistance [21]. Artemisinin resistance to Pf is associated with a mutation in the PfKelch13 gene, a primary marker for artemisinin resistance [22]. In the Plasmodium food vacuole, chloroquine efflux is due to a mutation in the PfCRT gene imparting chloroquine resistance in Pf strains [23]. Amphotericin B (Amp B) resistance to L. donovani promastigotes showed a notable change in the parasite’s sterol profile of the plasma membrane [24]. Overexpression of the P-glycoprotein of L. tropica displays cross-resistance to alkyl-lysophospholipid (ALP) miltefosine and edelfosine [25]. T. brucei resistance to nitroheterocyclic drugs such as nifurtimox and fexinidazole have reduced nitroreductase expression levels by loss of gene or gene expression contributing towards resistance [26]. Activation of pentamidine resistance protein1 (PRP1), a member of ATP- binding cassette transporters, confers pentamidine resistance in leishmania promastigotes [27]. Decoquinate and atovaquone drugs inhibit oxygen uptake by affecting the mitochondrial b1 complex in T. gondii, a mutation in these complex confers resistance [28]. An in vivo study showed that nifurtimox-resistance in T. brucei parasite displayed cross-resistance to fexinidazole [29]. In African trypanosomiasis, arsenical–diamidine cross-resistance is mediated through alterations in an unusual adenosine transporter [30]. The overexpression and amplification of Pfmdr1 gene provided cross-resistance to quinine in mefloquine-resistant K1 and W2 Pf strains [31,32]. Such a scenario stimulates an urgent need for new therapeutics to tackle the spread of infection and clinical resistance to commonly used antiparasitic drugs. The impact of COVID-19 has immensely interrupted many control programs against parasitic vector-borne diseases, followed by the co-occurrence of SARS-CoV-2 and human parasites, and a considerable effect on the outbreak of endemic cutaneous leishmaniasis [33,34,35]. Such a situation provokes the need for developing or identifying a new class of antiparasitic agents. In this context, a novel therapeutic cationic lipid, stearylamine (SA) or octadecylamine incorporated in lipid vesicles (liposome) displays a pronounced killing activity against clinically important human parasites such as Plasmodium, Leishmania, Trypanosoma and Toxoplasma. This review attempts to uncover how SA incorporated in lipid vesicles mediates its anti-parasitic effect through its possible mechanistic action. Moreover, delivering anti-parasitic agents using SA-based lipid vesicles may show a substantial inhibitory effect and enhanced synergistic efficacy as a novel therapeutic strategy for tackling deadly human parasites.
2. Stearylamine Liposome
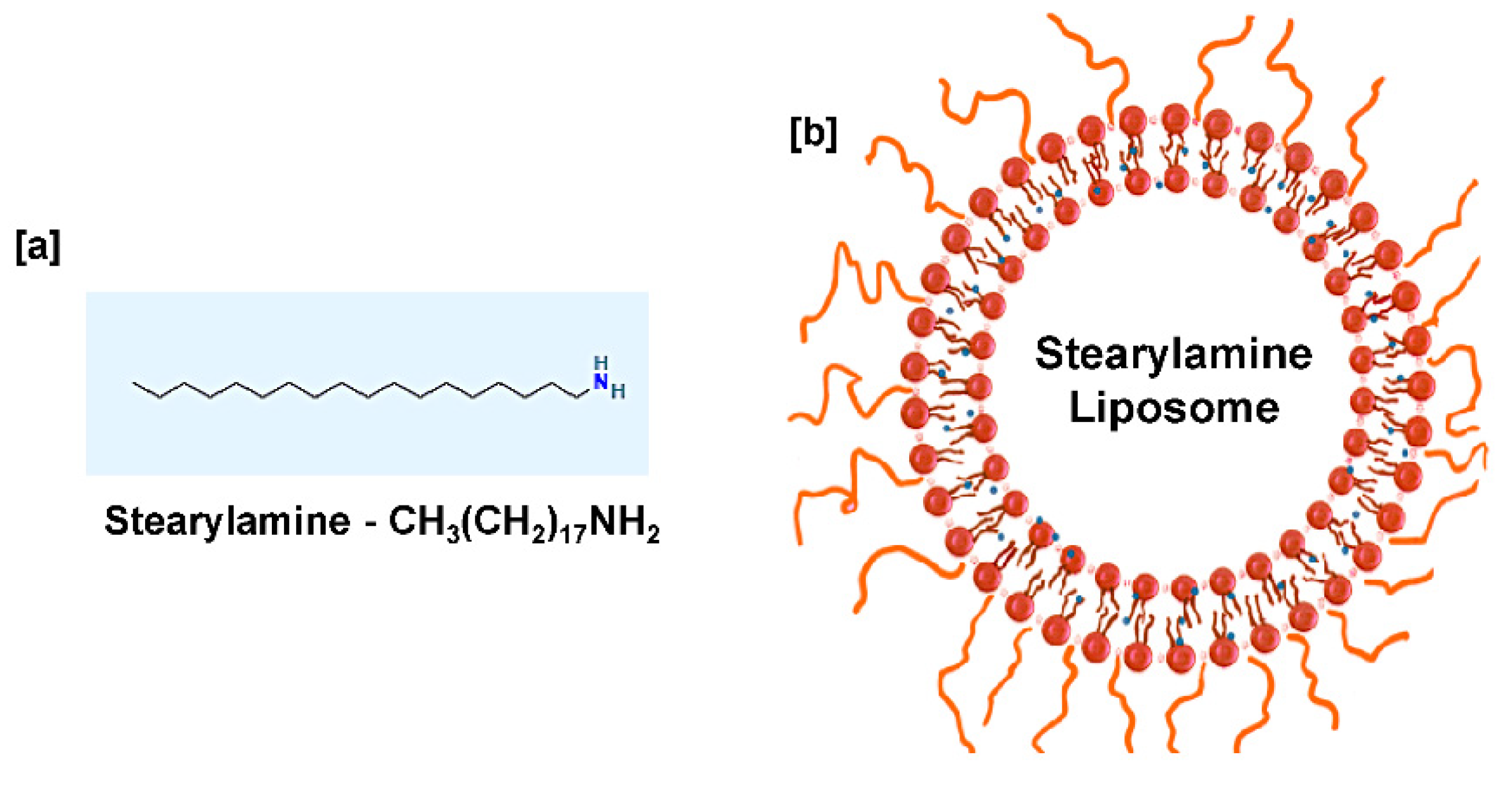
SA (C18H39N) is a cationic lipid [36] that exhibits a lipophilic and hydrophobic nature [37,38], having a molecular weight of 269.5 g/mol [39]. SA is a positively charged molecule at neutral pH with a pk value of 10.5 [40]. To enhance the SA solubility and understand its biological activity, SA is mixed with phospholipids to form lipid vesicles (liposomes) resembling biological membranes, as shown in Figure 1. Liposomes are spherical vesicles with particle sizes ranging from 30 nm to several micrometers comprising one or more lipid bilayers enclosing aqueous compartments. Majorly, phosphatidylcholine (PC) is widely used to prepare liposomes extracted from different sources such as EPC (egg PC, animal derivative) and SPC (soya PC, plant derivative). It has been reported that the choice of bilayer components determines the ‘rigidity’ or ‘fluidity’ and the net charge of the bilayer. Thus, PC is known to be the core element of all liposomes, whether conventional/stealth/charged or stimuli-responsive liposomes. The formation of aggregates and the property to adopt various microstructures makes phospholipids the most preferable for liposome preparation [41,42,43]. Liposome drug delivery systems are considered efficient due to their high biocompatibility, ability to enhance efficacy, reduce toxicity, and possess non-existing immunogenicity [44,45]. Based on their structural parameters, liposomes are characterized by multilamellar vesicles (MLVs), large unilamellar vesicles (LUVs), and small unilamellar vesicles (SUVs). A small amount of SA in DPPC liposomes changes the shape from MLVs to LUVs with a trapping efficiency of ten times that of DPPC liposomes [46].
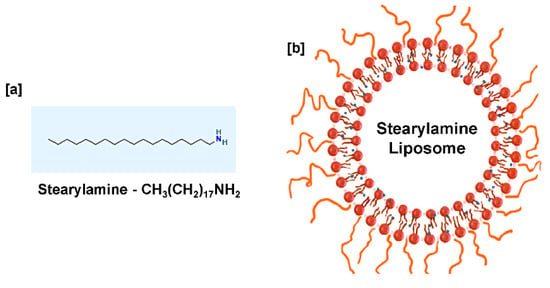
Figure 1.
(a) Chemical structure of stearylamine (b) Proposed liposome formulation containing stearylamine in a lipid bilayer and PEG-coated liposomal surface.
When SA is incorporated into the liposome bilayer, the size of lipid vesicles increases by repulsion and enhances drug entrapment efficiency [47]. Similarly, the liposome size is increased by raising the concentration of SA [48]. When the content of SA in liposomes increases, the zeta potential (ζ) value shows a high net positive charge of +52 mV for SA-PC (20 mol%) formulation having a polydispersity index of 0.2 [49,50]. In a study, the macrophages effectively uptake multilamellar SA liposomes compared with negative or neutral liposomes [51]. In liposomes, the structure of the hydrophilic group determines the toxic effect of the cationic lipids [52]. Amphotericin B in lipid nano-emulsions was studied against L. amazonensis in murine macrophages (J774). The lipid vesicle bearing 0.2% of SA showed higher cytotoxic effects when compared to 0.1% SA. This finding suggests that increasing the SA content on the liposome surface shows significant inhibitory action on L. amazonensis promastigotes [53]. Similarly, a study showed that an appropriate amount of SA improves liposome stability and therapeutic potential for delivering clinical drugs to diseased sites. Excess amounts of SA possess toxic effects and decrease liposome stability and efficacy [54]. Liposome containing 10 mol% of SA prevents verapamil and prochlorperazine leakage and retains their stability [55]. In a finding, prolonged exposure (90 min) of SA liposome to neutrophils resulted in segmentation and enlargement of the nucleus, and decondensation of chromatin. The SA-induced morphological changes in neutrophils decipher its therapeutic potential against tumor-associated neutrophils [56].
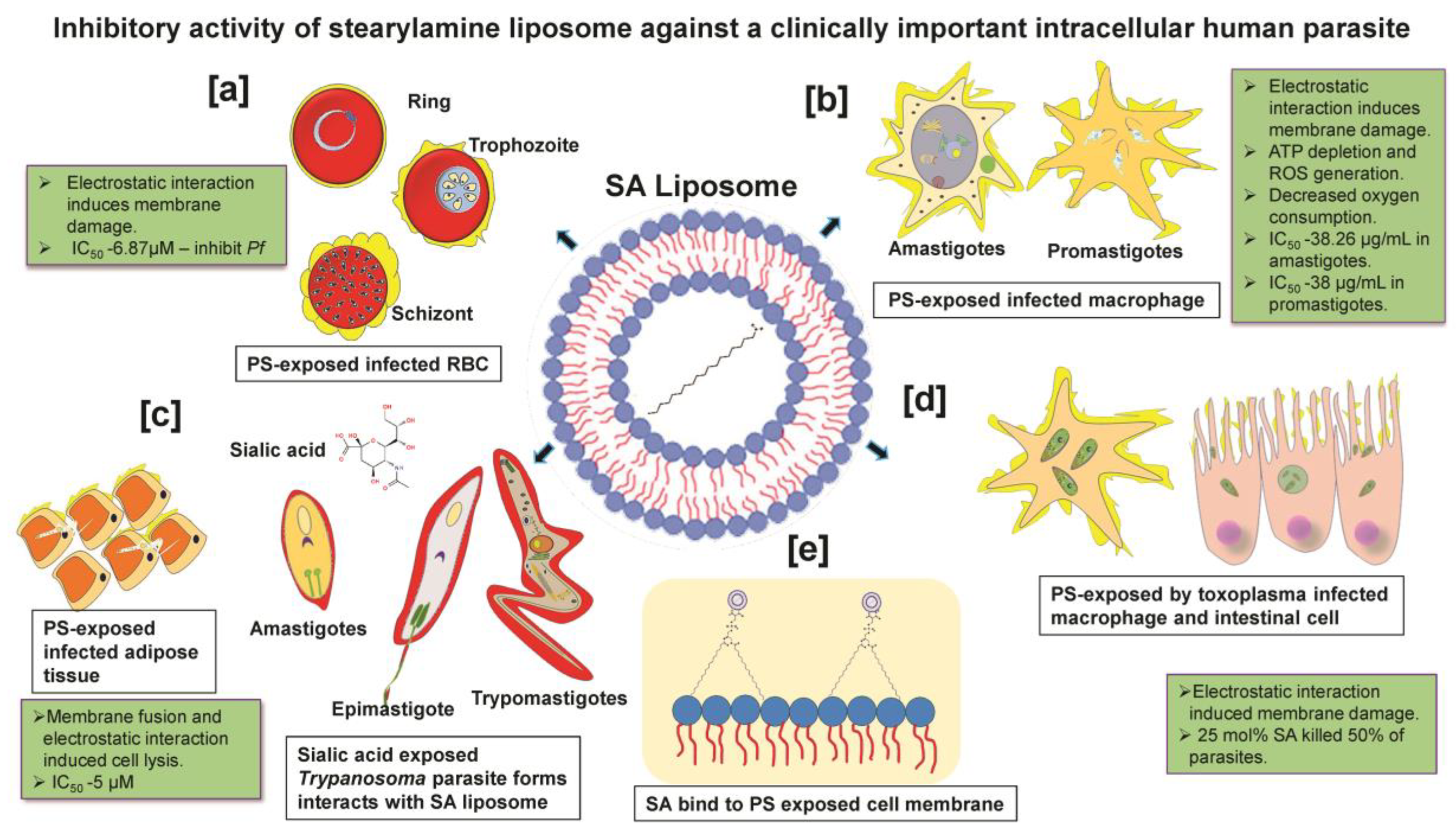
More importantly, targeting host death factors to kill infected cells is a potential therapeutic option for treating intracellular parasites [57]. A finding reported that SA-liposome interaction with WEHI231 cells activates the programmed cell death pathway of apoptosis, whereas anionic and neutral liposomes failed to activate [58,59]. SA liposome- induced reactive oxygen species (ROS) generation in cultured macrophages [60,61]. Substantial shreds of evidence have furnished that positively charged SA liposomes elicit anti-parasitic effects against Plasmodium, Trypanosoma, Toxoplasma, and Leishmania species through interaction with negatively charged parasite membrane (sialic acid) or infected host cells exposing phosphatidylserine (PS) on their surface, as shown in Figure 2 [62,63,64,65,66]. The PS is a negatively charged lipid localized in the eukaryotic membrane [67], restricted to the inner leaflet in normal conditions [68]. The floppase and flippase are ATP-dependent enzymes that translocate PS from the inner to outer leaflet during diseased conditions [69]. PS is a vital ligand that regulates cell death signaling [70] and regulates cell surface charge by inducing protein aggregation [71,72]. In this context, SA liposomes showed cytolytic effects by binding to acidic phospholipids having high affinity [73]. Additionally, SA liposomes have been proven to modulate the immune response relating to anti-parasitic activity without affecting the host cell [74]. SA-modified liposome containing ovalbumin as an oral vaccine adjuvant-induced significantly higher IgG and IgA antibody levels in mice sera. The incorporation of SA in liposome formulations also increased the production of IFN-γ and IL-4 in splenic cells of mice compared to unmodified liposomes [75]. SA liposomes as vaccine adjuvants combined with antigens significantly modulated the immune response due to the efficient internalization in macrophages. On the contrary, neutral and negatively charged liposomes with the exact quantities of antigens failed to show a comparable immunological response [51].
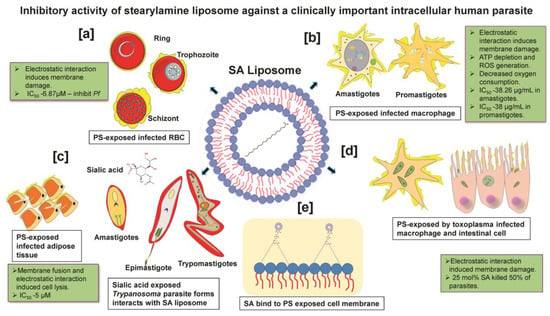
Figure 2.
Inhibitory activity of stearylamine liposome against clinically important intracellular human parasites: (a) Plasmodium: SA liposome interacts with PS-exposed infected RBC of intracellular Plasmodium during blood-stage infection. (b) Leishmania: Interaction of SA liposome with PS-exposed intracellular promastigotes and amastigotes of Leishmania parasite in macrophage. (c) Trypanosoma: Interaction of SA liposome with sialic acid exposing the developmental stage of the Trypanosomal parasite (amastigotes, epimastigotes and trypomastigote) and SA liposome interacts with PS-exposed Trypanosoma residing infected adipose cells. (d) Toxoplasma: Interaction of SA liposome with PS-exposed Toxoplasma residing in host nucleated cells and intracellular macrophages. (e) All four human parasites are most likely targeted via PS-exposed host cells as well, the presence of sialic acid residues in free-living parasites (without host) in circulation.
Recent studies have reported that SA exhibits anti-cancer potential. A study claims that the alkyl amine moiety of SA is responsible for disrupting the growth of various cancer cells with selective killing ability in cultured cancer cells and tumor mouse models. SA-PC liposome is comparatively more effective than other cationic liposome formulations such as PC-DTAB, PC-DOTAP and PC-DDAB against B16F10 cancer cells [49]. Delivering Anti-miR-191 loaded SA liposome formulation enhanced the inhibitory activity by suppressing tumor migration and inducing apoptosis against MCF-7 and ZR-75-1, having IC50 of 37.10 µM (SI = 5) and 33.39 µM (SI = 5.5), respectively, while IC50 of normal mouse fibroblast cell is 185.5 µM [76]. To confirm whether PS could be an ideal target for killing cancer cells, an experiment revealed that PC-SA liposomes failed to interact with cancer cells (B16F10) in the presence of anti-PS antibodies, confirming that SA-PC is PS-specific. Therefore, SA-PC targets cancer cells directly by interacting with PS. Also, PC-SA inhibited the growth of acute myeloid leukemia and acute promyelocytic leukemia cells isolated from patient samples displayed IC50 of 72.5 µg/mL and 69 µg/mL. SA-PC acts on various cancer cells by inducing apoptosis and ROS generation [49]. Intriguingly, SA liposome was reported to have antiviral properties, where SA liposome binds to the cell membrane of the A549 host cells which blocks the entry of herpes simplex type I virus, with comparable efficacy to standard antiviral acyclovir [77]. Therefore, the SA liposome perturbs the host cell membrane, a similar mechanism to that reported against intracellular parasites.
3. Stearylamine Liposome Activity on Plasmodium
Five Plasmodium species include Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi [78]. A parasite–RBC invasion is an important event in the Plasmodium life cycle that attributes to malarial pathogenesis [79]. Pf invades RBCs through two alternative invasion pathways, such as sialic acid-dependent (SAD) and sialic acid-independent (SAID) pathways [80]. In the SAD pathway, RBC sialoglycoproteins such as glycophorins, act as invasion receptors interacting with parasite ligands such as EBA-175, EBL-1, and EBA-140 [81,82,83,84,85,86,87,88,89,90]. In the SAID pathway, the hypothetical RBC receptors X and Z interact with an undefined Pf merozoite protein and Pf Rh2b [80]. A study confirmed that liposomes containing either stearyl alcohol (without amino group) or spermidine (without alkyl group) lack antimalarial activity. Thus, amino and alkyl groups are crucial for antiplasmodial action against Pf3D7. The presence of 20 mol% of SA in liposome showed IC50 −6.87µM, compared with 5 mol% having IC50 −41.15 µM, respectively. Therefore, 20 mol% of SA is optimal for antiplasmodial activity leading to growth arrest of Pf3D7 having a SI value of 27 by determining the CC50 of erythrocyte/IC50 of infected erythrocytes, suggesting minimal interaction with host erythrocytes [62,76]. The most likely accepted mechanism is the electrostatic interaction between positively charged SA liposomes and negatively charged infected RBC exposing increased levels of PS on the outer membrane [91]. However, it was reported that the exposed PS levels of iRBC on blood-stage forms of the ring are less. In contrast, trophozoites and schizont (mature forms) display increased levels by binding with anti-PS antibodies, responsible for cytoadherence of P. falciparum infected RBC to CD36 and thrombospondin [92,93]. Thus, blocking the interaction of PS-exposed infected RBC with endothelial cells using SA-PC may aid in preventing the severe form of complicated cerebral malaria. In another study, doxycycline (DOXY)-loaded SA liposome (10 mol%) showed a 38-fold enhancement against Pf3D7 in culture. In contrast, DOXY loaded with neutral liposomes showed a 16-fold enhancement relative to the free form of DOXY. When tested in P. berghei infection in murine malaria, the same formulations showed significant suppression in parasitemia, whereas DOXY-SA-liposome showed superior efficacy [94]. In another study, SA liposome (10 mol%) loaded with sodium ionophore monensin showed pronounced inhibitory effects compared to neutral or negatively charged liposomes against Pf3D7 and P. berghei infection in a mouse model [95]. Both studies underscore the presence of SA in liposomes combined with antimalarial drugs showing a synergistic antimalarial effect compared to SA-free liposomes. In in vitro studies, the IC50 value of CQ alone showed 48.66 ng/mL in Pf3D7 (CQ-sensitive strain) and 1488.33 ng/mL in PfRKL9 (CQ-resistant strain). However, the CQ incorporated in a nanostructured lipid carrier composed of 5 mol% of SA and 5 mol% of phosphatidylethanolamine showed IC50 of 12.8 ng/mL for Pf3D7, and 51.86 ng/mL for PfRKL9 strain. This finding demonstrates that CQ combined with SA is very effective against drug-resistant Pf, although the mechanism involved in CQ-sensitization in resistant strains remains to be explored [96]. Consistent with the experimental observation, further studies are warranted on whether SA liposome inhibition on Plasmodium growth is intervened by an effect on host cells and/or directly on the parasite. Further studies must explore whether SA-PC pre-treatment blocks the parasite entry during blood- and liver-stage infections.
4. Stearylamine Liposome Activity on Leishmania
The life cycle of Leishmania is digenic, and leishmaniasis is transmitted through metacyclic promastigotes, which are inhabited in the pharynx of the vector [97,98]. Leishmania species alter host macrophages and metabolic processes [99]. L. amazonensis amastigotes destabilize the host macrophages in the cell-cycling phase [100]. In L. mexicana, the presence of the cysteine proteinase gene is significant for host–parasite interaction and the survival of intracellular parasites [101]. SA-PC liposome showed anti-leishmanial activity by specific interaction with negatively charged PS on the promastigote and amastigote forms having ED50 of 33.41 µg/mL (SI-10.3) [102], 38 µg/mL (SI-9.07) [103], 38.26 µg/mL (SI-9) [104], which is absent in peritoneal macrophages (CC50- 344.69 µg/mL) [102]. The possible killing mode involves disruption of plasma membrane potential, resulting in depletion of cytosolic ATP and decreased oxygen consumption levels. To show whether PS could be an ideal target for SA, PS was masked with annexin-V, resulting in reduced killing activity, indicating that PS interacts with SA-PC liposomes and induces parasite death [102]. In another study, SA-PC liposome (22 mol%) killed 99% of L. donovani promastigotes within 60 min and 50% of the parasite in 13 min. On the other hand, PA (Phosphatidyl-acid)-PC and PC alone failed to inhibit the parasite. Thus, SA mediates its anti-leishmanial activity, selective to the parasites [105]. The SA-PC (88 µg/mL) loaded with sodium antimony gluconate (SAG) shows synergistic activity by eliminating chronic L. donovani infection from the spleen and liver in BALB/c mice. From the in vitro study, the EC50 value of SA-PC alone was 38.26 µg/mL and SAG 69.57 µg/mL, while 0.5 µg of SAG combined with 22.39 µg/mL of SA-PC/mL induced an equal effect against L. donovani amastigotes [104]. In SA-meglumine antimoniate (MA) liposomal formulation, SA improved the efficacy of MA liposome against amastigotes compared to promastigotes in culture conditions and BALB/c model of cutaneous leishmaniasis by reducing the splenic parasite burden and reduced lesion [48]. In an in vivo study, using SA-PC with sodium stibogluconate (SSG) liposome suppressed the parasite burden in the liver by about 97% in CK1R and 93% in GE1F8R in BALB/c mice [106]. Therefore, it seems reasonable to suggest that SA-PC mediates anti-parasitic effect by binding to PS-exposed cell membranes, followed by disruption of plasma membrane potential, depletion in cytosolic ATP levels, decreased oxygen consumption rate, ROS generation and apoptosis being the most likely reported mechanistic actions against leishmania parasite forms [102].
The in vitro study showed that 88 µg/mL SA-PC killed 95% of L. donovani amastigotes in 24 h, and in the in vivo study, a single dose of (55 mg) SA-PC liposome showed reduced hepatic parasite burden in BALB/c mice. This suggests that SA-PC liposomes are a new potential therapy for treating visceral leishmaniasis [63]. A single dose of paromomycin (16 mg/kg) associated with SA-PC (22 mg/mouse) killed the L. donovani parasite about 88 to 98% in the liver, spleen and bone marrow by downregulating IL-10 and TGF-β [103]. Thus, SA-PC plus paromomycin exhibits profound anti-leishmanial activity by direct parasite killing and its immunomodulatory effect. In the visceral leishmaniasis hamster model, Amp B alone showed 42.5% inhibition, and SA-PC showed 28.1%, but the combination of both showed 61.2% inhibition, indicating that SA enhances the effectiveness of Amp B [107]. In an in vitro study, curcumin analogue (HO-3867) loaded with SA-PC induced oxidative stress-induced apoptosis by PS exposure, depolarization of mitochondria, intracellular lipid accumulation, cell cycle arrest and morphological changes in L. donovani promastigotes. Additionally, it activated PARP-1 [poly (ADP)- ribose polymerase-1] and induced L. donovani metacaspase along with Sir2 gene downregulation which blocked the intracellular L. donovani amastigotes. Thus, parasite killing is mediated through aberrant nitric oxide levels and ROS [108]. Surface-modified SA liposome (SML) with Amp B showed effective killing activity against promastigote and amastigote forms of visceral leishmaniasis. The IC50 value of Amp B in conventional liposomes is 49.27 nM and SML (5% w/w mL) is 39.78 nM in promastigotes showing a ~1.34 fold enhancement [109]. Interestingly, a study showed that unilamellar SA liposomes of various sizes and different SA contents strongly influenced the interaction with macrophages. This finding correlates that SA liposome uptake is significantly higher in leishmanial parasite-infected peritoneal macrophages, resulting in reduced parasitic burden [110]. Based on the above findings, SA could be a potent anti-leishmanial agent with prophylactic and therapeutic properties.
5. Stearylamine Liposome Activity on Trypanosoma
Trypanosoma species completes its life cycle with two intermediate hosts, humans and vectors (tsetse fly stages) [111]. The host–parasite interaction of T. cruzi is based on their molecular specificity and invasion of host cells and tissues [112]. Under in vitro conditions, SA liposome (15 mol%) shows trypanocidal activity by inhibiting 99% of T. cruzi within 60 min of all the developmental stages [65]. Trypanosoma resides as a free-living blood form and host cells dependency for their survival. Trypomastigotes are the most severe form, which exposes higher amounts of negative charge than epimastigotes and amastigotes [113,114,115]. The free-living forms of Trypanosoma expose negatively charged sialic acid on their surface which facilitates the binding of SA liposomes, resulting in cytolytic activity [114]. Therefore, trypomastigotes are highly susceptible to SA-PC due to their increased sialic acid (negative charge) exposure. SA-PC liposome shows trypanocidal activity, and 15 mol% is optimal for killing more than 99% of T. brucei gambiense-infected cells within 30 min of treatment [116]. Amastigote forms display a minimal amount of sialic acid, making them less susceptible to SA-PC treatment [114]. An in vivo study revealed that diminazene encapsulated in SA-PC liposome (1 mol%) is more efficient than free drug (37% mortality) against T. brucei evansi [117]. Based on the above studies, it is clear that SA in liposomes displays a marked trypanocidal activity. Trypanocidal drugs loaded in SA liposomes exhibited enhanced killing potential toward the parasites. Further studies on its prophylactic effects remain to be determined.
6. Stearylamine Liposome Activity on Toxoplasma
Toxoplasmosis is caused by Toxoplasma gondii, an intracellular obligative parasite that replicates and invades the nucleated human cells and warm-blooded animals [118]. Tachyzoite and merozoites are replicative stages in the host, whereas bradyzoite and sporozoite are involved in the transmission of the disease [119]. Definitive and intermediate hosts are essential to completing their life cycle [120]. The polycationic property of SA is essential for cytocidal activity against T. gondii. In a study, 130 µg/mL (20 mol%) of SA-PC liposome curbed the growth of more than 95% of T. gondii within 90 min. Furthermore, 10 mg (30 mol%) of SA-PC liposome showed an inhibitory effect against 80% of a virulent strain of T. gondii in a mouse model [64]. Further studies are warranted to understand the prophylactic effect of SA-PC and combined therapy of antitrypanosomal drugs loaded in SA-PC against Toxoplasma infection.
7. Immunomodulatory Effect of SA Liposome
SA-PC liposomes not only act as a chemotherapeutic agent but have also been shown to modulate innate and adaptive immune responses. The immunomodulatory potential of SA liposome is well studied in the murine model of acute and chronic leishmaniasis. SA-PC-SSG directly kills the parasite by preferential uptake in L. donovani, downregulating the disease-promoting factors such as IL-10 and TGF-β, and upregulating Th1cytokines and macrophage microbial NO production [106]. Paromomycin loaded in SA-PC liposome induced the production of CD4+, CD8+ and IFN-γ by Th1-biased protective cell-mediated immunity which imparts protection against leishmaniasis [103]. Interestingly, prophylactic treatment of SA-PC loaded Amp B completely cleared the chronic L. donovani burden in bone marrow, spleen and hepatic cells in BALB/C mice by downregulation of IL-10 and upregulation of IL-12 and NO production [74]. Using an L. donovani-infected hamster model, the AmpB loaded with lipid polymer hybrid nanoparticles (LPNPS) containing SA showed a higher inhibitory effect (89.4%) compared with other lipid formulations without SA (AmpB-PNPS—63%, LAmpB—69.3%, dAmpB—56.3%). AmpB-LPNPS-SA treated groups showed increased IFN-γ and IL-12 (3.5-fold) and TNFα (2.4-fold) as well as suppressed IL-4, IL-10, and TNF-β expression. As a result, the triggered macrophage microbial molecules reversed the immunosuppressive condition towards the Th1-type immune response [121]. The immunomodulatory potential of SA liposome was explored for cancer immunotherapy. A finding reveals that SA-PC-loaded doxorubicin cures lung metastasis in mice by eliciting Th1 cytokine response-mediated anti-tumor immunity. The immune protective mechanism involves significant upregulation of IL-12 and NO production in splenic cultures and increased levels of IFN-γ, IL-2 and TNF-α in sera, suggesting that SA-PC imparts protection by directly acting on tumor growth and enhances the host immunity. Combined doxorubicin treatment with SA-PC liposome elicits Th1 cytokine response and cures metastatic tumors in the mouse model by enhancing lymph proliferation and T-cell response [122]. Therefore, SA liposomes possess chemotherapeutic and immunomodulatory potential, which may effectively tackle parasitic infections and metastatic cancer.
8. Safety and Biodistribution Profile of SA Liposome
An ex vivo study depicted 100% of relative hemolysis in various animal erythrocytes (guinea pig, rabbit and horse) exposed to 10 mol% of SA liposome for 60 min. On the other hand, 8% of hemolysis was reported in human RBC, suggesting its minimal cytotoxic effects [73]. However, increasing SA density (>20 mol%) on liposomes provokes increased toxicity and reduces the stability of liposomes. SPC-SA at 16 µg/mL failed to elicit any hemolytic activity in human RBC (2% hematocrit) up to 42 h [62]. Likewise, SPC/Chol/SA formulation induced hemolysis >50 µg/mL when incubated at 4% hematocrit for 1 h [76]. In another study, an SA-based formulation (SA-TDZA-liposome) did not provoke any hemolytic activity when tested at 2% hematocrit. Such studies underscore that SA liposome administration via the intravenous route is relatively safe and reliable [54]. Furthermore, in acute toxicity studies in mice models, intravenous administration of 220 mg of PC-SA liposomes showed no signs of mortality within 24 h and no apparent adverse effects were observed for 15 days. Histopathological organ toxicity revealed no signs of toxicity in vital organs compared to normal mice. This study advocates that 220 mg of PC-SA liposome is safe for administration and can be further extrapolated in clinical use. In addition, SA liposome specifically caused membrane damage to cancerous cells such as MCF-7, HepG2 and ZR-75-1, whereas no cytotoxic effects were observed in normal cells such as L929, HEK293 and human PBMCs at therapeutic concentrations having varied selectivity index values [49,76,123]. From this convincing evidence, SA-based liposomal formulations with optimal SA content may selectively act on diseased cells without causing harmful effects to normal cells and human erythrocytes. Further studies are warranted in higher model organisms before it enters clinical trials.
Intravenous administration of radiolabelled (125I- albumin) positively charged SA liposomes in rats showed a longer circulating time (3 h) as compared to negatively charged liposomes (30 min) and neutral liposomes (2 h) in plasma [124]. Though charged liposomes are eliminated from the reticuloendothelial system more quickly, adding PEG moiety on the surface may significantly enhance their circulation time. A finding states that fast clearance of charged liposomes was reversed by attaching optimal PEG density of varied chain length (750, 5000 kDa) having a retention time of >5 h in mice plasma [125]. To realize the full potential of anti-parasitic drugs in clinics, delivering through PEG-attached SA liposomes could be an ideal strategy to combat parasitic infections. SA-bearing pemetrexed disodium (PMX) liposomal formulation showed prolonged blood circulation and lung tissue retention with 2.85-fold and 2.35-fold higher than the conventional liposome and free PMX [126]. This study indicates that SA-based formulations exhibit a lung targeting effect which may be utilized for treating non-small cell lung cancer, metastasis cancer and respiratory pathogens. Another research finding highlighted that incorporating SA to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) liposome showed ~6.23-fold enhanced bioavailability of nintedanib-esylate compared to the free form [127]. From these studies, it is evident that the presence of SA in liposomes significantly improves the stability of the liposomes and has a targeted-based therapeutic effect.
9. Conclusions and Future Perspectives
SA displays remarkable activity against the four clinically important parasitic diseases. The significant advantage of SA-PC liposome is a positively charged component in delivery vehicles that display anti-parasitic activity and immunomodulatory potential. Thus, delivering anti-parasitic agents using SA-based liposomal formulations could further enhance the therapeutic efficacy and delay resistance development. Another approach involving the co-delivery of drugs in SA-PC vehicles could significantly curtail the parasite replication and block their transmission to a greater extent. However, no studies have yet been conducted to understand the efficacy of SA-PC liposomes on the survival of parasite vectors. Moreover, using SA-PC as a prophylactic agent against Plasmodium, Trypanosoma and Toxoplasma remain unexplored. Furthermore, the precise mechanistic action of SA targeting the parasite’s growth cycle or host imbalance is not well understood. As a result, this potent molecule can be explored as a multi-stage anti-protozoan agent in clinics at optimal dosages. Additionally, the effect of SA liposome on opportunistic pathogens leading to co-infection remains elusive. More studies are required to explore the therapeutic action of other clinical parasites. Overall, SA possesses minimal cytotoxic effects on mammalian cells, further strengthening the clinical utility of SA-PC liposome in humans.
Author Contributions
J.V., M.M. and V.R. contributed towards the literature survey, data interpretation and preparation of the figures. V.R. contributed to the overall supervision, design of the review, critical analysis of the intellectual content, revision of the manuscript and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research grant DST-INSPIRE Faculty Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The V.R. lab is supported by a research grant from the Department of Science & Technology (DST), INSPIRE-Faculty Project (DST/INSPIRE/04/2018/003541), Ministry of Science and Technology, Government of India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. A Global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014.
- Gupta, S.; Gazendam, N.; Farina, J.M.; Saldarriaga, C.; Mendoza, I.; López-Santi, R.; Pérez, G.E.; Martínez-Sellés, M.; Baranchuk, A. Malaria and the heart: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Bouyou-Akotet, M.K.; Ionete-Collard, D.E.; Mabika-Manfoumbi, M.; Kendjo, E.; Matsiegui, P.-B.; Mavoungou, E.; Kombila, M. Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar. J. 2003, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Staalsoe, T.; Lavstsen, T.; Jensen, A.T.; Sowa, M.K.; Arnot, D.E.; Hviid, L.; Theander, T.G. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003, 49, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Indari, O.; Chandramohanadas, R.; Jha, H.C. Epstein–Barr virus infection modulates blood–brain barrier cells and its co-infection with Plasmodium falciparum induces RBC adhesion. Pathog. Dis. 2021, 79, ftaa080. [Google Scholar] [CrossRef]
- Brooker, S.J.; Pullan, R.L.; Gitonga, C.W.; Ashton, R.A.; Kolaczinski, J.H.; Kabatereine, N.B.; Snow, R.W. Plasmodium–helminth coinfection and its sources of heterogeneity across east Africa. J. Infect. Dis. 2012, 205, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Akinbo, F.; Olowookere, T.; Oriakhi, M.O. Co-infection of Plasmodium falciparum and HIV among pregnant women in Edo State, Nigeria. J. Afr. Assoc. Physiol. Sci. 2019, 7, 53–58. [Google Scholar]
- Kamhawi, S. Phlebotomine sand flies and Leishmania parasites: Friends or foes? Trends Parasitol. 2006, 22, 439–445. [Google Scholar] [CrossRef]
- WHO. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 15 July 2022).
- Agrawal, Y.; Sinha, A.; Upadhyaya, P.; Kafle, S.; Rijal, S.; Khanal, B. Hematological profile in visceral leishmaniasis. Int. J. Infect. Microbiol. 2013, 2, 39–44. [Google Scholar] [CrossRef]
- Rathnayake, D.; Ranawake, R.R.; Sirimanna, G.; Siriwardhane, Y.; Karunaweera, N.; De Silva, R. Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int. J. Dermatol. 2010, 49, 549–551. [Google Scholar] [CrossRef]
- Gaifer, Z.; Boulassel, M.-R. Leishmania infantum and Epstein-Barr virus co-infection in a patient with hemophagocytosis. Infect. Dis. Rep. 2017, 8, 6545. [Google Scholar] [CrossRef]
- de Lourdes Higuchi, M.; De Brito, T.; Reis, M.M.; Barbosa, A.; Bellotti, G.; Pereira-Barreto, A.C.; Pileggi, F. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: Light microscopy and immunohistochemical findings. Cardiovasc. Pathol. 1993, 2, 101–106. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.B.; Troncon, L.E.A.; Dantas, R.O.; Meneghelli, U.G. Gastrointestinal manifestations of Chagas’ disease. Am. J. Gastroenterol. 1998, 93, 884–889. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 25 July 2022).
- Cordova, E.; Boschi, A.; Ambrosioni, J.; Cudos, C.; Corti, M. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int. J. Infect. Dis. 2008, 12, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.M.C.; Bezerra, R.C.; do S Guilherme, C.; Lopes, M.H.; Shikanai-Yasuda, M.A. Exacerbation of HIV viral load simultaneous with asymptomatic reactivation of chronic Chagas’ disease. Am. J. Trop. Med. Hyg. 2002, 67, 521–523. [Google Scholar] [CrossRef][Green Version]
- WHO. 2015. Available online: https://www.euro.who.int/__data/assets/pdf_file/0011/294599/Factsheet-Toxoplasmosis (accessed on 10 August 2022).
- Alsammani, M.A.; Ahmed, S.R.; Alsheeha, M.A.; Saadia, Z.; Khairi, S.A.; Research, G. Co-infection with Toxoplasma gondii and Clostridium perfringens in a postpartum woman with uterine gas gangrene: A case report. J. Obstet. Gynaecol. Res. 2012, 38, 1024–1027. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Oh, J.; Roos, D.S. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 2001, 45, 1271–1277. [Google Scholar] [CrossRef]
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.V.; Rizk, S.S.; Njimoh, D.L.; Ryan, Y.; Chotivanich, K.J.N. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef]
- Chinappi, M.; Via, A.; Marcatili, P.; Tramontano, A. On the mechanism of chloroquine resistance in Plasmodium falciparum. PLoS ONE 2010, 5, e14064. [Google Scholar] [CrossRef]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef]
- Pérez-Victoria, J.M.; Pérez-Victoria, F.J.; Parodi-Talice, A.; Jiménez, I.A.; Ravelo, A.G.; Castanys, S.; Gamarro, F. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 2001, 45, 2468–2474. [Google Scholar] [CrossRef]
- Wyllie, S.; Foth, B.J.; Kelner, A.; Sokolova, A.Y.; Berriman, M.; Fairlamb, A.H. Nitroheterocyclic drug resistance mechanisms in Trypanosoma brucei. J. Antimicrob. Chemother. 2016, 71, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.C.; Beverley, S.; Cotrim, P.C. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol. Biochem. Parasitol. 2003, 130, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, E.R.; Borotz, S.E.; Nothnagel, R.F. Mutants of Toxoplasma gondii Resistant to Atovaquone (566C80) or Decoquinate. J. Parasitol. 1993, 79, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.; Read, K.D.; Fairlamb, A.H. Cross-Resistance to Nitro Drugs and Implications for Treatment of Human African Trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900. [Google Scholar] [CrossRef]
- Barrett, M.; Fairlamb, A. The Biochemical Basis of Arsenical–Diamidine Crossresistance in African Trypanosomes. Parasitol. Today 1999, 15, 136–140. [Google Scholar] [CrossRef]
- Cowman, A.F.; Galatis, D.; Thompson, J.K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl. Acad. Sci. USA 1994, 91, 1143–1147. [Google Scholar] [CrossRef]
- Duraisingh, M.T.; Cowman, A.F. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005, 94, 181–190. [Google Scholar] [CrossRef]
- Aliee, M.; Castaño, S.; Davis, C.N.; Patel, S.; Miaka, E.M.; Spencer, S.E.F.; Keeling, M.J.; Chitnis, N.; Rock, K.S. Predicting the impact of COVID-19 interruptions on transmission of gambiense human African trypanosomiasis in two health zones of the Democratic Republic of Congo. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 245–252. [Google Scholar] [CrossRef]
- Sebastião, C.S.; Gaston, C.; Paixão, J.P.; Sacomboio, E.N.; Neto, Z.; de Vasconcelos, J.N.; Morais, J. Coinfection between SARS-CoV-2 and vector-borne diseases in Luanda, Angola. J. Med. Virol. 2022, 94, 366–371. [Google Scholar] [CrossRef]
- Mazaherifar, S.; Solhjoo, K.; Abdoli, A. Outbreak of cutaneous leishmaniasis before and during the COVID-19 pandemic in Jahrom, an endemic region in southwest of Iran: Cutaneous leishmaniasis and the COVID-19 pandemic. Emerg. Microbes Infect. 2022, 11, 2218–2221. [Google Scholar] [CrossRef]
- Teixeira, H.; Rosilio, V.; Laigle, A.; Lepault, J.; Erk, I.; Scherman, D.; Benita, S.; Couvreur, P.; Dubernet, C. Characterization of oligonucleotide/lipid interactions in submicron cationic emulsions: Influence of the cationic lipid structure and the presence of PEG-lipids. Biophys. Chem. 2001, 92, 169–181. [Google Scholar] [CrossRef]
- Manjunath, K.; Venkateswarlu, V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J. Control. Release 2005, 107, 215–228. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Amphiphilic chitosan nanosphere: Studies on formation, toxicity, and guest molecule incorporation. Bioorganic Med. Chem. 2008, 16, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Biophysical characterization of lutein or beta carotene-loaded cationic liposomes. RSC Adv. 2020, 10, 32409–32422. [Google Scholar] [CrossRef] [PubMed]
- Rosing, J.; Speijer, H.; Zwaal, R.F.A. Prothrombin activation on phospholipid membranes with positive electrostatic potential. Biochemistry 1988, 27, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Lasic, D.D.; Walker, S.; Bode, C.J.; Paquet, K.-J. Kinetic and thermodynamic effects on the structure and formation of phosphatidylcholine vesicles. Hepatology 1991, 13, 1010–1013. [Google Scholar] [CrossRef]
- Ulrich, A.S. Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep. 2002, 22, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, M.; Jaiswal, M.K.; Sarma, H.D.; Bahadur, D.; Banerjee, R. Biocompatibility and therapeutic evaluation of magnetic liposomes designed for self-controlled cancer hyperthermia and chemotherapy. Integr. Biol. 2017, 9, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Téllez, J.; Echeverry, M.C.; Romero, I.; Guatibonza, A.; Ramos, G.S.; De Oliveira, A.C.B.; Frézard, F.; Demicheli, C. Use of liposomal nanoformulations in antileishmania therapy: Challenges and perspectives. J. Liposome Res. 2020, 31, 169–176. [Google Scholar] [CrossRef]
- Yokoyama, S.; Inagaki, A.; Tsuchiya, K.; Sakai, H.; Imura, T.; Ohkubo, T.; Tsubaki, N.; Abe, M. Stearylamine Changes the Liposomal Shape from MLVs to LUVs. J. Oleo Sci. 2005, 54, 251–254. [Google Scholar] [CrossRef]
- Soni, A.; Jain, V.; Jain, S.K.; Khangar, P.K. Preparation and characterization of amphotericin B mannosylated liposomes for effective management of visceral leishmaniasis. J. Drug Deliv. Ther. 2021, 11, 113–118. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Fallah, M.; Jaafari, M.R. The activity of encapsulated meglumine antimoniate in stearylamine-bearing liposomes against cutaneous leishmaniasis in BALB/c mice. Exp. Parasitol. 2019, 200, 30–35. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, S.; Sen, T.; Shadab, M.; Banerjee, I.; Basu, S.; Ali, N. A Novel Therapeutic Strategy for Cancer Using Phosphatidylserine Targeting Stearylamine-Bearing Cationic Liposomes. Mol. Ther. Nucleic Acids 2017, 10, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, V.; Manjunath, K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Release 2004, 95, 627–638. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kunisawa, J.; Hayashi, A.; Tsutsumi, Y.; Kubo, K.; Nakagawa, S.; Fujiwara, H.; Hamaoka, T.; Mayumi, T. Positively Charged Liposome Functions as an Efficient Immunoadjuvant in Inducing Immune Responses to Soluble Proteins. Biochem. Biophys. Res. Commun. 1997, 240, 793–797. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Matos, A.P.D.S.; Lopes, D.C.D.X.P.; Peixoto, M.L.H.; Cardoso, V.D.S.; Vermelho, A.B.; Santos-Oliveira, R.; Viçosa, A.L.; Holandino, C.; Ricci-Júnior, E. Development, characterization, and anti-leishmanial activity of topical amphotericin B nanoemulsions. Drug Deliv. Transl. Res. 2020, 10, 1552–1570. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, L.; Liu, J.; Huang, G. Construction and evaluation in vitro and in vivo of tedizolid phosphate loaded cationic liposomes. J. Liposome Res. 2017, 28, 322–330. [Google Scholar] [CrossRef]
- Webb, M.S.; Wheeler, J.J.; Bally, M.B.; Mayer, L.D. The cationic lipid stearylamine reduces the permeability of the cationic drugs verapamil and prochlorperazine to lipid bilayers: Implications for drug delivery. Biochim. Biophys. Acta (BBA) Biomembr. 1995, 1238, 147–155. [Google Scholar] [CrossRef]
- Lotosh, N.Y.; Aliaseva, S.O.; Malashenkova, I.K.; Sorokoumova, G.M.; Vasilov, R.G.; Selischeva, A.A. Cationic Liposomes Cause ROS Generation and Release of Neutrophil Extracellular Traps. Biochem. Suppl. Ser. A Membr. Cell Biol. 2019, 13, 40–49. [Google Scholar] [CrossRef]
- Naderer, T.; Fulcher, M.C. Targeting apoptosis pathways in infections. J. Leukoc. Biol. 2018, 103, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Takano, S.; Arima, H.; Tsuchiya, S. Induction of apoptosis in WEHI 231 cells by cationic liposomes. Pharm. Res. 2000, 17, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Induction of apoptosis in macrophages by cationic liposomes. FEBS Lett. 1999, 460, 472–476. [Google Scholar] [CrossRef]
- Takano, S.; Aramaki, Y.; Tsuchiya, S. Physicochemical Properties of Liposomes Affecting Apoptosis Induced by Cationic Liposomes in Macrophages. Pharm. Res. 2003, 20, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Aramaki, Y.; Tsuchiya, S. Lipoxygenase May Be Involved in Cationic Liposome-Induced Macrophage Apoptosis. Biochem. Biophys. Res. Commun. 2001, 288, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Hasan, G.M.; Garg, N.; Dogra, E.; Surolia, R.; Ghosh, P.C. Inhibition of the growth of Plasmodium falciparum in culture by stearylamine-phosphatidylcholine liposomes. J. Parasitol. Res. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Dey, T.; Anam, K.; Afrin, F.; Ali, N. Antileishmanial Activities of Stearylamine-Bearing Liposomes. Antimicrob. Agents Chemother. 2000, 44, 1739–1742. [Google Scholar] [CrossRef]
- Tachibana, H.; Yoshihara, E.; Kaneda, Y.; Nakae, T. Protection of Toxoplasma gondii-Infected Mice by Stearylamine-Bearing Liposomes. J. Parasitol. 1990, 76, 352–355. [Google Scholar] [CrossRef]
- Yoshihara, E.; Tachibana, H.; Nakae, T. Trypanocidal activity of the stearylamine-bearing liposome invitro. Life Sci. 1987, 40, 2153–2159. [Google Scholar] [CrossRef]
- Sinha, R.; Roychoudhury, J.; Palit, P.; Ali, N. Cationic liposomal sodium stibogluconate (SSG), a potent therapeutic tool for treatment of infection by SSG-sensitive and-resistant Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 344–355. [Google Scholar] [CrossRef]
- Antila, H.S.; Buslaev, P.; Favela-Rosales, F.; Ferreira, T.M.; Gushchin, I.; Javanainen, M.; Kav, B.; Madsen, J.J.; Melcr, J.; Miettinen, M.S.; et al. Headgroup Structure and Cation Binding in Phosphatidylserine Lipid Bilayers. J. Phys. Chem. B 2019, 123, 9066–9079. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.G.; Grinstein, S. Sensing Phosphatidylserine in Cellular Membranes. Sensors 2011, 11, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Kroemer, G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013, 23, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of Phospholipid Asymmetry and Surface Exposure of Phosphatidylserine Is Required for Phagocytosis of Apoptotic Cells by Macrophages and Fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane Phosphatidylserine Regulates Surface Charge and Protein Localization. Science 2008, 319, 210–213. [Google Scholar] [CrossRef]
- Zhao, H.; Tuominen, E.K.J.; Kinnunen, P.K.J. Formation of Amyloid Fibers Triggered by Phosphatidylserine-Containing Membranes. Biochemistry 2004, 43, 10302–10307. [Google Scholar] [CrossRef]
- Yoshihara, E.; Nakae, T. Cytolytic activity of liposomes containing stearylamine. Biochim. Biophys. Acta (BBA)-Biomembr. 1986, 854, 93–101. [Google Scholar] [CrossRef]
- Banerjee, A.; De, M.; Ali, N. Complete Cure of Experimental Visceral Leishmaniasis with Amphotericin B in Stearylamine-Bearing Cationic Liposomes Involves Down-Regulation of IL-10 and Favorable T Cell Responses. J. Immunol. 2008, 181, 1386–1398. [Google Scholar] [CrossRef]
- Watarai, S.; Sasaki, Y. Evaluation of stearylamine-modified liposomes for the oral vaccine adjuvant. J. Infect. Dis. Ther. 2014, 2, 1–6. [Google Scholar]
- Sharma, S.; Rajendran, V.; Kulshreshtha, R.; Ghosh, P.C. Enhanced efficacy of anti-miR-191 delivery through stearylamine liposome formulation for the treatment of breast cancer cells. Int. J. Pharm. 2017, 530, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Kobayashi, M.; Yoshida, S.; Onodera, R.; Inoue, N.; Takeuchi, H. Effects of cationic liposomes with stearylamine against virus infection. Int. J. Pharm. 2018, 543, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Galimberti, L.; Milazzo, L.; Corbellino, M. Biology of human malaria plasmodia including plasmodium knowlesi. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012013. [Google Scholar] [CrossRef]
- Gilson, P.R.; Crabb, B.S. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 2009, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.; Yamodo, I.; Ranjan, R.; Li, X.; Mines, G.; Marinkovic, M.; Hanada, T.; Oh, S.S.; Chishti, A.H. Human erythrocyte band 3 functions as a receptor for the sialic acid-independent invasion of Plasmodium falciparum. Role of the RhopH3–MSP1 complex. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2855–2870. [Google Scholar] [CrossRef]
- Orlandi, P.A.; Klotz, F.W.; Haynes, J.D. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac (alpha 2-3) Gal-sequences of glycophorin A. J. Cell Biol. 1992, 116, 901–909. [Google Scholar] [CrossRef]
- Sim, B.K.L.; Chitnis, C.E.; Wasniowska, K.; Hadley, T.J.; Miller, L.H. Receptor and Ligand Domains for Invasion of Erythrocytes by Plasmodium falciparum. Science 1994, 264, 1941–1944. [Google Scholar] [CrossRef]
- Duraisingh, M.T.; Maier, A.G.; Triglia, T.; Cowman, A.F. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 4796–4801. [Google Scholar] [CrossRef]
- Gilberger, T.-W.; Thompson, J.K.; Triglia, T.; Good, R.T.; Duraisingh, M.T.; Cowman, A.F. A Novel Erythrocyte Binding Antigen-175 Paralogue fromPlasmodium falciparum Defines a New Trypsin-resistant Receptor on Human Erythrocytes. J. Biol. Chem. 2003, 278, 14480–14486. [Google Scholar] [CrossRef]
- Mayer, D.C.G.; Cofie, J.; Jiang, L.; Hartl, D.L.; Tracy, E.; Kabat, J.; Mendoza, L.H.; Miller, L.H. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc. Natl. Acad. Sci. USA 2009, 106, 5348–5352. [Google Scholar] [CrossRef]
- Li, X.; Marinkovic, M.; Russo, C.; McKnight, C.J.; Coetzer, T.L.; Chishti, A.H. Identification of a specific region of Plasmodium falciparum EBL-1 that binds to host receptor glycophorin B and inhibits merozoite invasion in human red blood cells. Mol. Biochem. Parasitol. 2012, 183, 23–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lobo, C.-A.; Rodriguez, M.; Reid, M.; Lustigman, S. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 2003, 101, 4628–4631. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.C.G.; Kaneko, O.; Hudson-Taylor, D.E.; Reid, M.E.; Miller, L.H. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc. Natl. Acad. Sci. USA 2001, 98, 5222–5227. [Google Scholar] [CrossRef] [PubMed]
- Narum, D.L.; Fuhrmann, S.R.; Luu, T.; Sim, B.K.L. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol. Biochem. Parasitol. 2002, 119, 159–168. [Google Scholar] [CrossRef]
- Jaskiewicz, E.; Jodłowska, M.; Kaczmarek, R.; Zerka, A. Erythrocyte glycophorins as receptors for Plasmodium merozoites. Parasites Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Rajendran, V.; Rohra, S.; Raza, M.; Hasan, G.M.; Dutt, S.; Ghosh, P.C. Stearylamine Liposomal Delivery of Monensin in Combination with Free Artemisinin Eliminates Blood Stages of Plasmodium falciparum in Culture and P. berghei Infection in Murine Malaria. Antimicrob. Agents Chemother. 2016, 60, 1304–1318. [Google Scholar] [CrossRef]
- Fernandez-Arias, C.; Rivera-Correa, J.; Gallego-Delgado, J.; Rudlaff, R.; Fernandez, C.; Roussel, C.; Götz, A.; Gonzalez, S.; Mohanty, A.; Mohanty, S.; et al. Anti-Self Phosphatidylserine Antibodies Recognize Uninfected Erythrocytes Promoting Malarial Anemia. Cell Host Microbe 2016, 19, 194–203. [Google Scholar] [CrossRef]
- Eda, S.; Sherman, I.W. Cytoadherence of malaria-infected red blood cells involves exposure of phosphatidylserine. Cell. Physiol. Biochem. 2002, 12, 373–384. [Google Scholar] [CrossRef]
- Rajendran, V.; Singh, C.; Ghosh, P.C. Improved efficacy of doxycycline in liposomes against Plasmodium falciparum in culture and Plasmodium berghei infection in mice. Can. J. Physiol. Pharmacol. 2018, 96, 1145–1152. [Google Scholar] [CrossRef]
- Rajendran, M.P.A.P.C.G.V.; Pachauri, M.; Ghosh, P.C. Combinatorial Effects of Monensin in Liposome Formulations with Antimalarial Drugs Against Blood Stages of Plasmodium falciparum in Culture and P. berghei Infection. Curr. Drug Ther. 2018, 13, 74–82. [Google Scholar] [CrossRef]
- Baruah, U.K.; Gowthamarajan, K.; Ravisankar, V.; Karri, V.V.S.R.; Simhadri, P.K.; Singh, V. Optimisation of chloroquine phosphate loaded nanostructured lipid carriers using Box–Behnken design and its antimalarial efficacy. J. Drug Target. 2017, 26, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.B.; Das, M.; Sudhandiran, G.; Shaha, C. Increase in Cytosolic Ca2+ Levels through the Activation of Non-selective Cation Channels Induced by Oxidative Stress Causes Mitochondrial Depolarization Leading to Apoptosis-like Death in Leishmania donovaniPromastigotes. J. Biol. Chem. 2002, 277, 24717–24727. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Ghoshal, A.; Mandal, C.; Shaha, C. Leishmania cell surface prohibitin: Role in host–parasite interaction. Cell. Microbiol. 2010, 12, 432–452. [Google Scholar] [CrossRef]
- Rabhi, I.; Rabhi, S.; Ben-Othman, R.; Rasche, A.; Consortium, S.; Daskalaki, A.; Trentin, B.; Piquemal, D.; Regnault, B.; Descoteaux, A.; et al. Transcriptomic Signature of Leishmania Infected Mice Macrophages: A Metabolic Point of View. PLoS Negl. Trop. Dis. 2012, 6, e1763. [Google Scholar] [CrossRef] [PubMed]
- Fortéa, J.O.Y.; de La Llave, E.; Regnault, B.; Coppée, J.-Y.; Milon, G.; Lang, T.; Prina, E. Transcriptional signatures of BALB/c mouse macrophages housing multiplying Leishmania amazonensis amastigotes. BMC Genom. 2009, 10, 119. [Google Scholar] [CrossRef]
- Frame, M.J.; Mottram, J.C.; Coombs, G.H. Analysis of the roles of cysteine proteinases of Leishmania mexicana in the host–parasite interaction. Parasitology 2000, 121, 367–377. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, J.; Ali, N. Stearylamine-bearing cationic liposomes kill Leishmania parasites through surface exposed negatively charged phosphatidylserine. J. Antimicrob. Chemother. 2007, 61, 103–110. [Google Scholar] [CrossRef]
- Banerjee, A.; De, M.; Ali, N. Combination Therapy with Paromomycin-Associated Stearylamine-Bearing Liposomes Cures Experimental Visceral Leishmaniasis through Th1-Biased Immunomodulation. Antimicrob. Agents Chemother. 2011, 55, 1661–1670. [Google Scholar] [CrossRef]
- Pal, S.; Ravindran, R.; Ali, N. Combination Therapy Using Sodium Antimony Gluconate in Stearylamine-Bearing Liposomes against Established and Chronic Leishmania donovani Infection in BALB/c Mice. Antimicrob. Agents Chemother. 2004, 48, 3591–3593. [Google Scholar] [CrossRef]
- Afrin, F.; Dey, T.; Anam, K.; Ali, N. Leishmanicidal activity of stearylamine-bearing liposomes in vitro. J. Parasitol. 2001, 87, 188–193. [Google Scholar] [CrossRef]
- Roychoudhury, J.; Sinha, R.; Ali, N. Therapy with Sodium Stibogluconate in Stearylamine-Bearing Liposomes Confers Cure against SSG-Resistant Leishmania donovani in BALB/c Mice. PLoS ONE 2011, 6, e17376. [Google Scholar] [CrossRef]
- Rathore, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Khare, P.; Jain, A.; Jain, S.K. Mannosylated liposomes bearing Amphotericin B for effective management of visceral Leishmaniasis. J. Liposome Res. 2011, 21, 333–340. [Google Scholar] [CrossRef]
- Das, A.; Kamran, M.; Ali, N. HO-3867 Induces ROS-Dependent Stress Response and Apoptotic Cell Death in Leishmania donovani. Front. Cell. Infect. Microbiol. 2021, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Patere, S.N.; Pathak, P.O.; Shukla, A.K.; Singh, R.K.; Dubey, V.K.; Mehta, M.J.; Patil, A.G.; Gota, V.; Nagarsenker, M.S. Surface-Modified Liposomal Formulation of Amphotericin B: In vitro Evaluation of Potential Against Visceral Leishmaniasis. AAPS PharmSciTech 2016, 18, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, R.; Lagocki, P.; Rahman, Y. The effects of charge and size on the interaction of unilamellar liposomes with macrophages. Biochim. Biophys. Acta (BBA) Biomembr. 1984, 772, 93–101. [Google Scholar] [CrossRef]
- Herman, M.; Pérez-Morga, D.; Schtickzelle, N.; Michels, P.A. Turnover of glycosomes during life-cycle differentiation ofTrypanosoma brucei. Autophagy 2008, 4, 294–308. [Google Scholar] [CrossRef]
- Castillo, C.; Carrillo, I.; Libisch, G.; Juiz, N.; Schijman, A.; Robello, C.; Kemmerling, U. Host-parasite interaction: Changes in human placental gene expression induced by Trypanosoma cruzi. Parasites Vectors 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Souto-Padron, T.; De Carvalho, T.; Chiari, E.; De Souza, W. Further studies on the cell surface charge of Trypanosoma cruzi. Acta Trop. 1984, 41, 215–225. [Google Scholar]
- De Carvalho, T.U.; Souto-Padróon, T.; De Souza, W. Trypanosoma cruzi: Surface charge and freeze-fracture of amastigotes. Exp. Parasitol. 1985, 59, 12–23. [Google Scholar] [CrossRef]
- Souza, W.D.; Arguello, C.; Martinez-Palomo, A.; Trissl, D.; Gonzáles-Robles, A.; Chiari, E. Surface charge of Trypanosoma cruzi. Binding of cationized ferritin and measurement of cellular electrophoretic mobility. J. Protozool. 1977, 24, 411–415. [Google Scholar] [CrossRef]
- Tachibana, H.; Yoshihara, E.; Kaneda, Y.; Nakae, T. In vitro lysis of the bloodstream forms of Trypanosoma brucei gambiense by stearylamine-bearing liposomes. Antimicrob. Agents Chemother. 1988, 32, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Yongsheng, Y.; Yongchun, O.; Chengmai, R.; Yuanguo, C.; Fenqin, Z. Trypanocidal value of liposomal diminazene in experimental Trypanosoma brucei evansi infection in mice. Veter- Parasitol. 1996, 61, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int. J. Parasitol. 2004, 34, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 1998, 28, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Jaiswal, A.K.; Gupta, P.K.; Dube, A.; Chourasia, M.K. Th-1 biased immunomodulation and synergistic antileishmanial activity of stable cationic lipid–polymer hybrid nanoparticle: Biodistribution and toxicity assessment of encapsulated amphotericin B. Eur. J. Pharm. Biopharm. 2015, 89, 62–73. [Google Scholar] [CrossRef]
- De, M.; Ghosh, S.; Asad, M.; Banerjee, I.; Ali, N. Combining doxorubicin with stearylamine-bearing liposomes elicits Th1 cytokine responses and cures metastasis in a mouse model. Cancer Immunol. Immunother. 2020, 69, 1725–1735. [Google Scholar] [CrossRef]
- Pandita, D.; Ahuja, A.; Lather, V.; Dutta, T.; Velpandian, T.; Khar, R.K. Development, characterization and in vitro assessement of stearylamine-based lipid nanoparticles of paclitaxel. Die Pharm. 2011, 66, 171–177. [Google Scholar]
- Gregoriadis, G.; Neerunjun, D.E. Control of the rate of hepatic uptake and catabolism of liposome-entrapped proteins injected into rats. Possible therapeutic applications. Eur. J. Biochem. 1974, 47, 179–185. [Google Scholar] [CrossRef]
- Levchenko, T.S.; Rammohan, R.; Lukyanov, A.N.; Whiteman, K.R.; Torchilin, V.P. Liposome clearance in mice: The effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002, 240, 95–102. [Google Scholar] [CrossRef]
- He, K.; Liu, J.; Gao, Y.; Hao, Y.; Yang, X.; Huang, G. Preparation and Evaluation of Stearylamine-Bearing Pemetrexed Disodium-Loaded Cationic Liposomes In Vitro and In Vivo. AAPS PharmSciTech 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.G.; Chinni, S. Bioavailability enhancement of vitamin E TPGS liposomes of nintedanib esylate: Formulation optimization, cytotoxicity and pharmacokinetic studies. Drug Deliv. Transl. Res. 2022, 12, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).