Odorant Receptors and Cancer
Abstract
1. Introduction
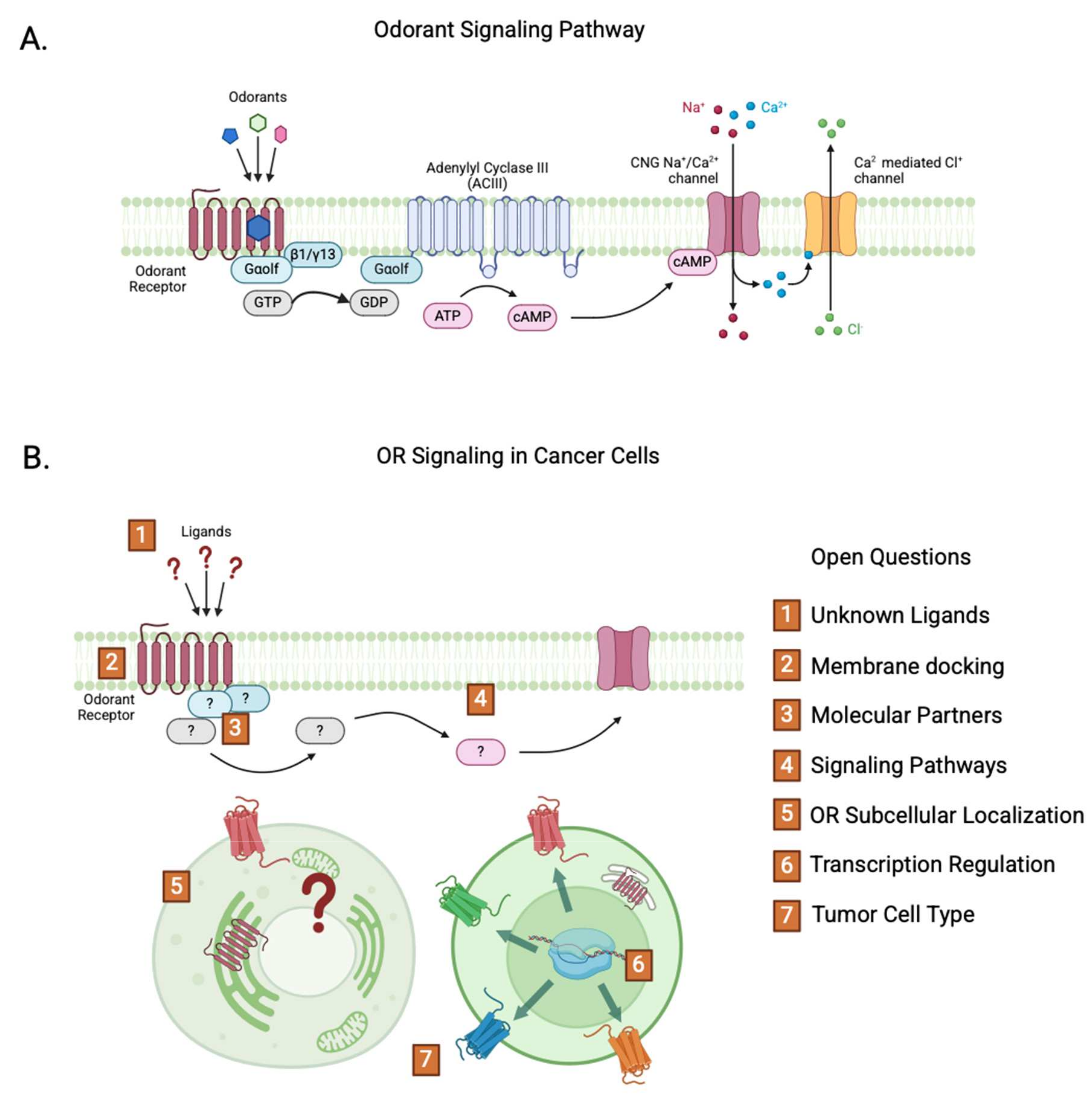
2. Odorant Receptors
2.1. ORs in Cancer
2.1.1. Prostate Cancer
2.1.2. Breast Cancer
2.1.3. Leukemia
2.1.4. Melanoma
2.1.5. Gliomas
2.1.6. Gastric Cancer
2.2. ORs and Tumor Microenvironment
2.3. ORs and Angiogenesis
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OR | Odorant receptor |
| GPCR | G protein coupled receptor |
| OSN | Olfactory sensory neuron |
| AML | Acute Myeloid Leukemia |
| TME | Tumor microenvironment |
| GBM | Glioblastoma |
| DLBCL | Diffuse large B-cell lymphoma |
| TAM | Tumor associated macrophages |
| LGG | Low-grade glioma |
| OS | Overall survival |
References
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gerard, C.; Perret, J.; et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 1992, 355, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef]
- Gelis, L.; Jovancevic, N.; Bechara, F.G.; Neuhaus, E.M.; Hatt, H. Functional expression of olfactory receptors in human primary melanoma and melanoma metastasis. Exp. Dermatol. 2017, 26, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, S.; Zwiebel, L.J.; Giorgio, T.D. Olfactory receptor gene abundance in invasive breast carcinoma. Sci. Rep. 2019, 9, 13736. [Google Scholar] [CrossRef]
- Orecchioni, M.; Kobiyama, K.; Winkels, H.; Ghosheh, Y.; McArdle, S.; Mikulski, Z.; Kiosses, W.B.; Fan, Z.; Wen, L.; Jung, Y.; et al. Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production. Science 2022, 375, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Poll, B.G.; Xu, J.; Gupta, K.; Shubitowski, T.B.; Pluznick, J.L. Olfactory receptor 78 modulates renin but not baseline blood pressure. Physiol. Rep. 2021, 9, e15017. [Google Scholar] [CrossRef]
- Xu, J.; Choi, R.; Gupta, K.; Warren, H.R.; Santhanam, L.; Pluznick, J.L. An evolutionarily conserved olfactory receptor is required for sex differences in blood pressure. Sci. Adv. 2024, 10, eadk1487. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, J.; Shang, P.; Sun, J.P.; Yu, X. Emerging roles of olfactory receptors in glucose metabolism. Trends Cell Biol. 2023, 33, 463–476. [Google Scholar] [CrossRef]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory receptor 10J5 responding to alpha-cedrene regulates hepatic steatosis via the cAMP-PKA pathway. Sci. Rep. 2017, 7, 9471. [Google Scholar] [CrossRef]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef]
- Colinas, O.; Mombaerts, P.; Lopez-Barneo, J.; Ortega-Saenz, P. Carotid Body Function in Tyrosine Hydroxylase Conditional Olfr78 Knockout Mice. Function 2024, 5, zqae010. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef] [PubMed]
- Flegel, C.; Vogel, F.; Hofreuter, A.; Schreiner, B.S.; Osthold, S.; Veitinger, S.; Becker, C.; Brockmeyer, N.H.; Muschol, M.; Wennemuth, G.; et al. Characterization of the Olfactory Receptors Expressed in Human Spermatozoa. Front. Mol. Biosci. 2015, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Ma, R.R.; Shi, D.B.; Wang, Y.W.; Li, X.M.; He, J.Y.; Wang, J.; Gao, P. The Olfactory Receptor Family 2, Subfamily T, Member 6 (OR2T6) Is Involved in Breast Cancer Progression via Initiating Epithelial-Mesenchymal Transition and MAPK/ERK Pathway. Front. Oncol. 2019, 9, 1210. [Google Scholar] [CrossRef]
- Rodriguez, M.; Siwko, S.; Zeng, L.; Li, J.; Yi, Z.; Liu, M. Prostate-specific G-protein-coupled receptor collaborates with loss of PTEN to promote prostate cancer progression. Oncogene 2016, 35, 1153–1162. [Google Scholar] [CrossRef]
- Xu, X.; Khater, M.; Wu, G. The olfactory receptor OR51E2 activates ERK1/2 through the Golgi-localized Gbetagamma-PI3Kgamma-ARF1 pathway in prostate cancer cells. Front. Pharmacol. 2022, 13, 1009380. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, H.H.; Wu, J.; Li, L.; Li, J.; Zhong, H.; Jin, Y.; Lei, T.Y.; Zhao, X.Y.; Xu, B.; et al. Prediction of Tumor Microenvironment Characteristics and Treatment Response in Lung Squamous Cell Carcinoma by Pseudogene OR7E47P-related Immune Genes. Curr. Med. Sci. 2023, 43, 1133–1150. [Google Scholar] [CrossRef]
- Weber, L.; Al-Refae, K.; Ebbert, J.; Jagers, P.; Altmuller, J.; Becker, C.; Hahn, S.; Gisselmann, G.; Hatt, H. Activation of odorant receptor in colorectal cancer cells leads to inhibition of cell proliferation and apoptosis. PLoS ONE 2017, 12, e0172491. [Google Scholar] [CrossRef]
- Naressi, R.G.; Schechtman, D.; Malnic, B. Odorant receptors as potential drug targets. Trends Pharmacol. Sci. 2023, 44, 11–14. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Zhang, X.; Firestein, S. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 2002, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2584–2589. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.; Lomvardas, S. Monoallelic expression of olfactory receptors. Annu. Rev. Cell Dev. Biol. 2015, 31, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.H.; Armelin-Correa, L.M.; Malnic, B. Monogenic and Monoallelic Expression of Odorant Receptors. Mol. Pharmacol. 2016, 90, 633–639. [Google Scholar] [CrossRef]
- Kurian, S.M.; Naressi, R.G.; Manoel, D.; Barwich, A.S.; Malnic, B.; Saraiva, L.R. Odor coding in the mammalian olfactory epithelium. Cell Tissue Res. 2021, 383, 445–456. [Google Scholar] [CrossRef]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial receptor codes for odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef]
- Gimelbrant, A.A.; Haley, S.L.; McClintock, T.S. Olfactory receptor trafficking involves conserved regulatory steps. J. Biol. Chem. 2001, 276, 7285–7290. [Google Scholar] [CrossRef]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell 2004, 119, 679–691. [Google Scholar] [CrossRef]
- Sharma, R.; Ishimaru, Y.; Davison, I.; Ikegami, K.; Chien, M.S.; You, H.; Chi, Q.; Kubota, M.; Yohda, M.; Ehlers, M.; et al. Olfactory receptor accessory proteins play crucial roles in receptor function and gene choice. Elife 2017, 6, e21895. [Google Scholar] [CrossRef]
- Zhuang, H.; Matsunami, H. Synergism of accessory factors in functional expression of mammalian odorant receptors. J. Biol. Chem. 2007, 282, 15284–15293. [Google Scholar] [CrossRef]
- Lalis, M.; Hladis, M.; Khalil, S.A.; Briand, L.; Fiorucci, S.; Topin, J. M2OR: A database of olfactory receptor-odorant pairs for understanding the molecular mechanisms of olfaction. Nucleic Acids Res. 2024, 52, D1370–D1379. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Bumbalo, R.; Bautze, V.; Strotmann, J.; Breer, H. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res. 2015, 361, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Shan, H.; Chen, L.; Long, A.; Zhang, Y.; Liu, Y.; Jia, L.; Wei, F.; Han, J.; Li, T.; et al. OLFR734 Mediates Glucose Metabolism as a Receptor of Asprosin. Cell Metab. 2019, 30, 319–328.e318. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Z.; Ge, X.Y.; Gao, M.X.; Meng, R.; Xu, X.; Zhang, Y.Q.; Li, R.Z.; Lin, J.Y.; Tian, Z.M.; et al. Autonomous sensing of the insulin peptide by an olfactory G protein-coupled receptor modulates glucose metabolism. Cell Metab. 2022, 34, 240–255.e210. [Google Scholar] [CrossRef] [PubMed]
- Billesbolle, C.B.; de March, C.A.; van der Velden, W.J.C.; Ma, N.; Tewari, J.; Del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural basis of odorant recognition by a human odorant receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Ranzani, M.; Iyer, V.; Ibarra-Soria, X.; Del Castillo Velasco-Herrera, M.; Garnett, M.; Logan, D.; Adams, D.J. Revisiting olfactory receptors as putative drivers of cancer. Wellcome Open Res. 2017, 2, 9. [Google Scholar] [CrossRef]
- Pronin, A.; Slepak, V. Ectopically expressed olfactory receptors OR51E1 and OR51E2 suppress proliferation and promote cell death in a prostate cancer cell line. J. Biol. Chem. 2021, 296, 100475. [Google Scholar] [CrossRef]
- Guardia, G.D.A.; Naressi, R.G.; Buzzato, V.C.; da Costa, J.B.; Zalcberg, I.; Ramires, J.; Malnic, B.; Gutiyama, L.M.; Galante, P.A.F. Acute Myeloid Leukemia Expresses a Specific Group of Olfactory Receptors. Cancers 2023, 15, 3073. [Google Scholar] [CrossRef]
- Glusman, G.; Yanai, I.; Rubin, I.; Lancet, D. The complete human olfactory subgenome. Genome Res. 2001, 11, 685–702. [Google Scholar] [CrossRef]
- Niimura, Y.; Nei, M. Evolution of olfactory receptor genes in the human genome. Proc. Natl. Acad. Sci. USA 2003, 100, 12235–12240. [Google Scholar] [CrossRef]
- Nakamura-Garcia, A.K.; Espinal-Enriquez, J. Pseudogenes in Cancer: State of the Art. Cancers 2023, 15, 4024. [Google Scholar] [CrossRef]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef]
- Buettner, J.A.; Glusman, G.; Ben-Arie, N.; Ramos, P.; Lancet, D.; Evans, G.A. Organization and evolution of olfactory receptor genes on human chromosome 11. Genomics 1998, 53, 56–68. [Google Scholar] [CrossRef]
- Olender, T.; Feldmesser, E.; Atarot, T.; Eisenstein, M.; Lancet, D. The olfactory receptor universe--from whole genome analysis to structure and evolution. Genet. Mol. Res. 2004, 3, 545–553. [Google Scholar]
- Meyer, C.; Larghero, P.; Almeida Lopes, B.; Burmeister, T.; Groger, D.; Sutton, R.; Venn, N.C.; Cazzaniga, G.; Corral Abascal, L.; Tsaur, G.; et al. The KMT2A recombinome of acute leukemias in 2023. Leukemia 2023, 37, 988–1005. [Google Scholar] [CrossRef]
- Ou, Z.; Stankiewicz, P.; Xia, Z.; Breman, A.M.; Dawson, B.; Wiszniewska, J.; Szafranski, P.; Cooper, M.L.; Rao, M.; Shao, L.; et al. Observation and prediction of recurrent human translocations mediated by NAHR between nonhomologous chromosomes. Genome Res. 2011, 21, 33–46. [Google Scholar] [CrossRef]
- Redaelli, S.; Grati, F.R.; Tritto, V.; Giannuzzi, G.; Recalcati, M.P.; Sala, E.; Villa, N.; Crosti, F.; Roversi, G.; Malvestiti, F.; et al. Olfactory receptor genes and chromosome 11 structural aberrations: Players or spectators? Hum. Genet. Genom. Adv. 2024, 5, 100261. [Google Scholar] [CrossRef]
- Cho, H.J.; Yeo, D.J.; Yang, H.; Koo, J. Comprehensive Transcriptomic Analysis Reveals Cell-Type-Specific Roles of Human Odorant Receptors in Glioblastoma and the Tumor Microenvironment. Int J Mol Sci 2024, 25, 13382. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Massberg, D.; Simon, A.; Haussinger, D.; Keitel, V.; Gisselmann, G.; Conrad, H.; Hatt, H. Monoterpene (-)-citronellal affects hepatocarcinoma cell signaling via an olfactory receptor. Arch. Biochem. Biophys. 2015, 566, 100–109. [Google Scholar] [CrossRef]
- Manteniotis, S.; Wojcik, S.; Brauhoff, P.; Mollmann, M.; Petersen, L.; Gothert, J.R.; Schmiegel, W.; Duhrsen, U.; Gisselmann, G.; Hatt, H. Functional characterization of the ectopically expressed olfactory receptor 2AT4 in human myelogenous leukemia. Cell Death Discov. 2016, 2, 15070. [Google Scholar] [CrossRef]
- Massberg, D.; Jovancevic, N.; Offermann, A.; Simon, A.; Baniahmad, A.; Perner, S.; Pungsrinont, T.; Luko, K.; Philippou, S.; Ubrig, B.; et al. The activation of OR51E1 causes growth suppression of human prostate cancer cells. Oncotarget 2016, 7, 48231–48249. [Google Scholar] [CrossRef]
- Kalbe, B.; Schulz, V.M.; Schlimm, M.; Philippou, S.; Jovancevic, N.; Jansen, F.; Scholz, P.; Lubbert, H.; Jarocki, M.; Faissner, A.; et al. Helional-induced activation of human olfactory receptor 2J3 promotes apoptosis and inhibits proliferation in a signanon-small-cell lung cancer cell line. Eur. J. Cell Biol. 2017, 96, 34–46. [Google Scholar] [CrossRef]
- Choi, Y.R.; Shim, J.; Park, J.H.; Kim, Y.S.; Kim, M.J. Discovery of Orphan Olfactory Receptor 6M1 as a New Anticancer Target in MCF-7 Cells by a Combination of Surface Plasmon Resonance-Based and Cell-Based Systems. Sensors 2021, 21, 3468. [Google Scholar] [CrossRef]
- Bao, Y.; Tang, Z.; Chen, R.; Yu, X.; Qi, X. Pan-cancer analysis identifies olfactory receptor family 7 subfamily A member 5 as a potential biomarker for glioma. PeerJ 2024, 12, e17631. [Google Scholar] [CrossRef]
- Asadi, M.; Ahmadi, N.; Ahmadvand, S.; Jafari, A.A.; Safaei, A.; Erfani, N.; Ramezani, A. Investigation of olfactory receptor family 51 subfamily j member 1 (OR51J1) gene susceptibility as a potential breast cancer-associated biomarker. PLoS ONE 2021, 16, e0246752. [Google Scholar] [CrossRef]
- Liu, G.; Hu, X.; Zhou, G. Long non-coding RNA OR3A4 promotes proliferation and migration in breast cancer. Biomed. Pharmacother. 2017, 96, 426–433. [Google Scholar] [CrossRef]
- Choi, Y.R.; Na, H.J.; Lee, J.A.; Kim, Y.; Kim, Y.S.; Kim, M.J. Discovery of (-)-epigallocatechin gallate, a novel olfactory receptor 2AT4 agonist that regulates proliferation and apoptosis in leukemia cells. Heliyon 2024, 10, e30298. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, B.; Wang, Y. FOXM1-induced upregulation of lncRNA OR3A4 promotes the progression of diffuse large B-cell lymphoma via Wnt/beta-catenin signaling pathway. Exp. Mol. Pathol. 2020, 115, 104451. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Zhi, Q.; Wang, D.; Guo, L.; Li, G.; Miao, R.; Shi, Y.; Kuang, Y. Long noncoding RNA OR3A4 promotes metastasis and tumorigenicity in gastric cancer. Oncotarget 2016, 7, 30276–30294. [Google Scholar] [CrossRef]
- Li, W.; Fu, Q.; Man, W.; Guo, H.; Yang, P. LncRNA OR3A4 participates in the angiogenesis of hepatocellular carcinoma through modulating AGGF1/akt/mTOR pathway. Eur. J. Pharmacol. 2019, 849, 106–114. [Google Scholar] [CrossRef]
- Giandomenico, V.; Cui, T.; Grimelius, L.; Oberg, K.; Pelosi, G.; Tsolakis, A.V. Olfactory receptor 51E1 as a novel target for diagnosis in somatostatin receptor-negative lung carcinoids. J. Mol. Endocrinol. 2013, 51, 277–286. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Zhao, Z. LncRNA OR3A4 Regulated the Growth of Osteosarcoma Cells by Modulating the miR-1207-5p/G6PD Signaling. Onco Targets Ther. 2020, 13, 3117–3128. [Google Scholar] [CrossRef]
- Guo, F.; Du, J.; Liu, L.; Gou, Y.; Zhang, M.; Sun, W.; Yu, H.; Fu, X. lncRNA OR3A4 Promotes the Proliferation and Metastasis of Ovarian Cancer Through KLF6 Pathway. Front. Pharmacol. 2021, 12, 727876. [Google Scholar] [CrossRef]
- Wei, P.; Tang, H.; Li, D. Insights into pancreatic cancer etiology from pathway analysis of genome-wide association study data. PLoS ONE 2012, 7, e46887. [Google Scholar] [CrossRef]
- Cui, T.; Tsolakis, A.V.; Li, S.C.; Cunningham, J.L.; Lind, T.; Oberg, K.; Giandomenico, V. Olfactory receptor 51E1 protein as a potential novel tissue biomarker for small intestine neuroendocrine carcinomas. Eur. J. Endocrinol. 2013, 168, 253–261. [Google Scholar] [CrossRef]
- Weber, L.; Schulz, W.A.; Philippou, S.; Eckardt, J.; Ubrig, B.; Hoffmann, M.J.; Tannapfel, A.; Kalbe, B.; Gisselmann, G.; Hatt, H. Characterization of the Olfactory Receptor OR10H1 in Human Urinary Bladder Cancer. Front. Physiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Xu, L.L.; Stackhouse, B.G.; Florence, K.; Zhang, W.; Shanmugam, N.; Sesterhenn, I.A.; Zou, Z.; Srikantan, V.; Augustus, M.; Roschke, V.; et al. PSGR, a novel prostate-specific gene with homology to a G protein-coupled receptor, is overexpressed in prostate cancer. Cancer Res. 2000, 60, 6568–6572. [Google Scholar]
- Neuhaus, E.M.; Zhang, W.; Gelis, L.; Deng, Y.; Noldus, J.; Hatt, H. Activation of an olfactory receptor inhibits proliferation of prostate cancer cells. J. Biol. Chem. 2009, 284, 16218–16225. [Google Scholar] [CrossRef]
- Abaffy, T.; Bain, J.R.; Muehlbauer, M.J.; Spasojevic, I.; Lodha, S.; Bruguera, E.; O’Neal, S.K.; Kim, S.Y.; Matsunami, H. A Testosterone Metabolite 19-Hydroxyandrostenedione Induces Neuroendocrine Trans-Differentiation of Prostate Cancer Cells via an Ectopic Olfactory Receptor. Front. Oncol. 2018, 8, 162. [Google Scholar] [CrossRef]
- Klauser, A.L.; Hirschfeld, M.; Ritter, A.; Rucker, G.; Jager, M.; Gundarova, J.; Weiss, D.; Juhasz-Boss, I.; Berner, K.; Erbes, T.; et al. Anticarcinogenic Effects of Odorant Substances Citral, Citrathal R and Cyclovertal on Breast Cancer in vitro. Breast Cancer Dove Med Press. 2021, 13, 659–673. [Google Scholar] [CrossRef]
- Li, M.; Schweiger, M.W.; Ryan, D.J.; Nakano, I.; Carvalho, L.A.; Tannous, B.A. Olfactory receptor 5B21 drives breast cancer metastasis. iScience 2021, 24, 103519. [Google Scholar] [CrossRef]
- Maiga, A.; Lemieux, S.; Pabst, C.; Lavallee, V.P.; Bouvier, M.; Sauvageau, G.; Hebert, J. Transcriptome analysis of G protein-coupled receptors in distinct genetic subgroups of acute myeloid leukemia: Identification of potential disease-specific targets. Blood Cancer J. 2016, 6, e431. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gunassekaran, G.R.; Lee, C.; Lee, N.; Lee, J.; Chae, S.; Park, J.Y.; Koo, J.; Lee, B. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc. Natl. Acad. Sci. USA 2021, 118, e2102434118. [Google Scholar] [CrossRef]
- Martin, A.L.; Anadon, C.M.; Biswas, S.; Mine, J.A.; Handley, K.F.; Payne, K.K.; Mandal, G.; Chaurio, R.A.; Powers, J.J.; Sprenger, K.B.; et al. Olfactory Receptor OR2H1 Is an Effective Target for CAR T Cells in Human Epithelial Tumors. Mol. Cancer Ther. 2022, 21, 1184–1194. [Google Scholar] [CrossRef]
| Cancer | OR | OS * | Ref. |
|---|---|---|---|
| Acute Myeloid Leukemia (AML) | OR2AE1, OR10A2, OR2G2 | Lower | [39] |
| OR5C1, OR1L6, OR10A4, OR13F1 | Higher | [39] | |
| OR52B6, OR2L3, OR52H1, OR2L5, OR2AK2, OR13D1, OR9A4, OR52K2, OR52K1, OR2G3, OR2B2, OR10A5 | - | [39] | |
| Adrenocortical carcinoma (ACC) | OR7A5 | Higher | [56] |
| Breast cancer (BRCA) | OR7A5 | Higher | [56] |
| OR2W3, OR2B6 | Lower | [4] | |
| OR5B21 | Higher | [57] | |
| OR6M1 | - | [55] | |
| OR2T6 | - | [14] | |
| OR51J1 | - | [58] | |
| OR3A4P | - | [59] | |
| Chronic Myeloid Leukemia (CML) | OR2AT4 | - | [52,60] |
| Colorectal Cancer (CRC) | OR3A4P | - | [61] |
| Diffuse Large B-Cell Lymphoma (DLBCL) | OR3A4P | - | [56] |
| Esophageal Cancer (EC) | OR3A4P | - | [61] |
| Gallbladder Cancer (GBC) | OR3A4P | - | [61] |
| Gastric Cancer (GC) | OR3A4P | - | [61] |
| Glioblastoma multiforme (GBM) | OR7A5 | Lower | [62] |
| OR7E156P | - | [49] | |
| OR7D2, OR7E14P, OR4N2 | - | [49] | |
| Hepatocellular carcinoma | OR3A4P | - | [63] |
| Kidney renal clear cell carcinoma (KIRC) | OR7A5 | Lower | [62] |
| Kidney renal papillary cell carcinoma (KIRP) | OR7A5 | Lower | [62] |
| Liver hepatocellular carcinoma (HCC) | OR7A5 | Higher | [62] |
| OR1A2 | - | [51] | |
| Low-grade glioma (LGG) | OR7A5 | Lower | [62] |
| Lung adenocarcinoma (LUAD) | OR7A5 | Lower | [62] |
| Lung squamous cell carcinoma (LUSC) | OR7A5 | Higher | [62] |
| OR51E1 | - | [64] | |
| Malignant Melanoma (MM) | OR2C3 | - | [37] |
| OR51E2 | - | [3] | |
| Non-small-cell lung cancer (NSCLC) | OR2J3 | - | [54] |
| Osteosarcoma | OR3A4P | - | [65] |
| Ovarian Cancer | OR3A4P | - | [66] |
| Ovarian serous cystadenocarcinoma (OSA) | OR7A5 | Lower | [62] |
| Pancreas (PDAC) | OR13C4 | - | [67] |
| OR3A4P | - | [61] | |
| Prostate (PCA) | OR51E1 | - | [38,53] |
| OR51E2 | - | [38] | |
| Small intestine neuroendocrine carcinomas (SI-NEC) | OR51E1 | - | [68] |
| Thymoma (THYM) | OR7A5 | Higher | [62] |
| Thyroid cancer (THCA) | OR7A5 | Higher | [62] |
| Urinary bladder cancer (BLCA) | OR10H1 | - | [69] |
| Uterine corpus endometrial carcinoma (UCEC) | OR7A5 | Lower | [62] |
| Therapeutic Potential | Challenges |
|---|---|
| Therapeutic activation by specific ligands |
|
| Biomarkers for diagnosis, prognosis, or minimum residual disease |
|
| Therapeutic targets for CAR-T or monoclonal antibodies |
|
| Regulatory function as RNA |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naressi, R.G.; Malnic, B.; Gutiyama, L.M. Odorant Receptors and Cancer. Receptors 2025, 4, 11. https://doi.org/10.3390/receptors4020011
Naressi RG, Malnic B, Gutiyama LM. Odorant Receptors and Cancer. Receptors. 2025; 4(2):11. https://doi.org/10.3390/receptors4020011
Chicago/Turabian StyleNaressi, Rafaella G., Bettina Malnic, and Luciana M. Gutiyama. 2025. "Odorant Receptors and Cancer" Receptors 4, no. 2: 11. https://doi.org/10.3390/receptors4020011
APA StyleNaressi, R. G., Malnic, B., & Gutiyama, L. M. (2025). Odorant Receptors and Cancer. Receptors, 4(2), 11. https://doi.org/10.3390/receptors4020011





