Vertebral Artery Sacrifice After Balloon Test Occlusion in Endovascular Repair of Subclavian Artery Aneurysm
Abstract
1. Introduction
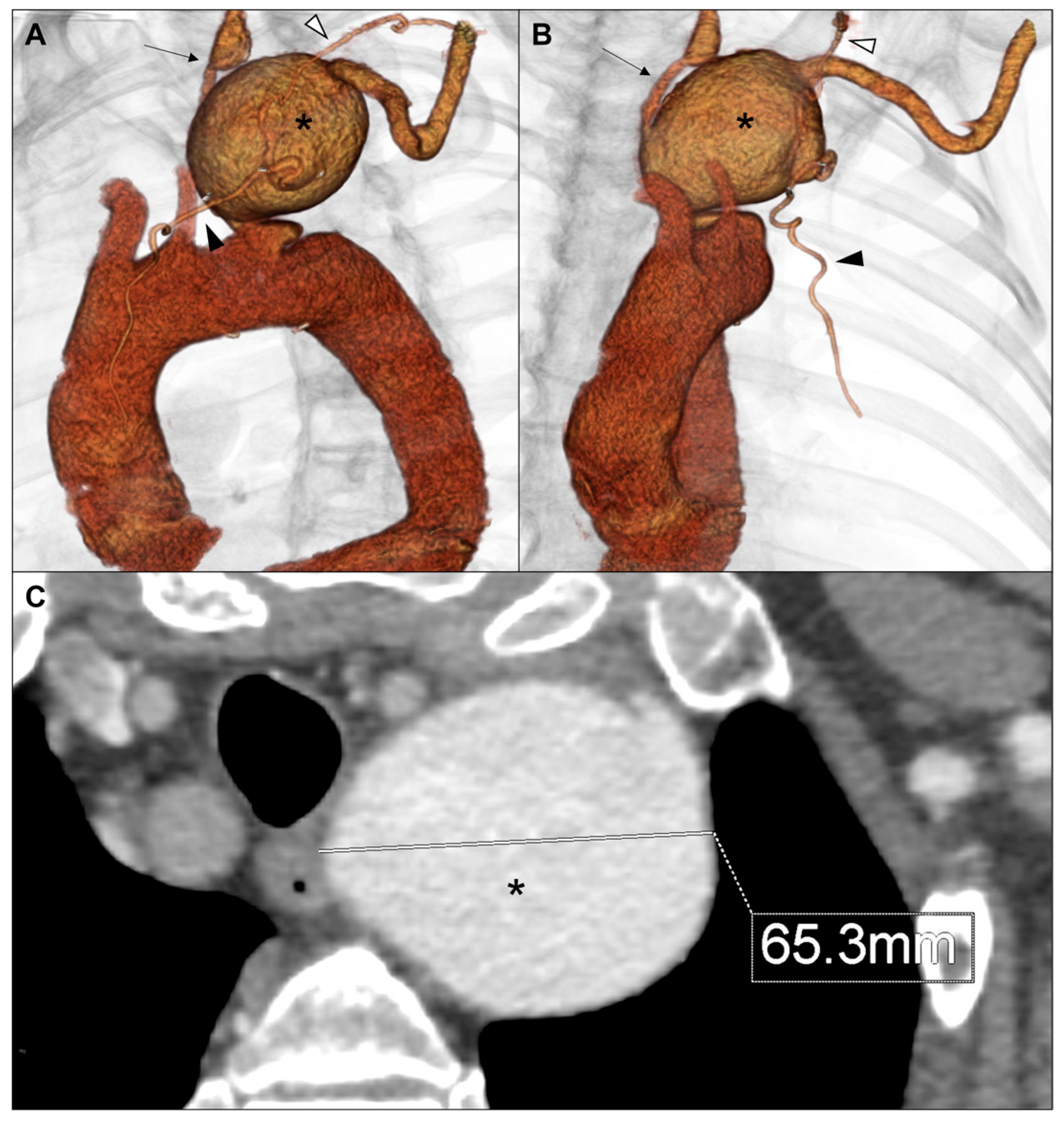
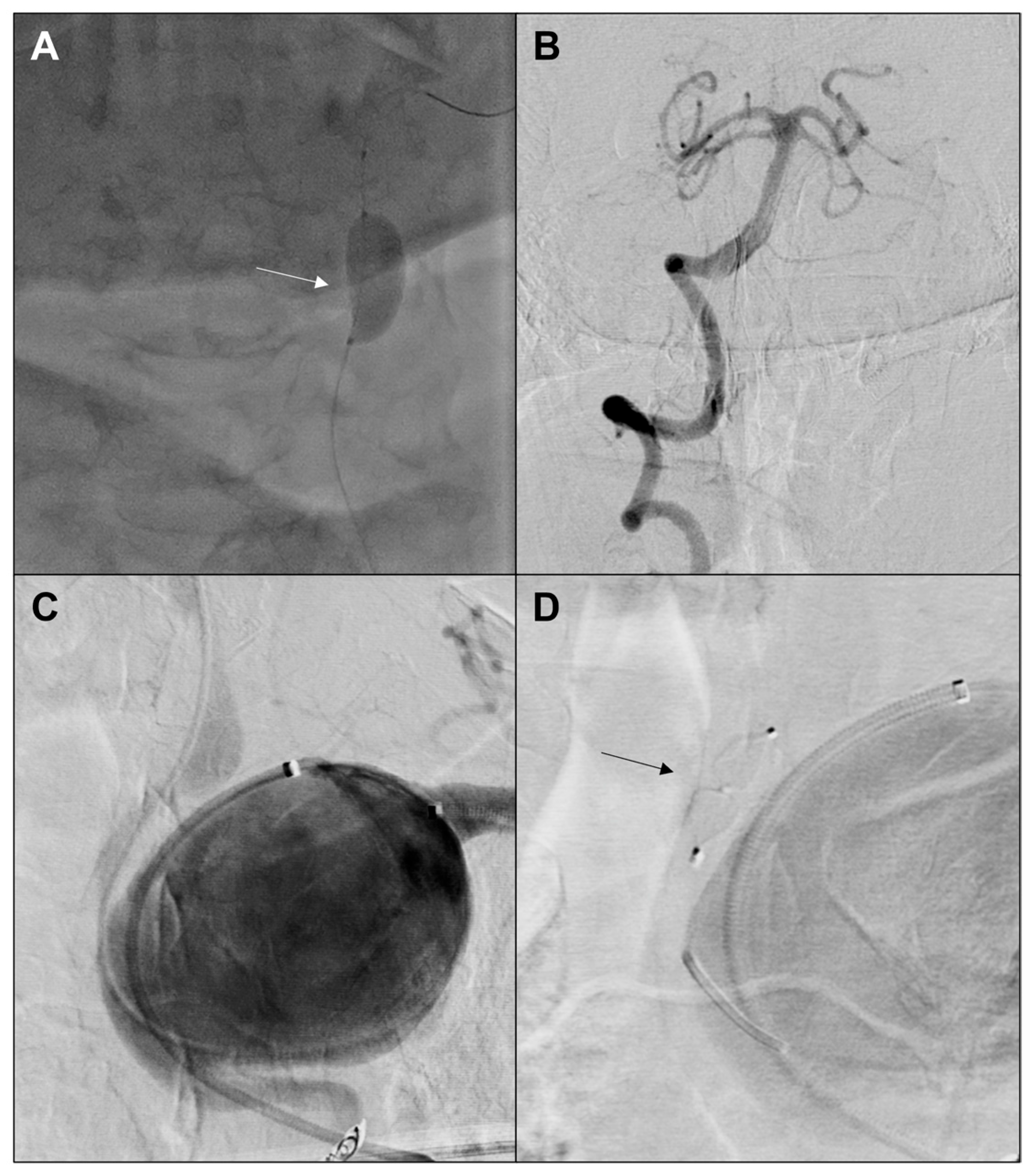
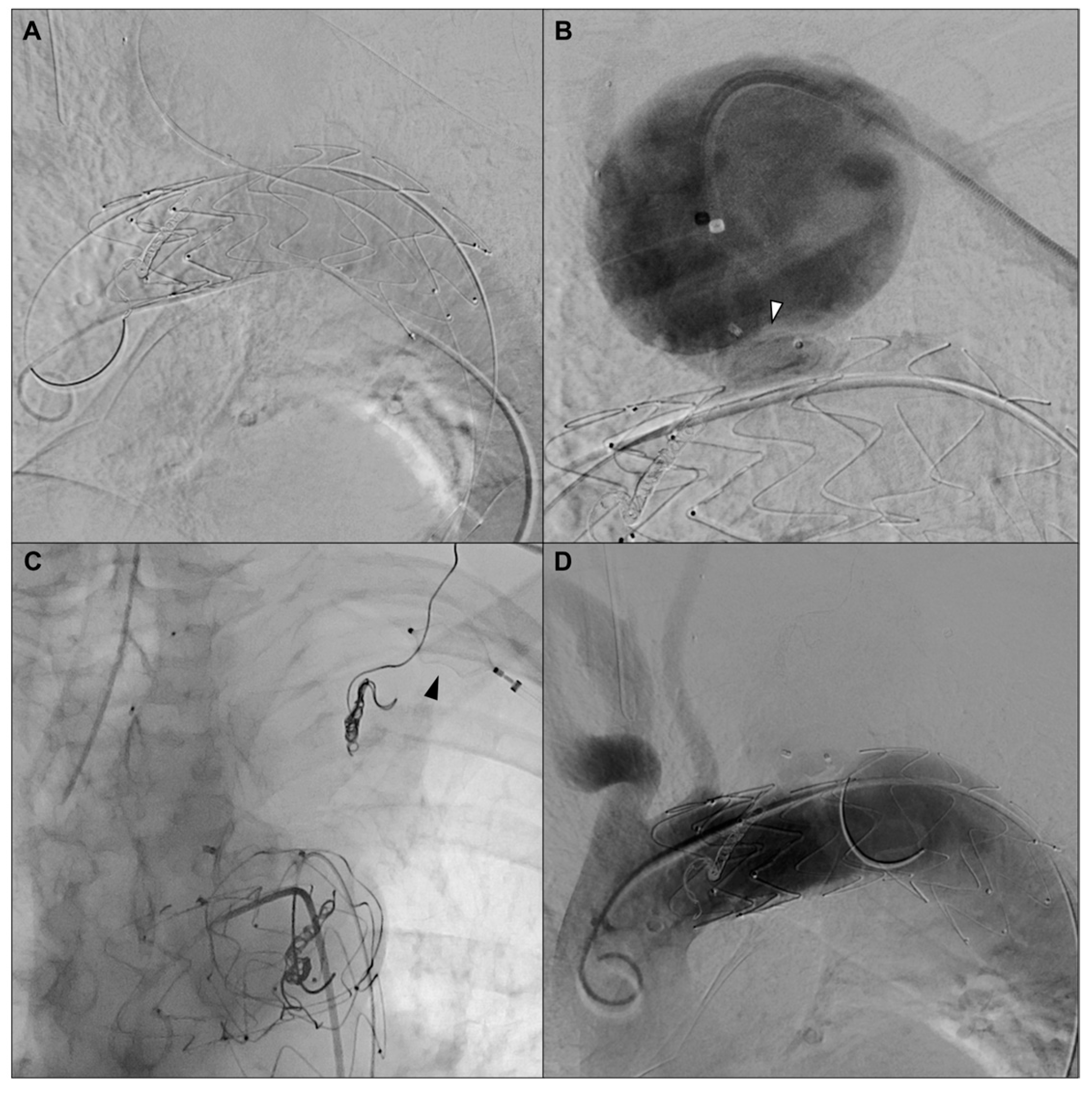
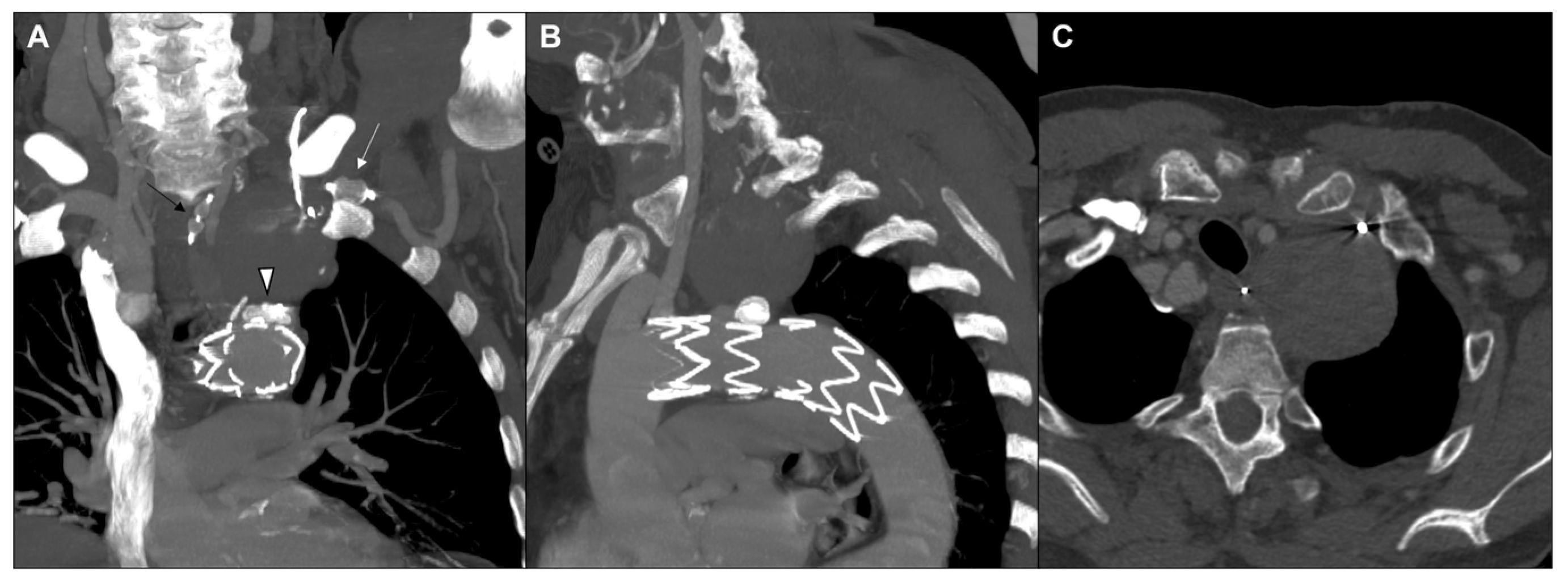
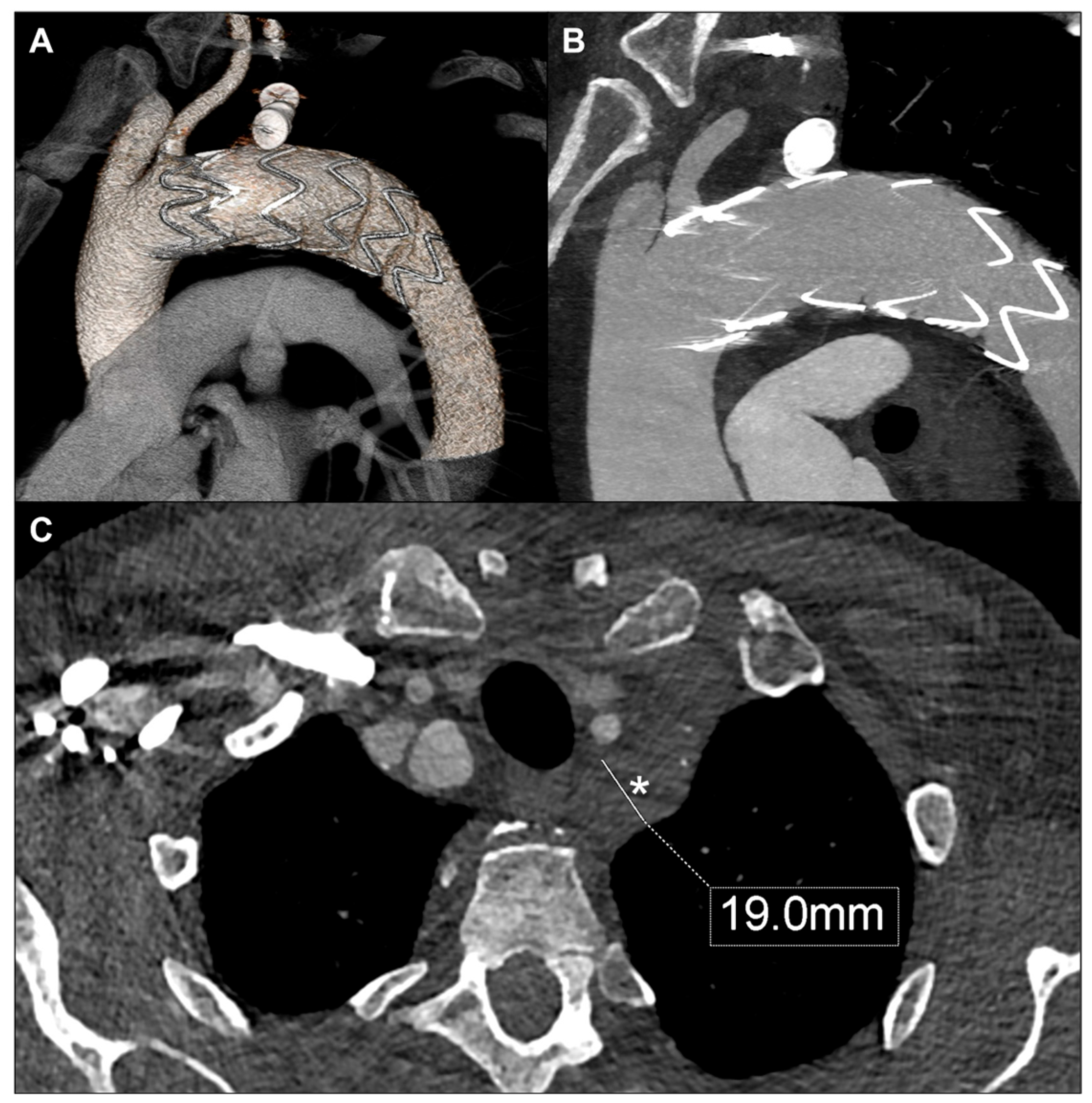
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vierhout, B.P.; Zeebregts, C.J.; van den Dungen, J.J.; Reijnen, M.M. Changing profiles of diagnostic and treatment options in subclavian artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, L.B.; Zlatanovic, P.; Ducic, S.; Koncar, I.; Cvetic, V.; Kuzmanovic, I. Single center experience in the management of a case series of subclavian artery aneurysms. Asian J. Surg. 2020, 43, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.D.; Barfield, M.E.; Hanna, J.M.; Shah, A.A.; Shortell, C.K.; McCann, R.L.; Hughes, G.C. Intrathoracic subclavian artery aneurysm repair in the thoracic endovascular aortic repair era. J. Vasc. Surg. 2013, 57, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Jung, Y.; Jeong, I.S.; Song, S.Y.; Na, K.J.; Oh, S.G. Open surgical treatment of subclavian artery pseudoaneurysm after endovascular repair: A case report. J. Cardiothorac. Surg. 2022, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, I.; Tomić, A.; Marić, N.; Pecarski, D.; Sarac, M.; Paunovic, D.; Rusovic, S. Endovascular treatment of the subclavian artery aneurysm in high-risk patient—A single-center experience. Vojnosanit. Pregl. 2016, 73, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Maskanakis, A.; Patelis, N.; Moris, D.; Tsilimigras, D.I.; Schizas, D.; Diakomi, M.; Bakoyiannis, C.; Georgopoulos, S.; Klonaris, C.; Liakakos, T. Stenting of Subclavian Artery True and False Aneurysms: A Systematic Review. Ann. Vasc. Surg. 2018, 47, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Leverich, M.; Nazzal, M.; Osman, M. Single-stage hybrid repair of a right subclavian artery aneurysm involving the origin of the right vertebral artery. Ann. Vasc. Surg. Brief. Rep. Innov. 2023, 3, 100173. [Google Scholar] [CrossRef]
- French, B.L.; Menghini, A.M.; Burke, C.R.; Byers, P.H.; Shalhub, S. Operative repair of right intrathoracic subclavian artery aneurysms in patients with genetic arteriopathy. J. Vasc. Surg. Cases Innov. Tech. 2022, 9, 101081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, A.M.; Neuhaus, A.A.; Hadley, G.; Balami, J.S.; Harston, G.W.; DeLuca, G.C.; Buchan, A.M. Posterior circulation ischaemic stroke diagnosis and management. Clin. Med. 2023, 23, 219–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.C.; Li, L.Q.; Guo, L.R.; Qi, L.X.; Guo, J.M.; Gu, Y.Q. Endovascular and hybrid treatments for subclavian artery aneurysms. Ann. Ital. Chir. 2021, 92, 709–714. [Google Scholar] [PubMed]
- Shergill, E.S.; Udwadia, F.R.; Grubisic, M.; Salata, K.; Misskey, J.; Faulds, J. Comparative study of left vertebral artery revascularization in patients with and without aberrant left vertebral anatomy. J. Vasc. Surg. 2024, 79, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Sorteberg, A.; Bakke, S.J.; Boysen, M.; Sorteberg, W. Angiographic balloon test occlusion and therapeutic sacrifice of major arteries to the brain. Neurosurgery 2008, 63, 651–660, dicussion 660-1. [Google Scholar] [CrossRef] [PubMed]
- Westbroek, E.M.; Pennington, Z.; Ehresman, J.; Ahmed, A.K.; Gailloud, P.; Sciubba, D.M. Vertebral Artery Sacrifice versus Skeletonization in the Setting of Cervical Spine Tumor Resection: Case Series. World Neurosurg. 2020, 139, e601–e607. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.B.; Dessesard Olijnyk, L.; Janelle, F.; Shedid, D.; Bojanowski, M.W.; Labidi, M. Surgical management of tumors of the cervical spine and craniovertebral junction involving the vertebral artery: A narrative review. Neurochirurgie 2024, 70, 101550. [Google Scholar] [CrossRef] [PubMed]
- Maitas, O.; Bob-Manuel, T.; Price, J.; Noor, A.; Obi, K.; Okoh, N.; Garikapati, K.; Kim, J.; Jahan, S.; Jenkins, J.S. Vertebral Artery Interventions: A Comprehensive Updated Review. Curr. Cardiol. Rev. 2023, 19, e170322202296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tansavatdi, K.; Dublin, A.B.; Donald, P.J.; Dahlin, B. Combined Balloon Test Occlusion and SPECT Analysis for Carotid Sacrifice: Angiographic Predictors for Success or Failure? J. Neurol. Surg. B Skull Base 2015, 76, 249–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coscarella, C.; Giudice, R.; Minucci, M.; Borlizzi, A.; Pennetta, F.F.; Orellana Davila, B.; Ferrer, C. Vertebral Artery Sacrifice After Balloon Test Occlusion in Endovascular Repair of Subclavian Artery Aneurysm. J. Vasc. Dis. 2025, 4, 35. https://doi.org/10.3390/jvd4030035
Coscarella C, Giudice R, Minucci M, Borlizzi A, Pennetta FF, Orellana Davila B, Ferrer C. Vertebral Artery Sacrifice After Balloon Test Occlusion in Endovascular Repair of Subclavian Artery Aneurysm. Journal of Vascular Diseases. 2025; 4(3):35. https://doi.org/10.3390/jvd4030035
Chicago/Turabian StyleCoscarella, Carlo, Rocco Giudice, Marta Minucci, Adelaide Borlizzi, Federico Francisco Pennetta, Bernardo Orellana Davila, and Ciro Ferrer. 2025. "Vertebral Artery Sacrifice After Balloon Test Occlusion in Endovascular Repair of Subclavian Artery Aneurysm" Journal of Vascular Diseases 4, no. 3: 35. https://doi.org/10.3390/jvd4030035
APA StyleCoscarella, C., Giudice, R., Minucci, M., Borlizzi, A., Pennetta, F. F., Orellana Davila, B., & Ferrer, C. (2025). Vertebral Artery Sacrifice After Balloon Test Occlusion in Endovascular Repair of Subclavian Artery Aneurysm. Journal of Vascular Diseases, 4(3), 35. https://doi.org/10.3390/jvd4030035





