Radial Hemostasis Devices and Post-Procedural Arterial Occlusion: Network Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
- Objectives
2. Materials and Methods
- CINHAL;
- Embase;
- PubMed;
- Cochrane Central Register of Controlled Trials.
2.1. Data Extraction
2.2. Quality Assessment
2.3. Statistics
2.4. Data Analysis
3. Results
3.1. General Results
3.2. Network Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Reasons for Studies Exclusion
- [32]. Reason: The operator used the TR-Band device arbitrarily without a defined inflation protocol.
- [33]. Reason: It is unclear how the TR-Band device was used (the amount of air inflated and the duration it remained in place are not specified).
- [34]. Reason: Small sample size; unclear how much the TR-Band was inflated.
- [35]. Reason: Small sample size; unclear how much the TR-Band was inflated.
- [36]. Reason: It is unclear how the TR-Band device was used (the amount of air inflated and the duration it remained in place are not specified).
- [37]. Reason: Small sample size with no reported RAO.
Appendix B. Quality Assessment
| Was The Assignment to Treatment Groups Truly Random? | Were Participants Blinded to the Treatment Allocation? | Was Allocation to Treatment Groups Concealed from the Allocator? | Were the Outcomes of People Who Withdrew Described and Included in the Analysis? | Were Those Assessing Outcomes Blind to the Treatment Allocation? | Were The Control And Treatment Groups Comparable at Entry? | Were Groups Treated Identically Other than for the Named Interventions? | Were Outcomes Measured in the Same Way for All Groups? | Were Outcomes Measured Reliably? | Was Appropriate Statistical Analysis used? | SCORE | ||
| 1 | Ayyaz Ul Haq et al. [21] | Unclear | No | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | 6 |
| 2 | Barbiero et al. [17] | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 3 | Campos et al. [16] | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 4 | Cong et al. [22] | Yes | No | No | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | 6 |
| 5 | Dangoisse et al. [10] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 6 | Dos Santos et al. [14] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 7 | Gorgulu et al. [18] | Yes | No | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 8 | Lavi et al. [11] | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 9 | Lavi et al. [12] | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 10 | Patel et al. [23] | Unclear | No | No | yes | no | Yes | Yes | Yes | Yes | Yes | 6 |
| 11 | Petroglou et al. [13] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 12 | Sanghvi et al. [24] | Unclear | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 6 |
| 13 | Shah et al. [25] | Unclear | No | No | Yes | no | Yes | Yes | Yes | Yes | Yes | 6 |
| 14 | Rathore et al. [15] | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 15 | Cubero et al. [19] | Unclear | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| 16 | Dai et al. [20] | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 17 | Pancholy et al. [6] | Unclear | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
Appendix C. Different Devices and Protocols Included in the Review Studies
| 1 | Tr Band™ 1 h | 11 | SafeGuard | 21 | Manual compression |
| 2 | Tr Band™, 10 cc, 4 h | 12 | Ankaferd Blood Stopper | 22 | Vitatech pressure bandage |
| 3 | Tr Band™, 15 cc, 4 h | 13 | WORK™ device | 23 | Helix device |
| 4 | Tr Band™, 2 h | 14 | RadAR 20 min | 24 | Helix device + pad |
| 5 | Tr Band™, 3 h | 15 | RadAr 60 min | 25 | Compressive dressing 30 min |
| 6 | Tr Band™, 1,5 h | 16 | RadAr 10 min | 26 | Compressive dressing 1 h |
| 7 | Tr Band™, 30 min | 17 | RadAr 30 min | 27 | Compressive dressing 2 h |
| 8 | Tr Band™ Accelerated Protocol (20 min) | 18 | VasoBand | ||
| 9 | Tr Band™ Coagulation-based protocol | 19 | Chitosan-based pad | ||
| 10 | Tr Band™ (MAP guided) | 20 | Pneumatic compression device inflated with 15 cc of air |
References
- Damluji, A.A.; Nelson, D.W.; Valgimigli, M.; Windecker, S.; Byrne, R.A.; Cohen, F.; Patel, T.; Brilakis, E.S.; Banerjee, S.; Mayol, J.; et al. Transfemoral Approach for Coronary Angiography and Intervention. JACC Cardiovasc. Interv. 2017, 10, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.G. Endovascular Treatment of Complications of Femoral Arterial Access. Yearb. Diagn. Radiol. 2011, 2011, 253–255. [Google Scholar] [CrossRef]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Rev. Española Cardiol. 2017, 70, 1082. [Google Scholar] [CrossRef]
- Hamon, M.; Pristipino, C.; Di Mario, C.; Nolan, J.; Ludwig, J.; Tubaro, M.; Sabate, M.; Mauri-Ferré, J.; Huber, K.; Niemelä, K.; et al. Consensus Document on the Radial Approach in Percutaneous Cardiovascular Interventions: Position Paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention 2013, 8, 1242–1251. [Google Scholar] [CrossRef]
- Kolkailah, A.A.; Alreshq, R.S.; Muhammed, A.M.; Zahran, M.E.; Anas El-Wegoud, M.; Nabhan, A.F. Transradial versus Transfemoral Approach for Diagnostic Coronary Angiography and Percutaneous Coronary Intervention in People with Coronary Artery Disease. Cochrane Database Syst. Rev. 2018, 2018, CD012318. [Google Scholar] [CrossRef]
- Pancholy, S.B.; Bernat, I.; Bertrand, O.F.; Patel, T.M. Prevention of Radial Artery Occlusion After Transradial Catheterization. JACC Cardiovasc. Interv. 2016, 9, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Pancholy, S.; Coppola, J.; Patel, T.; Roke-Thomas, M. Prevention of Radial Artery Occlusion—Patent Hemostasis Evaluation Trial (PROPHET Study): A Randomized Comparison of Traditional versus Patency Documented Hemostasis after Transradial Catheterization. Catheter. Cardiovasc. Interv. 2008, 72, 335–340. [Google Scholar] [CrossRef]
- Fernandez, R.S.; Lee, A. Effects of Methods Used to Achieve Hemostasis on Radial Artery Occlusion Following Percutaneous Coronary Procedures: A Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 738–764. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Dangoisse, V.; Guédès, A.; Chenu, P.; Hanet, C.; Albert, C.; Robin, V.; Tavier, L.; Dury, C.; Piraux, O.; Domange, J.; et al. Usefulness of a Gentle and Short Hemostasis Using the Transradial Band Device after Transradial Access for Percutaneous Coronary Angiography and Interventions to Reduce the Radial Artery Occlusion Rate (from the Prospective and Randomized CRASOC I, II, and III Studies). Am. J. Cardiol. 2017, 120, 374–379. [Google Scholar] [CrossRef]
- Lavi, S.; Cheema, A.; Yadegari, A.; Israeli, Z.; Levi, Y.; Wall, S.; Alemayehu, M.; Parviz, Y.; Murariu, B.; McPherson, T.; et al. Randomized Trial of Compression Duration After Transradial Cardiac Catheterization and Intervention. J. Am. Heart Assoc. 2017, 6, e005029. [Google Scholar] [CrossRef]
- Lavi, S.; Mehta, S.R.; Bajwa, R.; Taleb, H.; Bakar, S.N.; Sachedina, A.; Wagner, C.; Solomonica, A.; Awan, K.; Garg, P.; et al. Short durations of compression hemostatic device application post trans-radial cardiac catheterization: The practical-2 trial. J. Am. Coll. Cardiol. 2020, 75, 1130. [Google Scholar] [CrossRef]
- Petroglou, D.; Didagelos, M.; Chalikias, G.; Tziakas, D.; Tsigkas, G.; Hahalis, G.; Koutouzis, M.; Ntatsios, A.; Tsiafoutis, I.; Hamilos, M.; et al. Manual Versus Mechanical Compression of the Radial Artery After Transradial Coronary Angiography. JACC Cardiovasc. Interv. 2018, 11, 1050–1058. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Wainstein, R.V.; Valle, F.H.; Corrêa, C.L.; Aliti, G.B.; Ruschel, K.B.; Gonçalves, S.C.; Wainstein, M.V.; Rabelo-Silva, E.R. Two HEmostasis Methods After TransradIal Catheterization: THEMATIC Randomized Clinical Trial. J. Cardiovasc. Nurs. 2020, 35, 217–222. [Google Scholar] [CrossRef]
- Rathore, S.; Stables, R.H.; Pauriah, M.; Hakeem, A.; Mills, J.D.; Palmer, N.D.; Perry, R.A.; Morris, J.L. A Randomized Comparison of TR Band and Radistop Hemostatic Compression Devices after Transradial Coronary Intervention. Catheter. Cardiovasc. Interv. 2010, 76, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.D.C.; Alves, C.M.R.; Tsunemi, M.H.; Peterlini, M.A.S.; Avelar, A.F.M. Randomized Clinical Study on Radial Artery Compression Time after Elective Coronary Angiography. Rev. Lat. Am. Enferm. 2018, 26, e3084. [Google Scholar] [CrossRef]
- Barbiero, J.; Tumelero, R.; Tognon, A.; Duda, N.; Trentin, F.; Cadore, D.; Macedo, J. Comparison between Compression Dressing and Hemostatic Wristband after Cardiac Procedures Using the Radial Approach. J. Transcatheter Interv. 2018, 26, eA0015. [Google Scholar] [CrossRef]
- Gorgulu, S.; Norgaz, T.; Sipahi, I. Ankaferd Blood Stopper as a New Strategy to Avoid Early Complications after Transradial Procedures: A Randomized Clinical Trial. J. Interv. Cardiol. 2018, 31, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Cubero, J.M.; Lombardo, J.; Pedrosa, C.; Diaz-Bejarano, D.; Sanchez, B.; Fernandez, V.; Gomez, C.; Vazquez, R.; Molano, F.J.; Pastor, L.F. Radial Compression Guided by Mean Artery Pressure versus Standard Compression with a Pneumatic Device (RACOMAP). Catheter. Cardiovasc. Interv. 2009, 73, 467–472. [Google Scholar] [CrossRef]
- Dai, N.; Xu, D.; Hou, L.; Peng, W.; Wei, Y.; Xu, Y.-W. A Comparison of 2 Devices for Radial Artery Hemostasis After Transradial Coronary Intervention. J. Cardiovasc. Nurs. 2015, 30, 192–196. [Google Scholar] [CrossRef]
- Ayyaz Ul Haq, M.; Nazir, S.A.; Rashid, M.; Kwok, C.S.; Mubashiruddin, S.; Alisiddiq, Z.; Shoaib, A.; Ratib, K.; Mamas, M.A.; Nolan, J. Accelerated Patent Hemostasis Using a Procoagulant Disk; a Protocol Designed to Minimize the Risk of Radial Artery Occlusion Following Cardiac Catheterization. Cardiovasc. Revasc. Med. 2019, 20, 137–142. [Google Scholar] [CrossRef]
- Cong, X.; Huang, Z.; Wu, J.; Wang, J.; Wen, F.; Fang, L.; Fan, M.; Liang, C. Randomized Comparison of 3 Hemostasis Techniques After Transradial Coronary Intervention. J. Cardiovasc. Nurs. 2016, 31, 445–451. [Google Scholar] [CrossRef]
- Patel, G.; Shah, S.; Patel, B.; Patel, T. Randomized COmparison of Isolated Radial Artery ComPrEssioN Versus Radial and Ipsilateral Ulnar Artery Compression in Achieving Radial Artery Patency: The OPEN-Radial Trial. J. Am. Coll. Cardiol. 2020, 32, 476–482. [Google Scholar] [CrossRef]
- Sanghvi, K.A.; Montgomery, M.; Varghese, V. Effect of Hemostatic Device on Radial Artery Occlusion: A Randomized Comparison of Compression Devices in the Radial Hemostasis Study. Cardiovasc. Revasc. Med. 2018, 19, 934–938. [Google Scholar] [CrossRef]
- Shah, S.; Gindi, R.; Basir, M.B.; Khandelwal, A.; Alqarqaz, M.; Zaidan, M.; Voeltz, M.; Koenig, G.; Kim, H.E.; O’Neill, W.W.; et al. Optimal TR-Band Weaning Strategy While Minimizing Vascular Access Site Complications. Cardiovasc. Revasc. Med. 2019, 20, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Schwarzer, G. Ranking Treatments in Frequentist Network Meta-Analysis Works without Resampling Methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef]
- Van Valkenhoef, G.; Lu, G.; De Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating Network Meta-analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Choi, E.Y.; Ko, Y.G.; Kim, J.B.; Rhee, J.; Park, S.; Choi, D.; Jang, Y.; Shim, W.H.; Cho, S.Y. Hemostatic efficacy of hydrophilic wound dressing after transradial catheterization. J. Invasive Cardiol. 2005, 17, 459–462. [Google Scholar]
- Politi, L.; Aprile, A.; Paganelli, C.; Amato, A.; Zoccai, G.B.; Sgura, F.; Monopoli, D.; Rossi, R.; Modena, M.G.; Sangiorgi, G.M. Randomized Clinical Trial on Short-Time Compression with Kaolin-Filled Pad: A New Strategy to Avoid Early Bleeding and Subacute Radial Artery Occlusion after Percutaneous Coronary Intervention: SHORT-TIME RADIAL COMPRESSION WITH KAOLIN-FILLED PAD. J. Interv. Cardiol. 2011, 24, 65–72. [Google Scholar]
- Yang, Q.; Zhou, Y.J.; Nie, B.; Liu, X.L.; Cheng, W.J.; Wang, J.L. [Effects of bandage compression and the specific radial hemostasis in patients undergoing transradial coronary intervention]. Zhonghua Xin Xue Guan Bing Za Zhi 2010, 38, 720–723. [Google Scholar]
- Due-Tønnessen, N.; Egeland, C.H.; Meyerdierks, O.J.; Opdahl, A. Is radial artery occlusion and local vascular complications following transradial coronary procedures affected by the type of haemostasis device used? A non-inferiority Randomized Controlled Trial (RadCom trial). Eur. J. Cardiovasc. Nurs. 2021, 20, 580–587. [Google Scholar] [PubMed]
- Minor, R.L.; Maley, T.; Jenkins, D.; Li, Y.H. Randomized Trial of VasoStat Versus TR Band Following Radial Artery Access for Catheterization Procedures. J. Invasive Cardiol. 2021, 33, E84–E90. [Google Scholar]
- Roberts, J.S.; Niu, J.; Pastor-Cervantes, J.A. Comparison of Hemostasis Times with a Chitosan-Based Hemostatic Pad (Clo-SurPlus RadialTM) vs Mechanical Compression (TR Band®) Following Transradial Access: A pilot Study. Cardiovasc. Revascularization Med. 2019, 20, 871–874. [Google Scholar]
- Roberts, J.; Niu, J.; Shah, S.; Cianferra, E.; Pastor-Cervantes, J. TCT-780 Randomized Comparison of Hemostasis Times Following Transradial Access with a Kaolin-Based Hemostatic Pad (QuikClot® Radial®) vs. Mechanical Compression (TR Band®). J. Am. Coll. Cardiol. 2019, 74, B764. [Google Scholar]
- Safirstein, J.G.; Elfandi, A.; Reid, N.; Clark, T. HAND PERFUSION DURING RADIAL COMPRESSION WITH VASOSTAT VERSUS TR BAND: A RANDOMIZED TRIAL. J. Am. Coll. Cardiol. 2019, 73, 1135. [Google Scholar]
- Voon, V.; AyyazUlHaq, M.; Cahill, C.; Mannix, K.; Ahern, C.; Hennessy, T.; SamerArnous;Kiernan, T. Randomized study comparing incidence of radial artery occlusion post-percutaneous coronary intervention between two conventional compression devices using a novel air-inflation technique. WJC 2017, 9, 807–812. [Google Scholar]
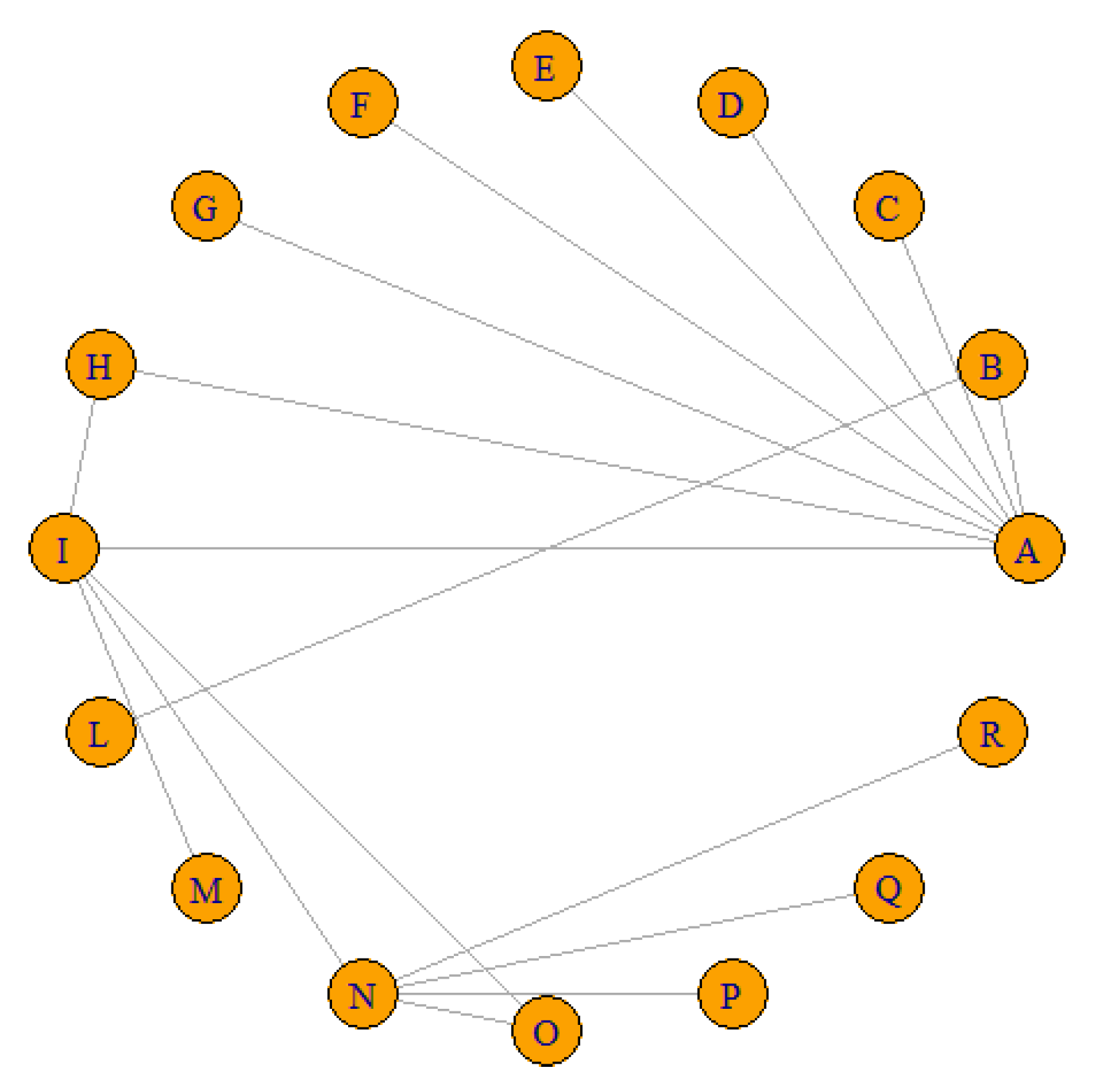
| ID | Treat1 | Treat2 | RR | IC 95% |
|---|---|---|---|---|
| Dangoisse, 2017 | A | B | 0.8597 | [0.2163; 3.4169] |
| Dangoisse, 2017 (2) | A | C | 3.1229 | [0.1963; 49.6759] |
| Dos Santos, 2020 | A | D | 1.2548 | [0.7024; 2.2415] |
| Patel, 2020 | A | E | 6.4488 | [1.4855; 27.9961] |
| Sanghvi, 2018 | A | F | 0.6155 | [0.2292; 1.6527] |
| Shah, 2019 | A | G | 0.517 | [0.1394; 1.9174] |
| Gorgulu, 2018 | A | H | 1.9614 | [0.1792; 21.4643] |
| Gorgulu, 2018 | H | I | 0.5074 | [0.0464; 5.5501] |
| Gorgulu, 2018 | A | I | 0.9952 | [0.1415; 6.9985] |
| Cubero, 2009 | B | L | 10.56 | [2.5139; 44.3580] |
| Campos, 2018 | I | M | 1.1038 | [0.6084; 2.0025] |
| Cong, 2016 | I | N | 0.4066 | [0.2753; 0.6005] |
| Cong, 2016 | N | O | 3.1101 | [2.0215; 4.7849] |
| Cong, 2016 | I | O | 1.2646 | [0.7591; 2.1066] |
| Pancholy, 2008 | N | R | 2.4321 | [1.2377; 4.7790] |
| Rathore, 2010 | N | P | 1.0857 | [0.7010; 1.6815] |
| Dai, 2015 | N | Q | 2.0588 | [1.1797; 3.5930] |
| Number of studies: | k = 13 | |||
| Number of treatments: | n = 16 | |||
| Number of pairwise comparisons: | m = 17 | |||
| Number of designs: | d = 13 | |||
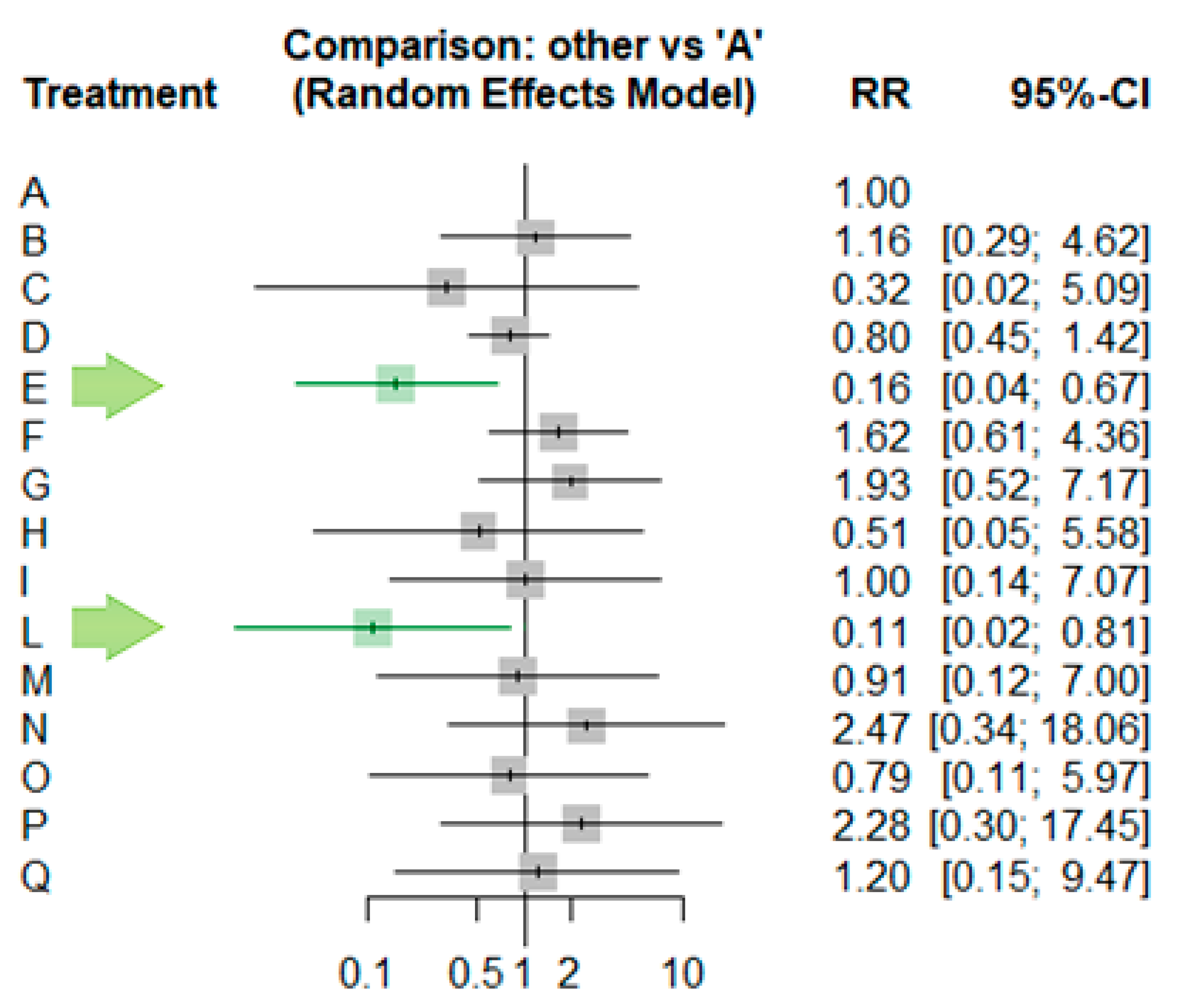
| ID | RR | IC | z | p-Value |
|---|---|---|---|---|
| A | . | . | . | |
| B | 1.1632 | [0.2927; 4.6232] | 0.21 | 0.83 |
| C | 0.3202 | [0.0201; 5.0937] | −0.81 | 0.4198 |
| D | 0.7969 | [0.4461; 1.4236] | −0.77 | 0.4432 |
| E | 0.1551 | [0.0357; 0.6732] | −2.49 | 0.0128 |
| F | 1.6247 | [0.6051; 4.3624] | 0.96 | 0.3355 |
| G | 1.9342 | [0.5215; 7.1734] | 0.99 | 0.3239 |
| H | 0.5098 | [0.0466; 0.5784] | −0.55 | 0.5811 |
| I | 1.0048 | [0.1429; 7.0662] | 0 | 0.9961 |
| L | 0.1102 | [0.015; 0.8066] | −2.17 | 0.0299 |
| M | 0.9103 | [0.1184; 6.997] | −0.09 | 0.9281 |
| N | 2.4713 | [0.3381; 18.0625] | 0.89 | 0.3727 |
| O | 0.7946 | [0.1058; 5.967] | −0.22 | 0.8231 |
| P | 2.2762 | [0.297; 17.4468] | 0.79 | 0.4286 |
| Q | 1.2003 | [0.1521; 9.4706] | 0.17 | 0.8624 |
| R | 1.0161 | [0.1243; 8.3033] | 0.01 | 0.9881 |
| ID | P-Score |
|---|---|
| L | 0.9157 |
| E | 0.8925 |
| C | 0.7121 |
| H | 0.6497 |
| O | 0.6071 |
| D | 0.5654 |
| M | 0.5352 |
| R | 0.4856 |
| I | 0.4841 |
| A | 0.47 |
| B | 0.4161 |
| Q | 0.4084 |
| F | 0.2977 |
| G | 0.2592 |
| P | 0.168 |
| N | 0.133 |
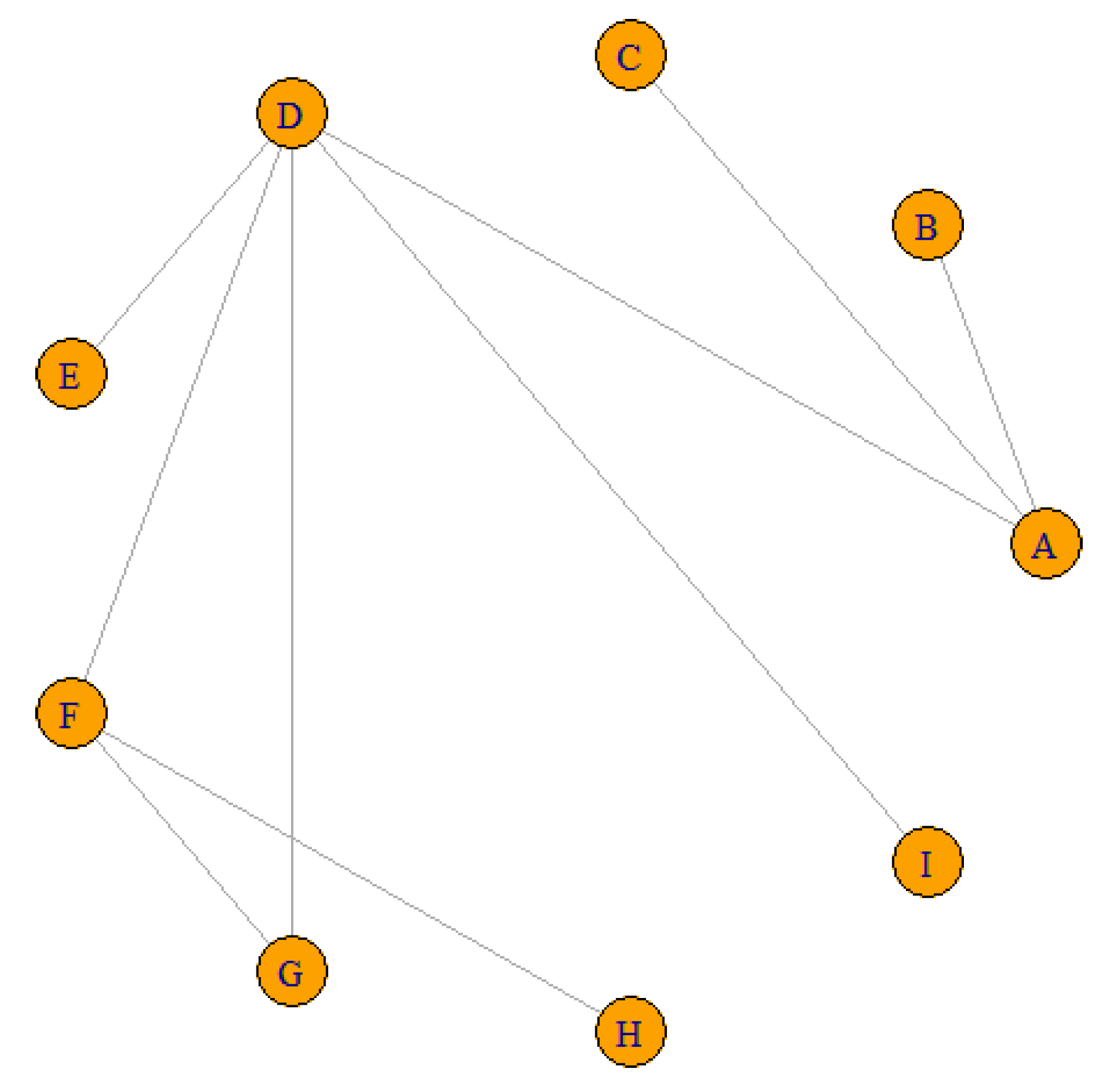
| ID | Treat1 | Treat2 | RR | 95% IC |
|---|---|---|---|---|
| Dos Santos, 2020 | A | B | 0.8333 | [0.2623;2.6473] |
| Sanghvi, 2018 | A | C | 0.7737 | [0.176;3.4015] |
| Barbiero, 2018 | A | D | 1.5833 | [0.7101;3.5303] |
| Campos, 2018 | D | E | 0.6835 | [0.2729;1.7119] |
| Cong, 2016 | D | F | 4.4485 | [2.5252;7.8365] |
| Cong, 2016 | F | G | 1.3047 | [0.5978;2.8473] |
| Cong, 2016 | D | G | 5.8038 | [3.0942;10.886] |
| Pancholy, 2008 | D | I | 3.9635 | [1.3467;11.665] |
| Dai, 2015 | F | H | 2.1794 | [1.1268;4.2153] |
| Number of studies | k = 7 | |||
| Number of treatments | n = 9 | |||
| Number of pairwise comparisons: | m = 9 | |||
| Number of designs: | d = 7 | |||
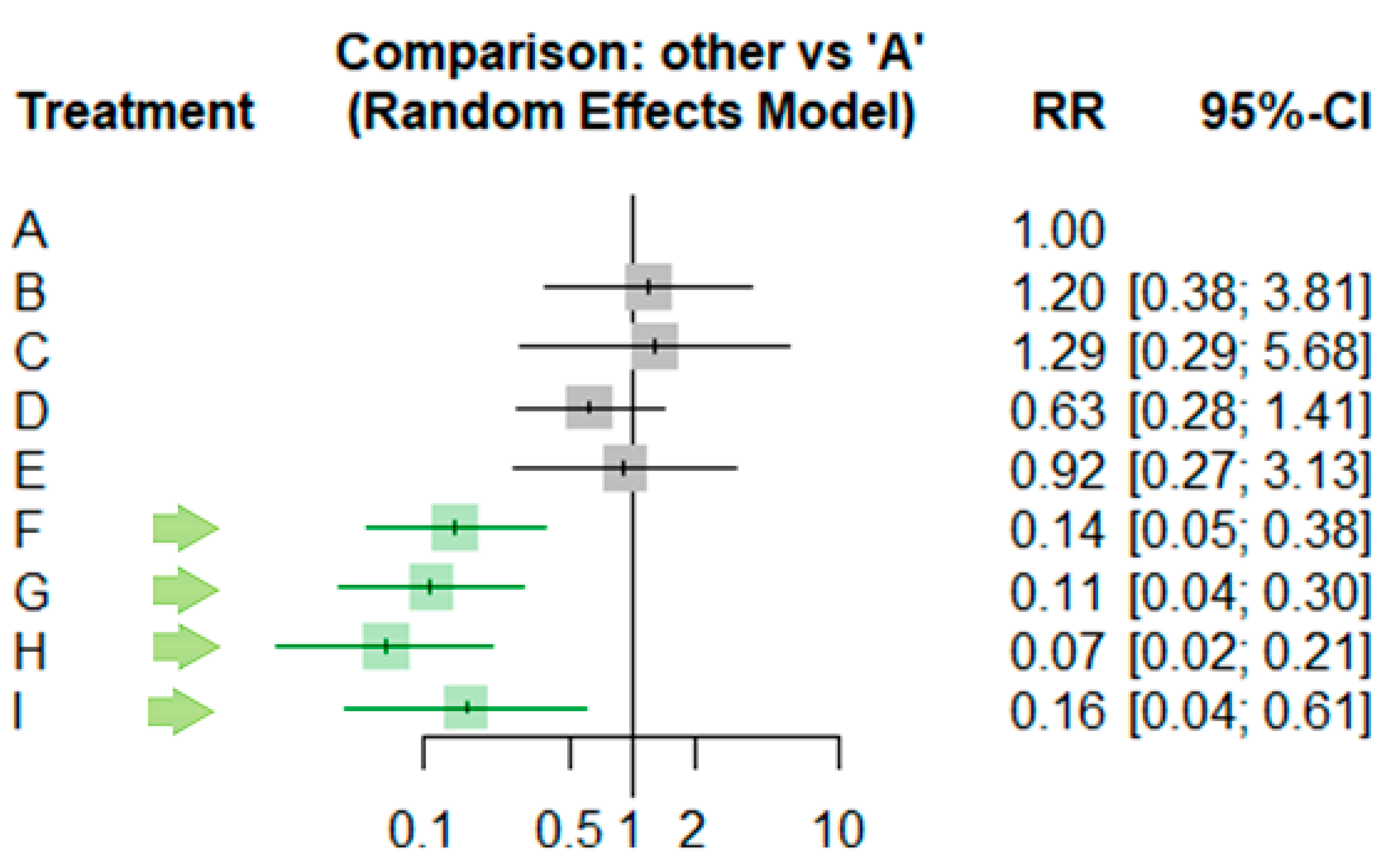
| ID | RR | 95% CI | z | p-Value |
|---|---|---|---|---|
| A | . | . | . | |
| B | 1.2 | [0.3777;3.8124] | 0.31 | 0.7572 |
| C | 1.2925 | [0.294;5.6823] | 0.34 | 0.7342 |
| D | 0.6316 | [0.2833;1.4083] | −1.12 | 0.2614 |
| E | 0.9241 | [0.2731;3.1269] | −0.13 | 0.8989 |
| F | 0.142 | [0.0532;0.3789] | −3.9 | <0.0001 |
| G | 0.1088 | [0.0393;0.3015] | −4.27 | <0.0001 |
| H | 0.0651 | [0.02;0.2126] | −4.53 | <0.0001 |
| I | 0.1594 | [0.0415;0.6114] | −2.68 | 0.0074 |
| ID | P-Score |
|---|---|
| H | 0.9654 |
| G | 0.8289 |
| F | 0.7282 |
| I | 0.7192 |
| D | 0.4099 |
| E | 0.2526 |
| A | 0.2298 |
| B | 0.1865 |
| C | 0.1795 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parozzi, M.; Bonacaro, A.; Bozzetti, M.; Cangelosi, G.; Bertuol, M.; Mozzarelli, F.; Ferrara, P.; Mancin, S.; Terzoni, S. Radial Hemostasis Devices and Post-Procedural Arterial Occlusion: Network Meta-Analysis of Randomized Controlled Trials. J. Vasc. Dis. 2025, 4, 25. https://doi.org/10.3390/jvd4030025
Parozzi M, Bonacaro A, Bozzetti M, Cangelosi G, Bertuol M, Mozzarelli F, Ferrara P, Mancin S, Terzoni S. Radial Hemostasis Devices and Post-Procedural Arterial Occlusion: Network Meta-Analysis of Randomized Controlled Trials. Journal of Vascular Diseases. 2025; 4(3):25. https://doi.org/10.3390/jvd4030025
Chicago/Turabian StyleParozzi, Mauro, Antonio Bonacaro, Mattia Bozzetti, Giovanni Cangelosi, Maria Bertuol, Fabio Mozzarelli, Paolo Ferrara, Stefano Mancin, and Stefano Terzoni. 2025. "Radial Hemostasis Devices and Post-Procedural Arterial Occlusion: Network Meta-Analysis of Randomized Controlled Trials" Journal of Vascular Diseases 4, no. 3: 25. https://doi.org/10.3390/jvd4030025
APA StyleParozzi, M., Bonacaro, A., Bozzetti, M., Cangelosi, G., Bertuol, M., Mozzarelli, F., Ferrara, P., Mancin, S., & Terzoni, S. (2025). Radial Hemostasis Devices and Post-Procedural Arterial Occlusion: Network Meta-Analysis of Randomized Controlled Trials. Journal of Vascular Diseases, 4(3), 25. https://doi.org/10.3390/jvd4030025








