Nuclear Receptors: Mechanistic Insights into Endocrine Resistance in Prostate and Breast Cancers
Abstract
1. Introduction
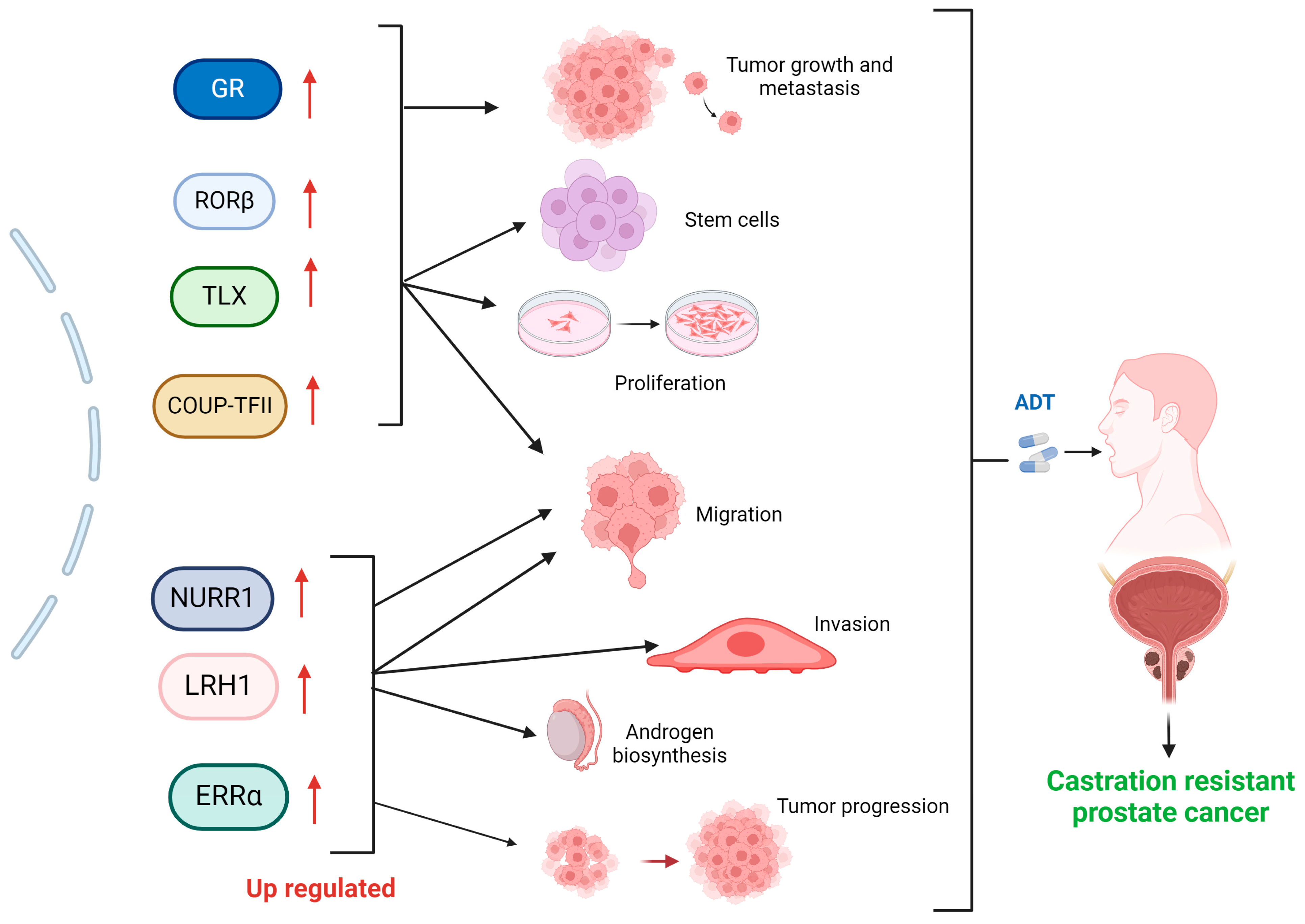
2. Nuclear Receptors in Prostate Cancer and Castration Resistance
Other Nuclear Receptors Associated with Prostate Cancer Castration Resistance
3. Role of Nuclear Receptors in Endocrine Resistance of Breast Cancer
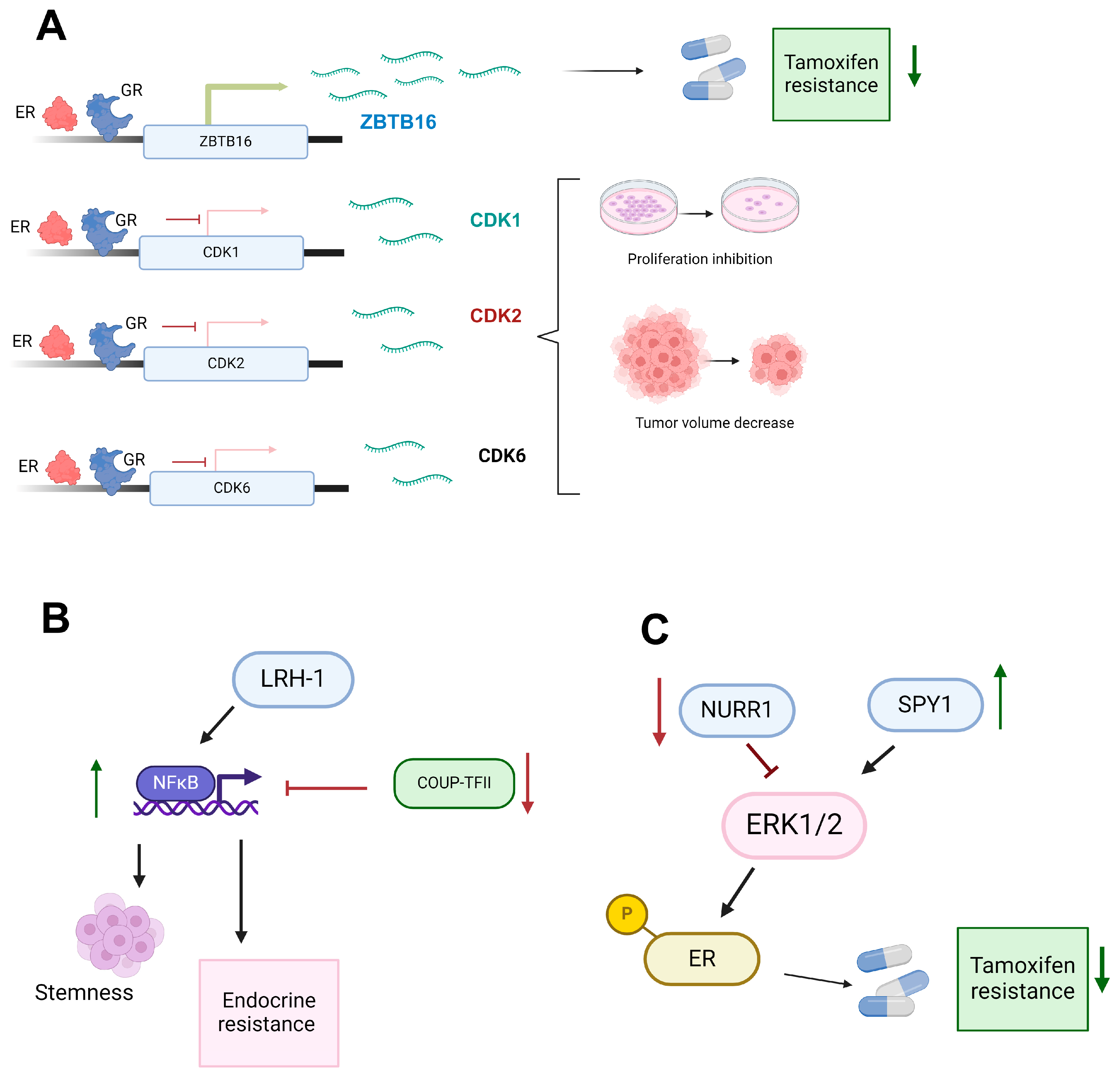
3.1. Variable Role of the Glucocorticoid Receptor in Breast Cancer Endocrine Resistance
3.2. UP-TFII’s Dual Role Modulating Angiogenesis, Metastasis, and Endocrine Therapy Resistance through NFκB Pathway Regulation in Cancer
3.3. The Oncogenic Role of LRH-1 in Endocrine-Sensitive and Endocrine-Resistant Breast Cancer: Mechanisms and Therapeutic Implications
3.4. Divergent Roles of NURR1 in Breast Cancer Progression: Implications for Endocrine Therapy and ERK Signaling Modulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Wu, D.; Ng, C.F.; Teoh, J.Y.; Yu, S.; Wang, Y.; Chan, F.L. Nuclear receptor profiling in prostatospheroids and castration-resistant prostate cancer. Endocr. Relat. Cancer 2018, 25, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, E.; Bergh, A.; Wikström, P. Clinical relevance of androgen receptor alterations in prostate cancer. Endocr. Connect. 2017, 6, R146–R161. [Google Scholar] [CrossRef]
- Wang, Q.; Carroll, J.S.; Brown, M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell 2005, 19, 631–642. [Google Scholar] [CrossRef]
- Sakellakis, M. Orphan receptors in prostate cancer. Prostate 2022, 82, 1016–1024. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Xu, K.; Hu, H.; Xu, Z.; Wu, D.; Wang, Z.; You, W.; Ng, C.F.; Yu, S.; et al. Nuclear Receptor LRH-1 Functions to Promote Castration-Resistant Growth of Prostate Cancer via Its Promotion of Intratumoral Androgen Biosynthesis. Cancer Res. 2018, 78, 2205–2218. [Google Scholar] [CrossRef]
- Jones, C.J.; Goetz, M.P.; Ingle, J.N.; Hawse, J.R. Glucocorticoid receptor activation inhibits proliferation of endoxifen-resistant breast cancer cells and resensitizes cells to hormonal therapy [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4–8; San Antonio, TX. Philadelphia (PA): AACR. Cancer Res. 2019, 79 (Suppl. 4), P5-04-05. [Google Scholar] [CrossRef]
- Shaik, S.; Campbell, H.; Williams, C. NURR1 Is Differentially Expressed in Breast Cancer According to Patient Racial Identity and Tumor Subtype. BioMedInformatics 2022, 2, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.; Brunelle, M.; Jangal, M.; Magnani, L.; Gévry, N. LRH-1 governs vital transcriptional programs in endocrine-sensitive and -resistant breast cancer cells. Cancer Res. 2014, 74, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, L.M.; Appana, S.N.; Datta, S.; Klinge, C.M. COUP-TFII inhibits NFkappaB activation in endocrine-resistant breast cancer cells. Mol. Cell Endocrinol. 2014, 382, 358–367. [Google Scholar] [CrossRef][Green Version]
- Burris, T.P.; de Vera, I.M.S.; Cote, I.; Flaveny, C.A.; Wanninayake, U.S.; Chatterjee, A.; Walker, J.K.; Steinauer, N.; Zhang, J.; Coons, L.A.; et al. International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily-Update 2023. Pharmacol. Rev. 2023, 75, 1233–1318. [Google Scholar] [CrossRef]
- Giguère, V. Orphan nuclear receptors: From gene to function. Endocr. Rev. 1999, 20, 689–725. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Glass, C.K. Nuclear receptors versus inflammation: Mechanisms of transrepression. Trends Endocrinol. Metab. 2006, 17, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The nuclear receptor superfamily: A structural perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, A.; Schmutz, C.; Askari, A.; Kuiper, J.H.; Middleton, J. Orphan receptor GPR15/BOB is up-regulated in rheumatoid arthritis. Cytokine 2014, 67, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Klepsch, V.; Moschen, A.R.; Tilg, H.; Baier, G.; Hermann-Kleiter, N. Nuclear Receptors Regulate Intestinal Inflammation in the Context of IBD. Front. Immunol. 2019, 10, 1070. [Google Scholar] [CrossRef]
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [CrossRef]
- Shah, R.B.; Mehra, R.; Chinnaiyan, A.M.; Shen, R.; Ghosh, D.; Zhou, M.; Macvicar, G.R.; Varambally, S.; Harwood, J.; Bismar, T.A.; et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: Lessons from a rapid autopsy program. Cancer Res. 2004, 64, 9209–9216. [Google Scholar] [CrossRef]
- Hu, C.D.; Choo, R.; Huang, J. Neuroendocrine differentiation in prostate cancer: A mechanism of radioresistance and treatment failure. Front. Oncol. 2015, 5, 90. [Google Scholar] [CrossRef]
- Liu, W.; Xie, C.C.; Zhu, Y.; Li, T.; Sun, J.; Cheng, Y.; Ewing, C.M.; Dalrymple, S.; Turner, A.R.; Isaacs, J.T.; et al. Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 2008, 10, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ibaragi, S.; Hu, G.F. Angiogenin as a molecular target for the treatment of prostate cancer. Curr. Cancer Ther. Rev. 2011, 7, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Duff, J.; McEwan, I.J. Mutation of histidine 874 in the androgen receptor ligand-binding domain leads to promiscuous ligand activation and altered p160 coactivator interactions. Mol. Endocrinol. 2005, 19, 2943–2954. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Watanabe, T.; Okada, M.; Inoue, K.; Ueda, T.; Takada, I.; Watabe, T.; Yamamoto, Y.; Fukuda, T.; Nakamura, T.; et al. Noncanonical Wnt signaling mediates androgen-dependent tumor growth in a mouse model of prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 4938–4943. [Google Scholar] [CrossRef]
- Giwercman, Y.L.; Abrahamsson, P.A.; Giwercman, A.; Gadaleanu, V.; Ahlgren, G. The 5alpha-reductase type II A49T and V89L high-activity allelic variants are more common in men with prostate cancer compared with the general population. Eur. Urol. 2005, 48, 679–685. [Google Scholar] [CrossRef]
- Crowley, F.; Sterpi, M.; Buckley, C.; Margetich, L.; Handa, S.; Dovey, Z. A Review of the Pathophysiological Mechanisms Underlying Castration-resistant Prostate Cancer. Res. Rep. Urol. 2021, 13, 457–472. [Google Scholar] [CrossRef]
- Ni, L.; Yang, C.S.; Gioeli, D.; Frierson, H.; Toft, D.O.; Paschal, B.M. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol. Cell Biol. 2010, 30, 1243–1253. [Google Scholar] [CrossRef]
- Lavaud, P.; Dumont, C.; Thibault, C.; Albiges, L.; Baciarello, G.; Colomba, E.; Flippot, R.; Fuerea, A.; Loriot, Y.; Fizazi, K. Next-generation androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920978134. [Google Scholar] [CrossRef]
- Podgoršek, E.; Mehra, N.; van Oort, I.M.; Somford, D.M.; Boerrigter, E.; van Erp, N.P. Clinical Pharmacokinetics and Pharmacodynamics of the Next Generation Androgen Receptor Inhibitor-Darolutamide. Clin. Pharmacokinet. 2023, 62, 1049–1061. [Google Scholar] [CrossRef]
- Sugawara, T.; Baumgart, S.J.; Nevedomskaya, E.; Reichert, K.; Steuber, H.; Lejeune, P.; Mumberg, D.; Haendler, B. Darolutamide is a potent androgen receptor antagonist with strong efficacy in prostate cancer models. Int. J. Cancer 2019, 145, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Censits, J.; Kelly, W.K. Enzalutamide: A novel antiandrogen for patients with castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhao, L.J.; Wang, Y.T. Synthesis and clinical application of small-molecule drugs approved to treat prostatic cancer. Eur. J. Med. Chem. 2023, 262, 115925. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, P.; Hu, M.; Yang, L.; Yan, G.; Xu, R.; Deng, Y.; Li, X.; Chen, Y. Discovery and biological evaluation of darolutamide derivatives as inhibitors and down-regulators of wild-type AR and the mutants. Eur. J. Med. Chem. 2019, 182, 111608. [Google Scholar] [CrossRef]
- Joseph, J.D.; Lu, N.; Qian, J.; Sensintaffar, J.; Shao, G.; Brigham, D.; Moon, M.; Maneval, E.C.; Chen, I.; Darimont, B.; et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013, 3, 1020–1029. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, S.; Lou, W.; Yang, J.C.; Armstrong, C.M.; Lombard, A.P.; D’Abronzo, L.S.; Evans, C.P.; Gao, A.C.; Liu, C. Cross-Resistance among Next-Generation Antiandrogen Drugs through the AKR1C3/AR-V7 Axis in Advanced Prostate Cancer. Mol. Cancer Ther. 2020, 19, 1708–1718. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Yang, J.C.; Nadiminty, N.; Gaikwad, N.W.; Evans, C.P.; Gao, A.C. Intracrine Androgens and AKR1C3 Activation Confer Resistance to Enzalutamide in Prostate Cancer. Cancer Res. 2015, 75, 1413–1422. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.C.; Armstrong, C.M.; Lou, W.; Liu, L.; Qiu, X.; Zou, B.; Lombard, A.P.; D’Abronzo, L.S.; Evans, C.P.; et al. AKR1C3 Promotes AR-V7 Protein Stabilization and Confers Resistance to AR-Targeted Therapies in Advanced Prostate Cancer. Mol. Cancer Ther. 2019, 18, 1875–1886. [Google Scholar] [CrossRef]
- Giguère, V.; Tini, M.; Flock, G.; Ong, E.; Evans, R.M.; Otulakowski, G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes. Dev. 1994, 8, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M.; Takeda, Y.; Slominski, A.; Kang, H.S. Retinoic acid-related Orphan Receptor γ (RORγ): Connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr. Opin. Toxicol. 2018, 8, 66–80. [Google Scholar] [CrossRef]
- Chang, M.R.; Griffin, P.R. RORβ modulates a gene program that is protective against articular cartilage damage. PLoS ONE 2022, 17, e0268663. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Chen, M.; Guo, W.; Guo, K.; Du, P.; Fang, Y.; Gao, M.; Wang, Q. RORβ suppresses the stemness of gastric cancer cells by downregulating the activity of the Wnt signaling pathway. Oncol. Rep. 2021, 46, 180. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zou, Y.; He, W.; Gage, F.H.; Evans, R.M. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 2008, 451, 1004–1007. [Google Scholar] [CrossRef]
- Jia, L.; Wu, D.; Wang, Y.; You, W.; Wang, Z.; Xiao, L.; Cai, G.; Xu, Z.; Zou, C.; Wang, F.; et al. Orphan nuclear receptor TLX contributes to androgen insensitivity in castration-resistant prostate cancer via its repression of androgen receptor transcription. Oncogene 2018, 37, 3340–3355. [Google Scholar] [CrossRef]
- Chen, X.; Qin, J.; Cheng, C.M.; Tsai, M.J.; Tsai, S.Y. COUP-TFII is a major regulator of cell cycle and Notch signaling pathways. Mol. Endocrinol. 2012, 26, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Koibuchi, N. COUP-TFII in Kidneys, from Embryos to Sick Adults. Diagnostics 2022, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Zou, Y.; Huang, G.L.; He, Z.W. Orphan nuclear receptor nurr1 as a potential novel marker for progression in human prostate cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2023–2028. [Google Scholar] [CrossRef]
- Saucedo-Cardenas, O.; Quintana-Hau, J.D.; Le, W.D.; Smidt, M.P.; Cox, J.J.; De Mayo, F.; Burbach, J.P.; Conneely, O.M. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 4013–4018. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Wang, Y.; Zhao, H.; Wang, Z.; Chan, F.L. Nuclear receptor NURR1 functions to promote stemness and epithelial-mesenchymal transition in prostate cancer via its targeting of Wnt/β-catenin signaling pathway. Cell Death Dis. 2024, 15, 234. [Google Scholar] [CrossRef]
- Wang, J.; Zou, J.X.; Xue, X.; Cai, D.; Zhang, Y.; Duan, Z.; Xiang, Q.; Yang, J.C.; Louie, M.C.; Borowsky, A.D.; et al. ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat. Med. 2016, 22, 488–496. [Google Scholar] [CrossRef]
- Fang, W.; Zheng, J.; Deng, L.; An, Y.; Rong, D.; Wei, J.; Xiong, X.F.; Wang, J.; Wang, Y. Discovery of the First-in-Class RORγ Covalent Inhibitors for Treatment of Castration-Resistant Prostate Cancer. J. Med. Chem. 2024, 67, 1481–1499. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Goodwin, B.; Chung, A.C.; Xu, X.; Wheeler, D.A.; Price, R.R.; Galardi, C.; Peng, L.; Latour, A.M.; Koller, B.H.; et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell Biol. 2005, 25, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.; Jangal, M.; Garneau, D.; Gévry, N. LRH-1 controls proliferation in breast tumor cells by regulating CDKN1A gene expression. Oncogene 2015, 34, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.B.; Lai, C.F.; Patel, H.; Periyasamy, M.; Lin, M.L.; Feller, S.M.; Fuller-Pace, F.V.; Meek, D.W.; Ali, S.; Buluwela, L. LRH-1 drives colon cancer cell growth by repressing the expression of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res. 2016, 44, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Aihara, A.; Chung, W.; Li, Y.; Chen, X.; Huang, Z.; Weng, S.; Carlson, R.I.; Nadolny, C.; Wands, J.R.; et al. LRH1 promotes pancreatic cancer metastasis. Cancer Lett. 2014, 350, 15–24. [Google Scholar] [CrossRef]
- You, W.; Wang, Y.; Chan, L. Functional cross-talk between nuclear receptor LRH-1 and androgen receptor signaling in prostate cancer [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14–18; Chicago, IL. Philadelphia (PA): AACR. Cancer Res. 2018, 78 (Suppl. 13), nr 3740. [Google Scholar] [CrossRef]
- Alaynick, W.A. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion 2008, 8, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.M.; Giguère, V. The NR3B subgroup: An ovERRview. Nucl. Recept. Signal 2007, 5, e009. [Google Scholar] [CrossRef]
- Duellman, S.J.; Calaoagan, J.M.; Sato, B.G.; Fine, R.; Klebansky, B.; Chao, W.R.; Hobbs, P.; Collins, N.; Sambucetti, L.; Laderoute, K.R. A novel steroidal inhibitor of estrogen-related receptor alpha (ERR alpha). Biochem. Pharmacol. 2010, 80, 819–826. [Google Scholar] [CrossRef]
- Cheung, C.P.; Yu, S.; Wong, K.B.; Chan, L.W.; Lai, F.M.; Wang, X.; Suetsugi, M.; Chen, S.; Chan, F.L. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J. Clin. Endocrinol. Metab. 2005, 90, 1830–1844. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, T.; Zhou, J.; Gao, W.; Li, Y.; Yu, S.; Wang, Y.; Chan, F.L. Nuclear receptor ERRα contributes to castration-resistant growth of prostate cancer via its regulation of intratumoral androgen biosynthesis. Theranostics 2020, 10, 4201–4216. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Xiao, Z.G.; Zou, C.; Zhang, X.; Wang, Z.; Wu, D.; Yu, S.; Chan, F.L. Nuclear receptor ERRα and transcription factor ERG form a reciprocal loop in the regulation of TMPRSS2:ERG fusion gene in prostate cancer. Oncogene 2018, 37, 6259–6274. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.Z.; Wardell, S.E.; Burnstein, K.L.; Defranco, D.; Fuller, P.J.; Giguere, V.; Hochberg, R.B.; McKay, L.; Renoir, J.M.; Weigel, N.L.; et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 2006, 58, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Jaiswal, P.K.; Harkless, R.V.; Long, T.M.; Gao, N.; Vandenburg, B.; Selman, P.; Durdana, I.; Lastra, R.R.; Vander Griend, D.; et al. Glucocorticoid Receptor (GR) Activation Is Associated with Increased cAMP/PKA Signaling in Castration-Resistant Prostate Cancer. Mol. Cancer Ther. 2024, 23, 552–563. [Google Scholar] [CrossRef]
- Pollack, A.; Bae, K.; Khor, L.Y.; Al-Saleem, T.; Hammond, M.E.; Venkatesan, V.; Byhardt, R.W.; Asbell, S.O.; Shipley, W.U.; Sandler, H.M. The importance of protein kinase A in prostate cancer: Relationship to patient outcome in Radiation Therapy Oncology Group trial 92-02. Clin. Cancer Res. 2009, 15, 5478–5484. [Google Scholar] [CrossRef]
- Li, J.; Alyamani, M.; Zhang, A.; Chang, K.H.; Berk, M.; Li, Z.; Zhu, Z.; Petro, M.; Magi-Galluzzi, C.; Taplin, M.E.; et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife 2017, 6, e20183. [Google Scholar] [CrossRef]
- Isikbay, M.; Otto, K.; Kregel, S.; Kach, J.; Cai, Y.; Vander Griend, D.J.; Conzen, S.D.; Szmulewitz, R.Z. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm. Cancer 2014, 5, 72–89. [Google Scholar] [CrossRef]
- Ali, S.; Rasool, M.; Chaoudhry, H.; Pushparaj, P.; Jha, P.; Hafiz, A.; Mahfooz, M.; Abdus Sami, G.; Azhar Kamal, M.; Bashir, S.; et al. Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation 2016, 12, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Rondón-Lagos, M.; Villegas, V.E.; Rangel, N.; Sánchez, M.C.; Zaphiropoulos, P.G. Tamoxifen Resistance: Emerging Molecular Targets. Int. J. Mol. Sci. 2016, 17, 1357. [Google Scholar] [CrossRef]
- Blasiak, J.; Chojnacki, J.; Pawlowska, E.; Jablkowska, A.; Chojnacki, C. Vitamin D May Protect against Breast Cancer through the Regulation of Long Noncoding RNAs by VDR Signaling. Int. J. Mol. Sci. 2022, 23, 3189. [Google Scholar] [CrossRef]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xin, Z.; Ren, P.; Wu, H. The Role of PPARs in Breast Cancer. Cells 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Augimeri, G.; Giordano, C.; Gelsomino, L.; Plastina, P.; Barone, I.; Catalano, S.; Andò, S.; Bonofiglio, D. The Role of PPARγ Ligands in Breast Cancer: From Basic Research to Clinical Studies. Cancers 2020, 12, 2623. [Google Scholar] [CrossRef] [PubMed]
- Kensler, K.H.; Poole, E.M.; Heng, Y.J.; Collins, L.C.; Glass, B.; Beck, A.H.; Hazra, A.; Rosner, B.A.; Eliassen, A.H.; Hankinson, S.E.; et al. Androgen Receptor Expression and Breast Cancer Survival: Results From the Nurses’ Health Studies. J. Natl. Cancer Inst. 2019, 111, 700–708. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Palla, S.L.; Carey, M.; Agarwal, R.; Meric-Berstam, F.; Traina, T.A.; Hudis, C.; Hortobagyi, G.N.; Gerald, W.L.; et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin. Cancer Res. 2009, 15, 2472–2478. [Google Scholar] [CrossRef]
- Abigail, B.C.; Suzanne, D.C. Glucocorticoid receptor-mediated oncogenic activity is dependent on breast cancer subtype. J. Steroid Biochem. Mol. Biol. 2024, 243, 106518. [Google Scholar] [CrossRef]
- Prekovic, S.; Chalkiadakis, T.; Roest, M.; Roden, D.; Lutz, C.; Schuurman, K.; Opdam, M.; Hoekman, L.; Abbott, N.; Tesselaar, T.; et al. Luminal breast cancer identity is determined by loss of glucocorticoid receptor activity. EMBO Mol. Med. 2023, 15, e17737. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Qian, K.; Li, L.; Zhang, C.; Fu, X.; Zhang, X.; Chen, H.; Liu, Q.; Cao, S.; et al. A novel tumor suppressor ZBTB1 regulates tamoxifen resistance and aerobic glycolysis through suppressing. J. Biol. Chem. 2020, 295, 14140–14152. [Google Scholar] [CrossRef] [PubMed]
- Tonsing-Carter, E.; Hernandez, K.M.; Kim, C.R.; Harkless, R.V.; Oh, A.; Bowie, K.R.; West-Szymanski, D.C.; Betancourt-Ponce, M.A.; Green, B.D.; Lastra, R.R.; et al. Glucocorticoid receptor modulation decreases ER-positive breast cancer cell proliferation and suppresses wild-type and mutant ER chromatin association. Breast Cancer Res. 2019, 21, 82. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Łukasik, P.; Baranowska-Bosiacka, I.; Kulczycka, K.; Gutowska, I. Inhibitors of Cyclin-Dependent Kinases: Types and Their Mechanism of Action. Int. J. Mol. Sci. 2021, 22, 2806. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Trub, A.; Ahn, A.; Taylor, M.; Ambani, K.; Chan, K.T.; Lu, K.H.; Mahendra, C.A.; Blyth, C.; Coulson, R.; et al. INX-315, a Selective CDK2 Inhibitor, Induces Cell Cycle Arrest and Senescence in Solid Tumors. Cancer Discov. 2024, 14, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Z.; Bhatt, T.; Dickler, M.; Giri, D.; Scaltriti, M.; Baselga, J.; Rosen, N.; Chandarlapaty, S. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017, 36, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Acero-Bedoya, S.; Mykytyn, A.V.; Goetz, M.P.; Hawse, J.R. Glucocorticoid receptor signaling elicits anti-cancer effects in endocrine-resistant breast cancer via induction of AZGP1 [abstract]. In: Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10–14; San Antonio, TX. Philadelphia (PA): AACR. Cancer Res. 2020, 80 (Suppl. 4), P6-04-16. [Google Scholar] [CrossRef]
- Litchfield, L.M.; Klinge, C.M. Multiple roles of COUP-TFII in cancer initiation and progression. J. Mol. Endocrinol. 2012, 49, R135–R148. [Google Scholar] [CrossRef]
- Kastrati, I.; Joosten, S.E.P.; Semina, S.E.; Alejo, L.H.; Brovkovych, S.D.; Stender, J.D.; Horlings, H.M.; Kok, M.; Alarid, E.T.; Greene, G.L.; et al. The NF-κB Pathway Promotes Tamoxifen Tolerance and Disease Recurrence in Estrogen Receptor-Positive Breast Cancers. Mol. Cancer Res. 2020, 18, 1018–1027. [Google Scholar] [CrossRef]
- Al-Rayyan, N.; Litchfield, L.M.; Ivanova, M.M.; Radde, B.N.; Cheng, A.; Elbedewy, A.; Klinge, C.M. 5-Aza-2-deoxycytidine and trichostatin A increase COUP-TFII expression in antiestrogen-resistant breast cancer cell lines. Cancer Lett. 2014, 347, 139–150. [Google Scholar] [CrossRef]
- Fayard, E.; Auwerx, J.; Schoonjans, K. LRH-1: An orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004, 14, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Deng, K.; Huang, J.; Zeng, R.; Zuo, J. Progress in the Understanding of the Mechanism of Tamoxifen Resistance in Breast Cancer. Front. Pharmacol. 2020, 11, 592912. [Google Scholar] [CrossRef]
- Lai, C.F.; Flach, K.D.; Alexi, X.; Fox, S.P.; Ottaviani, S.; Thiruchelvam, P.T.; Kyle, F.J.; Thomas, R.S.; Launchbury, R.; Hua, H.; et al. Co-regulated gene expression by oestrogen receptor α and liver receptor homolog-1 is a feature of the oestrogen response in breast cancer cells. Nucleic Acids Res. 2013, 41, 10228–10240. [Google Scholar] [CrossRef]
- Ferraiuolo, R.M.; Tubman, J.; Sinha, I.; Hamm, C.; Porter, L.A. The cyclin-like protein, SPY1, regulates the ERα and ERK1/2 pathways promoting tamoxifen resistance. Oncotarget 2017, 8, 23337–23352. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kim, C.Y.; Oh, J.H.; Kim, M.H. NR4A1 Regulates Tamoxifen Resistance by Suppressing ERK Signaling in ER-Positive Breast Cancer. Cells 2021, 10, 1633. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.; Ross, A.E.; Parikh, R.B.; Nohria, A.; Morgans, A.K. How to Treat Prostate Cancer With Androgen Deprivation and Minimize Cardiovascular Risk: A Therapeutic Tightrope. JACC CardioOncol. 2021, 3, 737–741. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Cázares, M.B.; Nuñez-Olvera, S.I.; Hernández-Barrientos, R.; Cortés-Malagón, E.M.; Alvarez-Sánchez, M.E.; Puente-Rivera, J. Nuclear Receptors: Mechanistic Insights into Endocrine Resistance in Prostate and Breast Cancers. Receptors 2024, 3, 444-456. https://doi.org/10.3390/receptors3040022
Silva-Cázares MB, Nuñez-Olvera SI, Hernández-Barrientos R, Cortés-Malagón EM, Alvarez-Sánchez ME, Puente-Rivera J. Nuclear Receptors: Mechanistic Insights into Endocrine Resistance in Prostate and Breast Cancers. Receptors. 2024; 3(4):444-456. https://doi.org/10.3390/receptors3040022
Chicago/Turabian StyleSilva-Cázares, Macrina Beatriz, Stephanie I. Nuñez-Olvera, Ricardo Hernández-Barrientos, Enoc Mariano Cortés-Malagón, María Elizbeth Alvarez-Sánchez, and Jonathan Puente-Rivera. 2024. "Nuclear Receptors: Mechanistic Insights into Endocrine Resistance in Prostate and Breast Cancers" Receptors 3, no. 4: 444-456. https://doi.org/10.3390/receptors3040022
APA StyleSilva-Cázares, M. B., Nuñez-Olvera, S. I., Hernández-Barrientos, R., Cortés-Malagón, E. M., Alvarez-Sánchez, M. E., & Puente-Rivera, J. (2024). Nuclear Receptors: Mechanistic Insights into Endocrine Resistance in Prostate and Breast Cancers. Receptors, 3(4), 444-456. https://doi.org/10.3390/receptors3040022








