Extended Color Doppler Ultrasound in the Diagnosis of Giant Cell Arteritis: Clinical Insights and Literature Review with Emphasis on Posterior Circulation Involvement
Abstract
1. Introduction
- Cranial GCA (C-GCA) affects the superficial temporal arteries, presenting with classical symptoms such as new-onset headache, scalp tenderness, jaw claudication and vision loss.
2. Methods
- Original studies, systematic reviews and meta-analyses published in English or Italian from 2010 onward.
- Adult patients (age > 50 years) with suspected or confirmed GCA.
- The use of extended CDUS protocols, including assessment of temporal, axillary, subclavian, carotid and vertebral arteries.
- Outcome reporting diagnostic sensitivity, specificity, accuracy, or clinical impact (e.g., association with ischemic complications, especially posterior circulation stroke).
- Studies comparing CDUS with other imaging modalities (PET/CT, MRI) or with clinical reference standards.
- Studies on pediatric populations or vasculitides other than GCA.
- Studies lacking specific data on CDUS or not distinguishing between limited and extended protocols.
- Articles without relevant diagnostic or clinical outcome data.
3. Results
4. Discussion
- Tier 1: A CDUS of temporal, axillary and subclavian arteries and extension to carotid and vertebral vessels in high-risk patients.
- Tier 2: MRI or PET/CT, when CDUS is negative or inconclusive. Van Nieuwland et al. found these modalities to have comparable overall diagnostic accuracy, with differing artery-specific performance [25].
- Tier 3: Temporal artery biopsy in selected cases in the context of persistently high clinical suspicion despite negative imaging findings.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Horton, B.T.; Magath, T.B.; Brown, G.E. An undescribed form of arteritis of temporal vessels. Mayo. Clin. Proc. 1932, 7, 700–701. [Google Scholar]
- Weyand, C.M.; Goronzy, J.J. Giant-cell arteritis and polymyalgia rheumatica. N. Engl. J. Med. 2014, 371, 50–57. [Google Scholar] [CrossRef]
- Schimdt, W.A.; Nielsen, B.D. Imaging in large vessel vasculitis. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101589. [Google Scholar] [CrossRef]
- De Mornac, D.; Espitia, O.; Néel, A.; Connault, J.; Masseau, A.; Espitia-Thibault, A.; Artifoni, M.; Achille, A.; Wahbi, A.; Lacou, M.; et al. Large-vessel involvement is predictive of multiple relapses in giant cell arteritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211009029. [Google Scholar] [CrossRef]
- Schmidt, W.A.; Kraft, H.E.; Völker, L.; Vorpahl, K.; Gromnica-Ihle, E.J. Color duplex ultrasonography in the diagnosis of temporal arteritis. N. Engl. J. Med. 1997, 337, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.A.; Halloush, T.; Dejaco, C. Diagnosis vasculitis with ultrasound: Findings and pitfalls. Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720X241251742. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.; Bond, M.; Dejaco, C.; Ponte, C.; Mackie, S.L.; Falzon, L.; Schmdit, W.A.; Ramiro, S. Imaging in diagnosis, monitoring and outcome prediction of large vessel vasculitis: A systematic literature review and meta-analysis informing the 2023 update of the EULAR recommendations. RMD Open 2023, 9, e003379. [Google Scholar] [CrossRef] [PubMed]
- Kargiotis, O.; Psychogios, K.; Safouris, A.; Bakila, E.; Andreadou, E.; Karapanayiotides, T.; Finitsis, S.; Palaiodimou, L.; Giannopoulos, S.; Magoufis, G.; et al. Cervical duplex ultrasound for the diagnosis of giant cell arteritis with vertebral artery involvement. J. Neuroimaging 2021, 31, 656–664. [Google Scholar] [CrossRef]
- Burr-Haaversen, A.C.; Brekke, L.K.; Kermani, T.A.; Molberg, Ø.; Diamantopoulos, A.P. Extended ultrasound examination identifies more large vessel involvement in patients with giant cell arteritis. Rheumatology 2023, 62, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, K.S.; Sandovici, M.; Bley, T.A.B.; Stone, J.R.; Slart, R.H.; Brouwer, E. Large vessel giant cell arteritis. Lancet Rheumatol. 2023, 6, e397–e408. [Google Scholar] [CrossRef] [PubMed]
- Seitz, P.; Lötscher, F.; Bucher, S.; Bütikofer, L.; Maurer, B.; Hakim, A.; Seitz, L. Ultrasound intima-media thickness cut-off values for the diagnosis of giant cell arteritis using a dual clinical and MRI reference standard and cardiovascular risk stratification. Front. Med. 2024, 11, 1389655. [Google Scholar] [CrossRef] [PubMed]
- Coath, F.L.; Mukhthyar, C. Ultrasonography in the diagnosis and follow-up of giant cell arteritis. Rheumatology 2021, 60, 2528–2536. [Google Scholar] [CrossRef]
- Nakajima, E.; Moon, F.H.; Junior, N.C.; Macedo, C.R.; de Souza, A.W.S.; Iared, W. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: A systematic review and meta-analysis. Adv. Rheumatol. 2023, 8, 5. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Yip, A.; Jernberg, E.T.; Bardi, M.; Geiger, J.; Lohne, F.; Schmidt, W.A.; Mykebust, G.; Diamantopoulos, A.P. Magnetic resonance imaging compared to ultrasonography in giant cell arteritis: A cross-sectional study. Arthritis Res. Ther. 2020, 22, 247. [Google Scholar] [CrossRef]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021, 73, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Società Italiana di Reumatologia (SIR). Linee Guida Per la Diagnosi e il Trattamento Delle Vasculitis; SIR: Milano, Italy, 2020; Available online: https://www.reumatologia.it/obj/files/lineeguida/1.VASCULITI.pdf (accessed on 11 August 2025).
- Mackie, S.L.; Dejaco, C.; Appenzeller, S.; Camellino, D.; Duftner, C.; Gonzalez-Chiappe, S.; Mahr, A.; Mukhtyar, C.; Reynolds, G.; de Souza, A.W.S.; et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology 2020, 59, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Terslev, L.; Jensen, M.R.; Brittain, J.M.; Døhn, U.M.; Faber, C.; Heegaard, S.; Klefter, O.N.; Kønig, E.B.; Subhi, Y.; et al. Comparison of temporal artery ultrasound versus biopsy in the diagnosis of giant cell arteritis. Eye 2023, 37, 344–349. [Google Scholar] [CrossRef]
- Pacheco, M.; Sà Costa, R.; Soares, C.; Costa, A.; Azevedo, E. Ultrasonography of the superficial temporal and Axillary arteries in giant cell arteritis diagnosis. J. Stroke Cerebrovasc. Dis. 2024, 33, 107845. [Google Scholar] [CrossRef] [PubMed]
- Skoog, J.; Svensson, C.; Eriksson, P.; Sjöwall, C.; Zachrisson, H. The diagnostic performance of an extended ultrasound protocol in patients with clinically suspected giant cell arteritis. Front. Med. 2022, 8, 807996. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Floris, A.; Ponte, C.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Piper, J.; Luqmani, R. The use of ultrasound to assess giant cell arteritis: Review of the current evidence and practical guide for the rheumatologist. Rheumatology 2018, 57, 227–235. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Bond, M.; Bosch, P.; Ponte, C.; Mackie, S.L.; Bley, T.A.; Blockmans, D.; Brolin, S.; Bolek, E.C.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice: 2023 update. Ann. Rheum. Dis. 2024, 83, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Prünte, M.K.R.; Naumann, A.; Christ, M.; Naumann, M.; Bayas, A. Giant cell arteritis with vertebral artery involvement—Baseline characteristics and follow-up of a monocentric patient cohort. Front. Neurol. 2023, 14, 1188073. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwland, M.; Nienhuis, P.H.; Haagsma, C.; van der Geest, K.S.; Wagenaar, N.R.; Appelman, A.P.; Vijbrief, O.D.; van Bon, L.; Colin, E.M.; Brouwer, E.; et al. An in-depth comparison of vascular inflammation on ultrasound, FDG-PET/CT and MRI in patients with suspected giant cell arteritis. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Tomelleri, A.; Buttgereit, F.; Matteson, E.L.; Dejaco, C. Therapeutic Advances in Muscoloskeletal Disease. Sage J. 2022, 14, 1759720X221096366. [Google Scholar] [CrossRef]
- Chrysidis, S.; Døhn, U.M.; Terslev, L.; Fredberg, U.; Lorenzen, T.; Christensen, R.; Larsen, K.; Diamantopoulos, A.P. Diagnostic accuracy of vascular ultrasound in patients with suspected giant cell arteritis (EUREKA): A prospective, multicentre, non-interventional, cohort study. Lancet Rheumatol. 2021, 3, e865–e873. [Google Scholar] [CrossRef]
- van Nieuwland, M.; Colin, E.M.; Vermeer, M.; Wagenaar, N.R.; Vijlbrief, O.D.; van Zandwijk, J.K.; Slart, R.H.; Koffijberg, H.; Jebbink, E.G.; van der Geest, K.S.; et al. A direct comparison in diagnostic performance of CDUS, FDG-PET/CT and MRI in patients suspected of giant cell arteritis. Rheumatology 2025, 64, 1392–1399. [Google Scholar] [CrossRef] [PubMed]

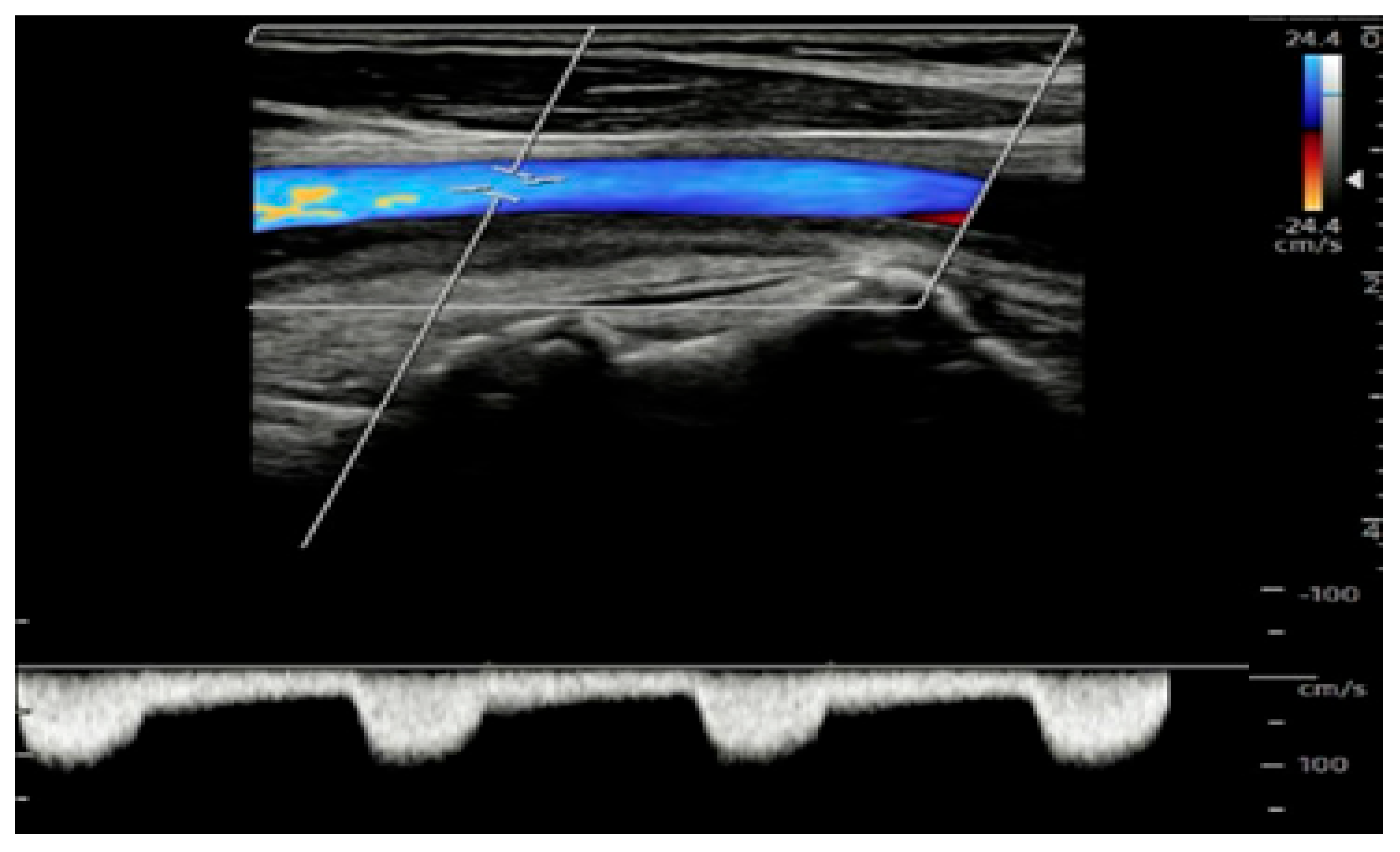
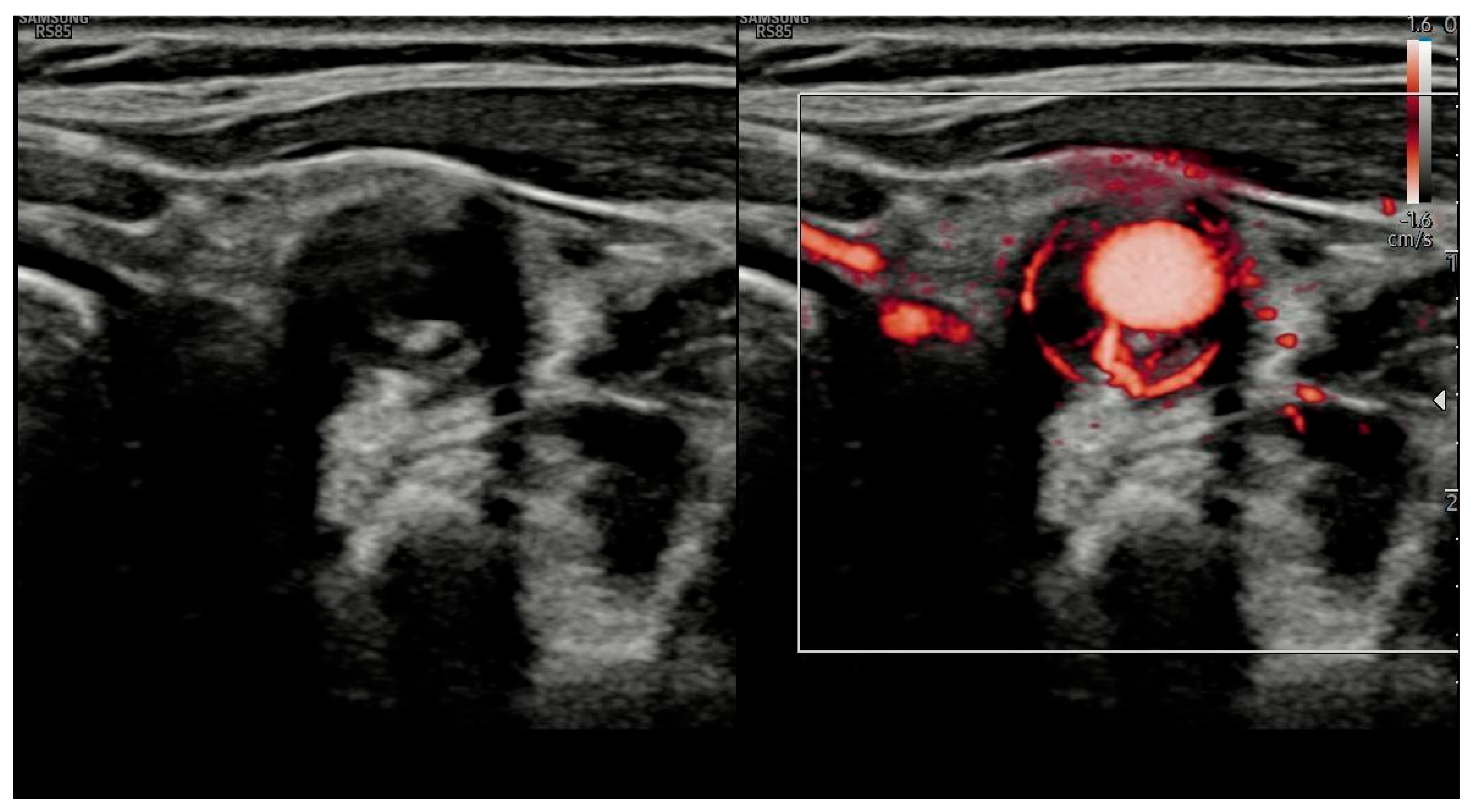
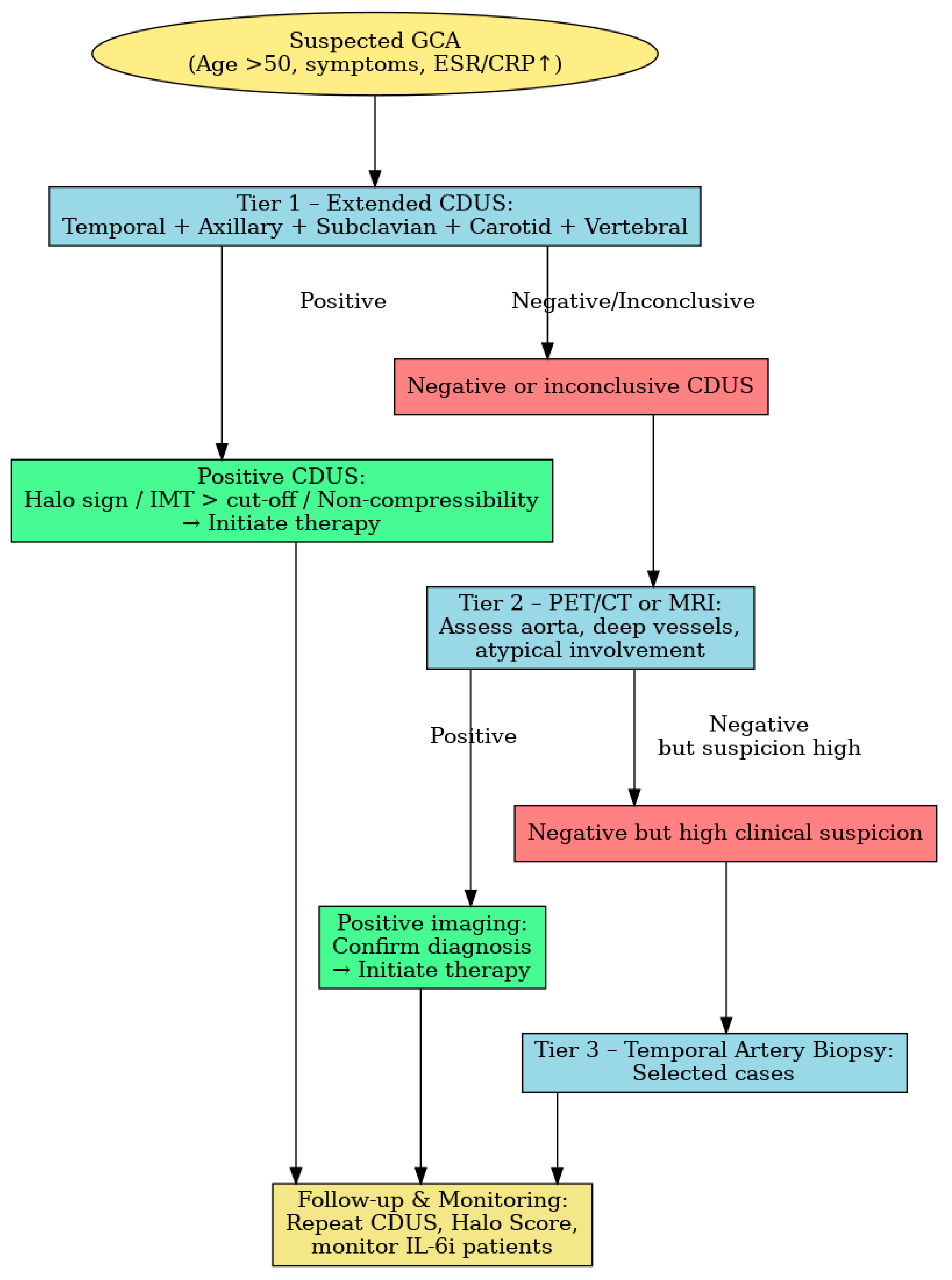
| Study/Protocol | Scope of Extension | Diagnostic Impact | Reference |
|---|---|---|---|
| Oxford A2 Protocol (2023) | Carotid, subclavian, axillary, vertebral arteries | Sensitivity increased from 42.1% to 69.9% | [9] |
| van der Geest et al. (2022) | Carotid and axillary arteries in addition to temporal arteries | Sensitivity rose to 95%, specificity maintained at 98% | [10] |
| Bosch et al., RMD Open (2023) | Vertebral and subclavian extension | Predicts higher relapse and steroid dependence | [7] |
| Guidelines | Main Recommendations |
|---|---|
| EULAR (2018 and 2023) [7,14] | -CDUS of temporal ± axillary arteries as initial test -Can replace biopsy if positive -Supports extended protocol -Diagnosis possible without biopsy |
| EULAR Points to Consider (2024) [11] | -Endorses extended CDUS including carotid and vertebral arteries -Supports biopsy sparing approach in expert centers -CDUS recommended for diagnosis, monitoring and relapse prediction |
| ACR/Vasculitis Foundation (2021) [16] | -CDUS as non-invasive first-line tool only in expert centers -Still prioritizes temporal artery biopsy -Additional imaging (PET/CT, MRA) after confirmation |
| SIR (Italian Society of Rheumatology) [17] | -Aligned with EULAR recommendations -CDUS as a first-line diagnostic tool -Often replaces biopsy -Supports fast-track pathways and vascular extension |
| BSR 2020 (British Society for Rheumatology) [18] | -CDUS preferred in fast-track clinics -TAB still considered where ultrasound expertise is lacking |
| Key Differences | -CDUS replacing biopsy -EULAR, SIR, BSR: supportive -ACR: cautious, prioritizes biopsy |
| Overall Consensus | -Extended CDUS is crucial in GCA diagnosis when performed by trained operators with proper protocols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Privitera, I.; Costanzo, L.; Magnano San LIo, P.; Romano, R.; Piro, S.; Romano, M. Extended Color Doppler Ultrasound in the Diagnosis of Giant Cell Arteritis: Clinical Insights and Literature Review with Emphasis on Posterior Circulation Involvement. J. Vasc. Dis. 2025, 4, 37. https://doi.org/10.3390/jvd4030037
Privitera I, Costanzo L, Magnano San LIo P, Romano R, Piro S, Romano M. Extended Color Doppler Ultrasound in the Diagnosis of Giant Cell Arteritis: Clinical Insights and Literature Review with Emphasis on Posterior Circulation Involvement. Journal of Vascular Diseases. 2025; 4(3):37. https://doi.org/10.3390/jvd4030037
Chicago/Turabian StylePrivitera, Ivan, Luca Costanzo, Paola Magnano San LIo, Raffaella Romano, Salvatore Piro, and Marcello Romano. 2025. "Extended Color Doppler Ultrasound in the Diagnosis of Giant Cell Arteritis: Clinical Insights and Literature Review with Emphasis on Posterior Circulation Involvement" Journal of Vascular Diseases 4, no. 3: 37. https://doi.org/10.3390/jvd4030037
APA StylePrivitera, I., Costanzo, L., Magnano San LIo, P., Romano, R., Piro, S., & Romano, M. (2025). Extended Color Doppler Ultrasound in the Diagnosis of Giant Cell Arteritis: Clinical Insights and Literature Review with Emphasis on Posterior Circulation Involvement. Journal of Vascular Diseases, 4(3), 37. https://doi.org/10.3390/jvd4030037







