Abstract
The aryl hydrocarbon receptor (AhR) is overexpressed in many tumor types and exhibits tumor-specific tumor promoter and tumor suppressor-like activity. In colon cancer, most but not all studies suggest that the AhR exhibits tumor suppressor activity which is enhanced by AhR ligands acting as agonists. Our studies investigated the role of the AhR in colon tumorigenesis using wild-type and AhR-knockout mice, the inflammation model of colon tumorigenesis using mice treated with azoxymethane (AOM)/dextran sodium sulfate (DSS) and APCS580/+; KrasG12D/+ mice all of which form intestinal tumors. The effects of tissue-specific AhR loss in the intestine of the tumor-forming mice on colonic stem cells, organoid-initiating capacity, colon tumor formation and mechanisms of AhR-mediated effects were investigated. Loss of AhR enhanced stem cell and tumor growth and in the AOM/DSS model AhR-dependent suppression of FOXM1 and downstream genes was important for AhR-dependent anticancer activity. Furthermore, the effectiveness of interleukin-22 (IL22) in colonic epithelial cells was also dependent on AhR expression. IL22 induced phosphorylation of STAT3, inhibited colonic organoid growth, promoted colonic cell proliferation in vivo and enhanced DNA repair in AOM/DSS-induced tumors. In this mouse model, the AhR suppressed SOCS3 expression and enhanced IL22-mediated activation of STAT3, whereas the loss of the AhR increased levels of SOCS3 which in turn inhibited IL22-induced STAT3 activation. In the APCS580/+; KrasG12D/+ mouse model, the loss of the AhR enhanced Wnt signaling and colon carcinogenesis. Results in both mouse models of colon carcinogenesis were complemented by single cell transcriptomics on colonic intestinal crypts which also showed that AhR deletion promoted expression of FOXM1-regulated genes in multiple colonic cell subtypes. These results support the role of the AhR as a tumor suppressor-like gene in the colon.
1. Introduction:
The aryl hydrocarbon receptor (AhR) is a basic helix-loop-helix protein that was initially discovered as the intracellular receptor that bound the environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) with high affinity [1]. Subsequent studies showed that TCDD and structurally related chlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and biphenyls (PCBs) also bound the AhR and there was a correlation between their receptor binding affinities and their toxic and biochemical potencies in cellular and animal models [2]. TCDD and related halogenated aromatics induced a common pattern of age, sex and species-dependent toxic responses including a wasting syndrome, chloracne, hepatic porphyria, thymic atrophy and teratogenicity [2]. The classical mechanism of action for dioxin-like compounds (DLCs) involves ligand binding to the cytosolic AhR, nuclear translocation and formation of a heterodimer with the AhR nuclear translocator (ARNT) protein and activation of gene expression through binding of the heterodimer to cis-acting AhR response elements (AhREs) in target gene promoters [3,4].
A number of subsequent studies demonstrated that the AhR was not only a receptor that mediated the toxic effects of a specific small set of structurally related toxicants but also had multiple endogenous functions for maintaining cellular homeostasis and pathophysiology [5,6]. It was also discovered that the AhR also bound and is activated by structurally diverse ligands including health promoting phytochemicals, microbial metabolites, endogenous biochemicals, pharmaceuticals and many other structurally diverse compounds [7,8,9]. Development of AhR knockout mice demonstrated that the AhR has multiple functions in organs/tissues [10,11,12,13,14]. Differences in the effects of AhR ligands are due, in part, to their activity as selective AhR modulators (SAhRMs) and the tissue persistence of the toxic AhR ligands (Figure 1).
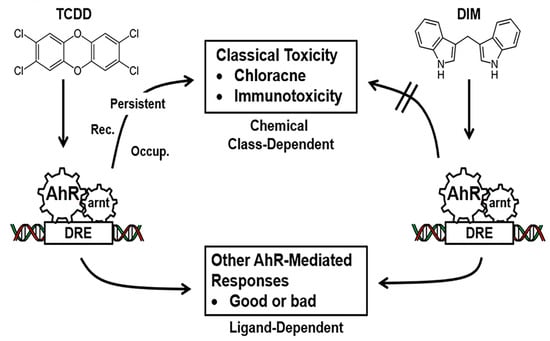
Figure 1.
AhR ligands as Selective AhR Modulators (SAhRMs). The toxicities associated with TCDD, and related compounds is associated with their persistence and as yet other unknown factors [7,8,9] whereas SAhRMs such as diindolylmethane (DIM) do not induce the toxic responses.
The AhR has emerged as a potential drug target for multiple diseases including cancer; however, there are still some conflicting reports regarding the pro-oncogenic or tumor suppressor-like activity of the receptor and its ligands and this is particularly true for breast cancer [15,16]. Some reports show that the AhR exhibits pro-oncogenic activity in colon cancer [17,18,19]; however, most studies on colon cancer indicate that the AhR is a tumor suppressor and tumor growth is inhibited by AhR agonists [20,21,22,23]. This paper will highlight the role of the AhR in colon cancer and the mechanisms associated with its anticancer activities.
2. AhR and Its Role in Colon Cancer
The role of the AhR in colonic inflammation models of inflammatory bowel disease and colon cancer have been previously investigated in cell culture and in in vivo models and with some exceptions, the AhR and selected agonists have been associated with decreased colonic inflammation and increased tumor suppression [20,21]. AhR−/− knockout mice develop cecal tumors with high accumulation of beta-catenin in the tumors whereas this response was not observed in heterozygous or wild type mice (20). Cecal carcinogenesis was also observed in AhR−/− and AhR+/− and AhR+/+ mice crossed with the Apcmin/+ mouse with a decreasing order of susceptibility, respectively, and cecal tumorigenesis was inhibited in AhR-expressing mice or after dietary treatment with AhR ligands indole-3-carbinol and diindolylmethane [0.1 and 0.01%, respectively, in the diet]. Cecal tumor formation is also enhanced by microbial bacteria and apoptosis-associated speck such as proteins containing a caspase recruitment domain (ASC) [21]. The AhR and AhR agonists also inhibited colon tumor formation in an inflammation model of colon cancer where mice are treated with the carcinogen azoxymethane (AOM) in combination with inflammatory stressor dextran sodium sulfate (DSS), and in a syngeneic mouse model using MC38 colon cancer cells injected into the right flank [22]. In contrast, some studies reported alternative results showing that the AhR exhibited pro-oncogenic activity primarily in colon cancer cell models [17,18,19]. Our studies investigated the role of the AhR and AhR ligands in various models of colon carcinogenesis and also focused on the mechanisms associated with the tumor suppressor-like activity of this receptor.
3. Inflammation-Associated Colon Carcinogenesis-Role of AhR
The inflammation-induced colon cancer mouse model [23,24,25] was used for investigating the mechanisms of AhR-mediated effects on colonic stem cells and colon tumor formation [23,24]. This model used AOM (10 mg/kg) as the carcinogen which is then promoted by three cycles of DSS followed by termination 6 weeks after the final dose of DSS (Figure 2A). A comparison of AhR+/+ and AhR−/− mice in the combined carcinogen-inflammation (AOM/DSS) model showed that significantly higher levels of overall tumor incidence, the number of adenomas per mouse, tumor volume and the number of adenocarcinomas per mouse were observed in the AhR knockout mice [25]. These data complement results of a previous study using this same model [23]. Since colon stem cells are precursors of intestinal tumors [26], we used an inducible deletion of the AhR in an Leucine-rich-containing G-protein coupled receptor 5 (LGR5) expressing model and examined the role of the AhR in colonic stem and progenitor cells. Loss of the AhR had dramatic effects on stem and progenitor cells and these included increased organoid forming efficiency and diameter whereas some parameters were decreased by treatment with 25 nM TCDD in wild type but not AhR−/− cells. The observation that the AhR and TCDD treatment decrease colonic stem and progenitor cells correlated with the AhR-dependent decrease of colonic tumor formation. RNAseq and subsequent pathway analysis of differentially expressed genes in stem and progenitor cells from AhR+/+ and AhR−/− mice demonstrated that the AhR repressed Forkhead box protein M1(FOXM1) expression which was further decreased by TCDD. AhR-dependent repression of FOXM1 was observed in crypts adjacent to colon tumors and tumors, stem and progenitor cells and chromatin immunoprecipitation showed that TCDD induced formation of the AhR:ARNT complex in regions of the FOXM1 promoter containing a cis-acting AhRE binding site (Figure 2B). The discovery that the AhR represses FOXM1 expression in the colon is consistent with a previous report showing that FOXM1 signaling contributes to formation and growth of colonic tumors (Figure 2A) [27].
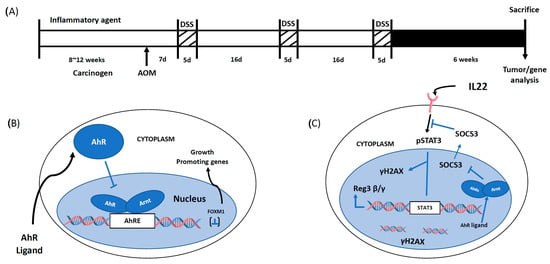
Figure 2.
Experimental protocol and mechanism of action of the AhR in colon cancer (A). Model for the AOM/DSS experimental protocol [25] (B). The AhR inhibits growth of colon tumors by suppressing expression of FOXM1 [25] (C). The AhR enhances IL22-mediated activation of STAT3 and downstream pathway by suppressing expression of SOCS3 [33]. The figures represent intestinal cells and IL22 is generated by ILCs and other immune cells.
Interleukin 22 (IL22) plays an important functional role in the gastrointestinal tract by maintaining gut barrier function, protecting against inflammation, enhancing wound associated regeneration and responsiveness to DNA damage. IL22 is produced by different types of immune cells including innate lymphoid cells (ILCs) and there is evidence that the AhR plays a role in increased cellular levels of IL22 [28,29]. The AhR or its ligands play a direct role in the induction of IL22 and amelioration of colonic inflammation and intestinal stem cell distress [30,31,32]. Loss of the AhR in the AOM/DSS mouse model for colon carcinogenesis was used to investigate potential interactions between the AhR and IL22 and mechanisms of this interaction and effects on colon organoids (Figure 2B) [33]. IL22 enhanced STAT3 phosphorylation in organoids and increased colonic cell proliferation in vivo. Loss of the AhR also decreased IL22-responsiveness and blunts the DNA damage response after treatment with AOM. Examination of RNAseq data from our initial study [25] and based on the known IL22 signaling pathways resulted in identification of Suppressor of cytokine signaling 3 (SOCS3) as a critical differentially enhanced gene after AhR knockout (Figure 2C) [33]. Subsequent studies showed that AhR deficiency in organoids resulted in SOCS3 induction and treatment with TCDD decreased SOCS3 levels in AhR+/+ but not AhR−/− mice and SOCS3 levels were also elevated in colonic crypts in the absence of AhR expression (33). The relationship between SOCS3 and the AhR was further investigated in AhR deficient mice in the AOM/DSS tumor model where SOCS3 levels were increased in tumors compared to uninvolved mucosa. Thus, AhR-SOCS3 interactions are inhibitory in intestinal cells and tumors resulting in enhanced pSTAT3 and downstream genes including the antimicrobial peptide Reg3β/γ peptide and γH2AX (33). Previous studies have also demonstrated important AhR-SOCS3 interactions associated with plasmodium burghei infection [34], hepatotoxicity [35] and carcinogen-induced lesions [36] and in all of these three examples the AhR and AhR ligands induced SOCS3. Moreover, the mechanism of AhR- dependent SOCS3 induction involved interaction of the AhR complex with cis-acting AhREs in the SOCS3 promoter [28]. In contrast the AhR represses SOCS3 expression in intestinal-derived cells and this results in IL22-induced pSTAT3 and downstream signaling pathways. In the absence of the AhR, IL22 /STAT3 responsiveness is inhibited, and this compromises the activity of IL22 in maintaining gut health (Figure 2C).
4. ApcS580/+; KrasG12D/+ Mice and Colon Cancer: Role of the AhR
The ApcS580/+; KrasG12D/+ mouse contains an inactivating mutation of the tumor suppressor APC gene and an activating mutation of the Kras oncogene in the intestine which enhances colon tumorigenesis, and our study investigated the role of the AhR in this model by comparing results in the mutant mice with or without intestinal AhR expression [37]. Functional effects of loss of AhR on this genetic mouse model were similar to that observed in the inflammation induced mouse model of colon carcinogenesis as described above. Loss of intestinal AhR increased organoid-forming efficiency of stem and progenitor cells, enhanced organoid size and number, increased tumor size and the number of tumors in the distal colon per mouse and cecum weight. Moreover, analysis of RNAseq data showed that AhR loss enhanced Wnt signaling. In mice expressing the AhR, treatment with TCDD, showed increased AhR-responsiveness and decreased FOXM1 in organoids. Thus, in this genetic mouse model for colon tumorigenesis, AhR-mediated suppression of the Wnt signaling pathways are major tumor suppressor-like responses.
5. Single Cell Analysis of Colon Crypt Cells
Despite recent progress recognizing the importance of AhR-dependent signaling in colon cancer initiation and progression, its role in regulating colonic crypt homeostasis has been the subject of much speculation. Recently, it has been demonstrated that single cell multi-omics enable the characterization of previously unapproachable clinical phenomena, such as “deep landscapes” of cell heterogeneity that reflect the dynamics of the intestinal crypt [38]. Thus, to further assess the effects of AhR on intestinal epithelial cell–cell communication, we utilized single-cell RNA sequencing (scRNAseq) to assess transcriptomics at the single cell level in wild-type and intestinal-specific AhR knockout mice [39]. Consistent with bulk RNA findings [35], AhR deletion increased FOXM1 regulated genes in crypt-associated epithelial cell types and subtypes of goblet cells and crypt secretory cells. In addition, AhR deletion elevated single-cell entropy (a measure of differentiation potency or cell stemness) and RNA velocity length (a measure of the rate of cell differentiation) in noncycling and cycling Lgr5+ stem cells. In general, intercellular signaling crosstalk via soluble and membrane-bound factors was perturbed in AhR null colonocytes. For example, with respect to epidermal growth factor (EGF) pathway, increased EGF receptor (EGFR) interactions involving enterocytes were detected following AhR deletion. Collectively, these findings provide new evidence linking AhR with the modulation of putative stem cell driver genes, colonic crypt potency lineage decisions and cell–cell communication in vivo.
6. Summary
The AhR regulates anti-inflammatory activities in the gut and both the receptor, and its ligands protect against intestinal inflammation and development of colon cancer. Our research has demonstrated that in an inflammation model of colon cancer where mice are treated with the carcinogen AOM and DSS (AOM/DSS), tumor development is enhanced with loss of the AhR and AhR ligands inhibit tumorigenesis in AhR+/+ mice. The AhR inhibits growth of colonic stem cells, and this is also consistent with the tumor suppressor like activity of this receptor. The mechanism of AhR-mediated anti-tumorigenic activity in the AOM/DSS model involves suppression of the growth-promoting gene FOXM1. Additionally, in this same model which incorporates IL22 as an anti-inflammatory agent generated from group three innate lymphoid cells (ILC3s), the AhR suppressed SOCS3 expression and enhanced IL22-dependent activation of pSTAT3 and downstream genes whereas in AhR deleted mice, SOCS3 expression is enhanced and inhibits pSTAT3. We also observed that in APCS580/+; KrasGD12/+ mutant mice that loss of AhR activates colon stem cells and colon cancer and Wnt signaling. Single cell sequencing of intestinal crypts from AhR wild type and intestinal specific AhR knockout mice demonstrated how the AhR shaped differentiation potency in the mouse colon. Deletion of the AhR enhanced expression of FOXM1- and FOXM1-regulated genes in crypt-associated canonical epithelial cells, deep crypt-secretory cells and subtypes of goblet cells. The overall results clearly confirm the tumor suppressor-like activity of the AhR in the colon and demonstrate the possible clinical applications of AhR agonists for treating this disease.
Author Contributions
Conceptualization, R.S.C., S.S. and H.H.; Experiment Design, S.S., H.H., A.J., L.A.D., C.D.A., I.I., Y.Y., J.J.C. and R.S.C.; Methodology, L.A.D., C.D.A., Y.Y., R.S.C., S.S. and J.J.C.; Software, J.J.C., I.I. and Y.Y.; Validation, R.S.C., H.H. and L.A.D.; Formal Analysis, I.I., R.S.C., H.H. And L.A.D.; Investigation, S.S., H.H., A.J., L.A.D., C.D.A., I.I., Y.Y., J.J.C. and R.S.C.; Resources, R.S.C. and S.S.; Data Curation, H.H., J.J.C., Y.Y., R.S.C. and L.A.D.; Writing—Original Draft Preparation, S.S. and R.S.C.; Writing—Review and Editing, S.S. and R.S.C.; Visualization, H.H., R.S.C. and J.J.C.; Project Administration, R.S.C. and S.S.; Funding Acquisition, R.S.C. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by Texas AgriLife Research, the Sid Kyle Chair Endowment, the Allen Endowed Chair in Nutrition & Chronic Disease Prevention, the Cancer Prevention Research Institute of Texas (RP160589), and the National Institutes of Health (R01-ES025713, R01-CA202697, R35-CA197707, P30-ES029067 and T32-CA090301).
Institutional Review Board Statement
All experiments involving mice were approved by Texas A&M University’s Animal Care and Use Committee; approval date 8 August 2022, approval code IACUC 2022-0165.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data already available in a publicly accessible repository and on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Poland, A.; Knutson, J.C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: Examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982, 22, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.P., Jr. Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharm. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Denison, M.S.; Faber, S.C. Additionally, Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr. Opin. Toxicol. 2017, 2, 124–131. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Safe, S.; Jin, U.H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef]
- Lahvis, G.P.; Bradfield, C.A. Ahr null alleles: Distinctive or different? Biochem. Pharmacol. 1998, 56, 781–787. [Google Scholar] [CrossRef]
- Fernandez-Salguero, P.M.; Ward, J.M.; Sundberg, J.P.; Gonzalez, F.J. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997, 34, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.V.; Su, G.H.; Reddy, J.K.; Simon, M.C.; Bradfield, C.A. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 1996, 93, 6731–6736. [Google Scholar] [CrossRef]
- Mimura, J.; Yamashita, K.; Nakamura, K.; Morita, M.; Takagi, T.N.; Nakao, K.; Ema, M.; Sogawa, K.; Yasuda, M.; Katsuki, M.; et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997, 2, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Fernandez-Salguero, P. The aryl hydrocarbon receptor: Studies using the AHR-null mice. Drug. Metab. Dispos. 1998, 26, 1194–1198. [Google Scholar] [PubMed]
- Kolluri, S.K.; Jin, U.H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef]
- Safe, S.; Zhang, L. The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer. Cancers 2022, 14, 5574. [Google Scholar] [CrossRef]
- Ikuta, T.; Kurosumi, M.; Yatsuoka, T.; Nishimura, Y. Tissue distribution of aryl hydrocarbon receptor in the intestine: Implication of putative roles in tumor suppression. Exp. Cell Res. 2016, 343, 126–134. [Google Scholar] [CrossRef]
- Bogoevska, V.; Wolters-Eisfeld, G.; Hofmann, B.T.; El Gammal, A.T.; Mercanoglu, B.; Gebauer, F.; Vashist, Y.K.; Bogoevski, D.; Perez, D.; Gagliani, N.; et al. HRG/HER2/HER3 signaling promotes AhR-mediated Memo-1 expression and migration in colorectal cancer. Oncogene 2017, 36, 2394–2404. [Google Scholar] [CrossRef]
- Xie, G.; Peng, Z.; Raufman, J.P. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1006–G1015. [Google Scholar] [CrossRef]
- Kawajiri, K.; Kobayashi, Y.; Ohtake, F.; Ikuta, T.; Matsushima, Y.; Mimura, J.; Pettersson, S.; Pollenz, R.S.; Sakaki, T.; Hirokawa, T.; et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 13481–13486. [Google Scholar] [CrossRef]
- Ikuta, T.; Kobayashi, Y.; Kitazawa, M.; Shiizaki, K.; Itano, N.; Noda, T.; Pettersson, S.; Poellinger, L.; Fujii-Kuriyama, Y.; Taniguchi, S.; et al. ASC-associated inflammation promotes cecal tumorigenesis in aryl hydrocarbon receptor-deficient mice. Carcinogenesis 2013, 34, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Yakkundi, P.; Gonsalves, E.; Galou-Lameyer, M.; Selby, M.J.; Chan, W.K. Aryl hydrocarbon receptor acts as a tumor suppressor in a syngeneic MC38 colon carcinoma tumor model. Hypoxia 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Díaz, C.J.; Ronnekleiv-Kelly, S.M.; Nukaya, M.; Geiger, P.G.; Balbo, S.; Dator, R.; Megna, B.W.; Carney, P.R.; Bradfield, C.A.; Kennedy, G.D. The Aryl Hydrocarbon Receptor is a Repressor of Inflammation-associated Colorectal Tumorigenesis in Mouse. Ann. Surg. 2016, 264, 429–436. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [PubMed]
- Han, H.; Davidson, L.A.; Fan, Y.; Goldsby, J.S.; Yoon, G.; Jin, U.; Wright, G.A.; Landrock, K.K.; Weeks, B.R.; Wright, R.C.; et al. Loss of aryl hydrocarbon receptor potentiates FoxM1 signaling to enhance self-renewal of colonic stem and progenitor cells. EMBO J. 2020, 39, e104319. [Google Scholar] [CrossRef]
- Barker, N.; Ridgway, R.A.; Van Es, J.H.; Van De Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Yoshida, Y.; Wang, I.C.; Yoder, H.M.; Davidson, N.O.; Costa, R.H. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology 2007, 132, 1420–1431. [Google Scholar] [CrossRef]
- Yeste, A.; Mascanfroni, I.D.; Nadeau, M.; Burns, E.J.; Tukpah, A.-M.; Santiago, A.; Wu, C.; Patel, B.; Kumar, D.; Quintana, F.J. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 2014, 5, 3753. [Google Scholar] [CrossRef]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.M.; Fish, K.; Fu, Y.X.; Zhou, L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, P237–P248.e1. [Google Scholar] [CrossRef]
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Gronke, K.; Hernández, P.P.; Zimmermann, J.; Klose, C.S.N.; Kofoed-Branzk, M.; Guendel, F.; Witkowski, M.; Tizian, C.; Amann, L.; Schumacher, F.; et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Davidson, L.A.; Fan, Y.-Y.; Landrock, K.K.; Jayaraman, A.; Safe, S.H.; Chapkin, R.S. Loss of aryl hydrocarbon receptor suppresses the response of colonic epithelial cells to IL22 signaling by upregulating SOCS3. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G93–G106. [Google Scholar] [CrossRef] [PubMed]
- Brant, F.; Miranda, A.S.; Esper, L.; Rodrigues, D.H.; Kangussu, L.M.; Bonaventura, D.; Soriani, F.M.; Pinho, V.; Souza, D.G.; Rachid, M.A.; et al. Role of the aryl hydrocarbon receptor in the immune response profile and development of pathology during Plasmodium berghei Anka infection. Infect. Immun. 2014, 82, 3127–3140. [Google Scholar] [CrossRef]
- Wada, T.; Sunaga, H.; Miyata, K.; Shirasaki, H.; Uchiyama, Y.; Shimba, S. Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression. J. Biol. Chem. 2016, 291, 7004–7016. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Lee, Y.; Li, C.H.; Cheng, Y.W.; Kang, J.J. Down-regulation of aryl hydrocarbon receptor intensifies carcinogen-induced retinal lesion via SOCS3-STAT3 signaling. Cell Biol. Toxicol. 2020, 36, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Davidson, L.A.; Hensel, M.; Yoon, G.; Landrock, K.; Allred, C.; Jayaraman, A.; Ivanov, I.; Safe, S.H.; Chapkin, R.S. Loss of Aryl Hydrocarbon Receptor Promotes Colon Tumorigenesis in ApcS580/+; KrasG12D/+ Mice. Mol. Cancer Res. 2021, 19, 771–783. [Google Scholar] [CrossRef]
- Burclaff, J.; Bliton, R.J.; Breau, K.A.; Ok, M.T.; Gomez-Martinez, I.; Ranek, J.S.; Bhatt, A.P.; Purvis, J.E.; Woosley, J.T.; Magness, S.T. A Proximal-to-Distal Survey of Healthy Adult Human Small Intestine and Colon Epithelium by Single-Cell Transcriptomics. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1554–1589. [Google Scholar] [CrossRef]
- Yang, Y.; Osorio, D.; Davidson, L.A.; Han, H.; Mullens, D.A.; Jayaraman, A.; Safe, S.; Ivanov, I.; Cai, J.J.; Chapkin, R.S. Single-cell RNA Sequencing Reveals How the Aryl Hydrocarbon Receptor Shapes Cellular Differentiation Potency in the Mouse Colon. Cancer Prev. Res. 2022, 15, 17–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).