Abstract
Aortic aneurysm is a vascular disease with a complex pathogenesis which is usually asymptomatic but can lead to high mortality with sudden rupture. This review comprehensively examines the molecular mechanisms of aortic aneurysms in the context of extracellular matrix destruction, smooth muscle cell apoptosis, chronic inflammation, oxidative stress, genetic mutations, and epigenetic regulations. In addition, the potential of molecular biomarkers in diagnosis and prognosis and targeted treatment strategies are evaluated. Animal models and translational findings form the basis for establishing a bridge between preclinical and clinical applications. This study aims to provide insight into the integration of molecular findings into clinical practice in light of the current literature and to guide future research.
1. Introduction
Aortic aneurysm is a primary vascular disease characterized by segmental dilatation of the aorta, the largest vessel in the arterial system. It is often asymptomatic and has a high mortality rate. This dilatation is usually defined as an increase in the aortic diameter to more than 50% of normal. Clinically, it is most commonly classified as abdominal aortic aneurysm (AAA) and thoracic aortic aneurysm (TAA). Since the disease is often silent, it cannot be diagnosed before life-threatening complications such as rupture or dissection occur. Since rupture can result in a high rate of death, early diagnosis and a good understanding of the pathophysiology of the disease are of critical importance [1,2]. In recent years, significant advances have been made in the diagnosis and treatment processes thanks to advanced imaging techniques (e.g., CT angiography and MR angiography) and endovascular surgical approaches [3,4]. However, these technical developments are insufficient to understand the mechanisms of the disease’s onset and ensure effective prevention. The pathogenesis of aortic aneurysm is too complex to be evaluated solely in terms of hemodynamic stresses; it involves the interaction of cellular, molecular, genetic, and environmental factors [5,6]. The standard structure of the aortic wall consists of three layers that provide vascular wall integrity: the tunica intima, the tunica media, and the tunica adventitia. These structures contain a complex organization of smooth muscle cells (vascular smooth muscle cells—SMCs), extracellular matrix (ECM) proteins (especially elastin and collagen), and connective tissue cells. Under homeostatic conditions, ECM synthesis and degradation are maintained in a delicate balance. However, during aneurysm formation, this balance is disrupted; ECM degradation increases, SMCs undergo apoptosis, infiltration of inflammatory cells intensifies, and oxidative stress factors such as reactive oxygen species come into play [7,8]. As a result of these processes, the aortic wall’s elasticity and mechanical strength decrease, paving the way for progressive dilation and possible rupture.
In addition, recent genetic and epigenetic studies have revealed that hereditary predisposition and changes in gene expression via epigenetic regulators play an important role in developing aortic aneurysms [9,10]. Genetic mutations seen in connective tissue diseases such as Marfan syndrome, Loeys–Dietz syndrome, and vascular-type Ehlers–Danlos syndrome disrupt the structural integrity of the aortic wall and cause aneurysm formation at an early age [11]. Thanks to advances in molecular biology techniques, biomarkers that facilitate diagnosis in the early stages of the disease, treatment options for specific targets and individualized monitoring strategies are promising [12,13]. This review aims to examine the molecular pathogenesis of aortic aneurysms in detail, evaluate the role of genetic and epigenetic factors in line with the current literature, and reveal the contributions of translational research with possible biomarkers that will shed light on future diagnostic and therapeutic approaches.
This narrative review was conducted based on a comprehensive literature search using the PubMed, Scopus, and Web of Science databases. The search included studies published between January 2010 and March 2025. The primary keywords used included the following: aortic aneurysm, pathogenesis, molecular mechanisms, genetics, epigenetics, inflammation, oxidative stress, microRNA, biomarkers, and translational research. Boolean operators (and, or) were applied to optimize search sensitivity and specificity. Only English-language, peer-reviewed articles were included. Studies were selected based on their relevance to the molecular pathogenesis, genetic and epigenetic mechanisms, and translational aspects of aortic aneurysms. Exclusion criteria were as follows: non-English publications, case reports, conference abstracts, and experimental animal studies without direct human translational implications. Priority was given to recent high-quality reviews, meta-analyses, and original research with robust methodologies.
2. Normal Histology and Molecular Structure of the Aortic Wall
The aorta, the main vessel of systemic circulation, is an artery with high elastic properties that delivers oxygenated blood from the heart to the body under high pressure. This feature requires the aortic wall to have a unique architecture, both structurally and functionally [14,15,16]. The aortic wall consists of three layers from the inside to the outside: the tunica intima, the tunica media, and the tunica adventitia. Each layer represents a complex organization containing different cellular components and extracellular matrix (ECM) proteins [14,17]. The tunica intima is a thin layer covered with endothelial cells in direct contact with the blood. These cells not only form a physical barrier but also play an active role in regulating vascular tone, inflammation, and hemostasis by secreting molecules such as nitric oxide and endothelin-1 [18,19]. Endothelial dysfunction is considered the first step of pathological changes in the vascular wall by facilitating the adhesion of inflammatory cells [20].
The tunica media is the thickest layer of the aortic wall and consists mainly of smooth muscle cells (SMCs) and ECM proteins such as elastin and collagen synthesized by these cells. Elastic lamellae allow the aorta to resist systolic pressure and maintain continuous blood flow by providing an elastic springback in diastole. Collagen increases mechanical strength [15,21]. SMCs in the media layer provide structural support, regulate the synthesis and degradation of ECM, respond to inflammatory stimuli, and exhibit phenotype changeability. In the development of aneurysms, apoptosis of these cells or the transition from the contractile phenotype to the synthetic phenotype increases ECM imbalance and disrupts wall integrity [22,23]. The tunica adventitia is the outer layer enriched with fibroblasts, macrophages, lymphocytes, and small vascular networks called the vasa vasorum. The vasa vasorum supplies the outer layers of large-diameter arteries and can expand with neovascularization under hypoxic conditions. The adventitia also forms a microenvironment where inflammatory cells migrate and chronic inflammation persists. During chronic inflammation, the adventitia thickens, and macrophage and lymphocyte infiltration increases, triggering MMP release and disrupting the medial layer [24,25]. The ECM components of the aortic wall are the basic structures that determine the mechanical and elastic properties of the tissue.
The main ECM proteins include elastin, type I and III collagen, fibrillin-1, decorin, fibronectin, and laminin. SMCs and fibroblasts synthesize these molecules, while their degradation is mediated by matrix metalloproteinases (MMPs) [14,26,27]. MMP activity is balanced by tissue inhibitors of metalloproteinases (TIMPs). This dynamic balance is critical for maintaining vascular wall homeostasis. However, disrupting the MMP-TIMP balance increases ECM destruction, leading to aneurysm formation [13,28]. Consequently, the aortic wall’s standard histological structure and molecular organization play a vital role in maintaining vascular integrity. When this complex structure is disrupted, the aneurysmal process begins with loss of elasticity and structural collapse. The molecular pathomechanisms behind this disruption will be discussed in detail in the following sections.
3. Pathogenesis and Molecular Mechanisms in Aortic Aneurysm
The pathogenesis of aortic aneurysm is not only the result of hemodynamic stress acting on the vessel wall, but also dysfunction of cellular structures in the vessel wall and disruptions in molecular signaling pathways. This multidimensional process progresses through the interaction of extracellular matrix (ECM) destruction, smooth muscle cell (SMC) apoptosis, inflammatory response, oxidative stress, and genetic/epigenetic regulations [5,29]. The continuity of these processes impairs the mechanical strength of the aortic wall, leading to progressive dilatation and ultimately the risk of rupture (Figure 1).
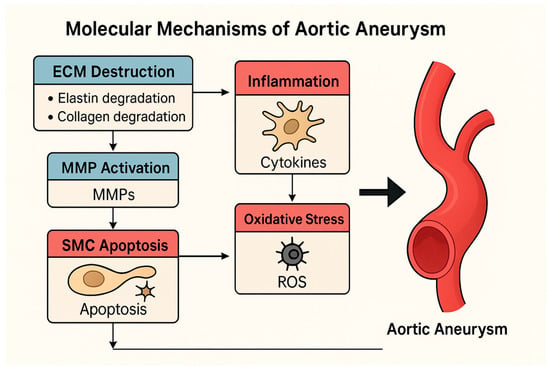
Figure 1.
Molecular mechanisms contributing to aortic aneurysm pathogenesis, including extracellular matrix (ECM) degradation, smooth muscle cell (SMC) apoptosis, oxidative stress, inflammation, and dysregulated TGF-β signaling, which collectively weaken the aortic wall.
This pathway involves multiple overlapping mechanisms such as VSMC apoptosis, ECM remodeling, inflammatory cytokine signaling, and activation of ROS-generating enzymes like NADPH oxidases. Downstream transcriptional effects are mediated via NF-κB, SMAD2/3, and p53 signaling, contributing to medial layer weakening and aortic wall dilatation.
3.1. Matrix Metalloproteinases (MMPs) and ECM Degradation
Matrix metalloproteinases (MMPs) play the most important role in the degradation of ECM proteins in the aortic wall. In particular, MMP-2 and MMP-9 are key enzymes in degrading structural proteins such as elastin and collagen. These enzymes are secreted primarily by macrophages, neutrophils, and synthetic vascular SMCs. Studies on aneurysmal aortic tissue have shown that the expression of these MMPs is increased while the levels of their natural inhibitors, TIMP-1 and TIMP-2, are decreased [30,31]. This imbalance accelerates the degradation of the ECM, disrupts the integrity of the elastic lamellae, and leads to irreversible structural weakening of the aortic wall [32].
3.2. Smooth Muscle Cell (SMC) Apoptosis and Phenotypic Change
Vascular smooth muscle cells provide structural support and are responsible for the production of ECM proteins, cytokine response, and vascular tone regulation. Apoptosis of SMCs significantly increases aneurysm development [28]. With the increase in apoptosis, ECM synthesis decreases and vascular wall integrity is weakened. In addition, SMCs show phenotypic change from the contractile phenotype to the synthetic phenotype, increasing MMP production and causing the expression of proinflammatory cytokines [17,33]. This transformation further exacerbates the ECM degradation cycle.
3.3. Inflammation and the Role of the Immune System
Aneurysm development is characterized by chronic inflammatory cell populations that settle in the vessel wall. Macrophages, T lymphocytes, and dendritic cells are densely located in the intima and adventitia layers. Cytokines such as IL-6, IL-1β, and TNF-α released from these cells increase both MMP expression and trigger SMC apoptosis [34,35]. In recent years, it has been shown that the NLRP3 inflammasome, one of the inflammatory signaling pathways, is activated in abdominal aortic aneurysms and plays a role in maintaining the inflammatory cascade [36,37]. This inflammatory response accelerates vascular weakening by increasing proteolytic activity in local tissue.
3.4. The Role of Oxidative Stress
Oxidative stress is involved in aneurysm pathogenesis as both a cause and a consequence. The increase in reactive oxygen species (ROS) is particularly associated with activating the NADPH oxidase complex [38]. ROS accumulation causes apoptosis of SMCs, impairs endothelial function, and triggers inflammation. At the same time, ROS promotes ECM degradation by increasing MMP gene expression [39]. Known risk factors such as aging, smoking, and hypertension contribute to aneurysm progression by increasing the level of oxidative stress [40].
3.5. Disruption of the TGF-β Signaling Pathway
Transforming Growth Factor-β (TGF-β) is a growth factor that normally supports vascular wall homeostasis. However, under pathological conditions, excessive activation of this signaling pathway or dysfunction due to mutations may result in imbalance in ECM synthesis and excessive fibrosis [41]. In connective tissue diseases such as Marfan and Loeys–Dietz syndromes, TGFBR1/2 and SMAD gene mutations disrupt the regulation of this pathway and increase the risk of thoracic aortic aneurysm [42,43]. In addition, TGF-β modulates inflammation and neovascularization, affecting disease progression [44]. All of these mechanisms do not work alone, but in interaction with each other. Increased MMP activity, in synergy with inflammatory cell infiltration and oxidative stress, causes the aortic wall to weaken and expand over time. Understanding this molecular cycle is of great importance for identifying new therapeutic targets.
3.6. Genetic Contributions to Aneurysm Formation
Genetic predisposition plays a significant role in aneurysm formation, particularly in thoracic aortic aneurysms. Mutations in genes such as FBN1, TGFBR1/2, SMAD3, ACTA2, MYH11, and LOX affect extracellular matrix integrity, TGF-β signaling, and smooth muscle cell function. These variants can impair vessel wall architecture and increase susceptibility to dissection. Genome-wide association studies (GWASs) have also identified common polymorphisms associated with sporadic aortic aneurysms, further supporting a polygenic component to disease susceptibility.
4. Genetic and Epigenetic Factors
Not only environmental factors, but also genetic predisposition and epigenetic regulators play a critical role in the development of aortic aneurysms. Thoracic aortic aneurysms (TAAs) in particular have a more distinct genetic basis compared to abdominal aortic aneurysms (AAAs) [45,46]. Familial clustering, inherited connective tissue diseases, and single-gene mutations underlie this difference. In addition, molecular biology studies in recent years have shown that epigenetic mechanisms (such as DNA methylation, microRNAs, and histone modifications) also have an effect on aortic wall biology [10,47].
4.1. Hereditary Connective Tissue Diseases and Monogenic Mutations
Some hereditary connective tissue syndromes are responsible for a significant portion of TAA cases that occur at a young age. The best-defined are Marfan syndrome, Loeys–Dietz syndrome, and vascular-type Ehlers–Danlos syndrome (Figure 2).
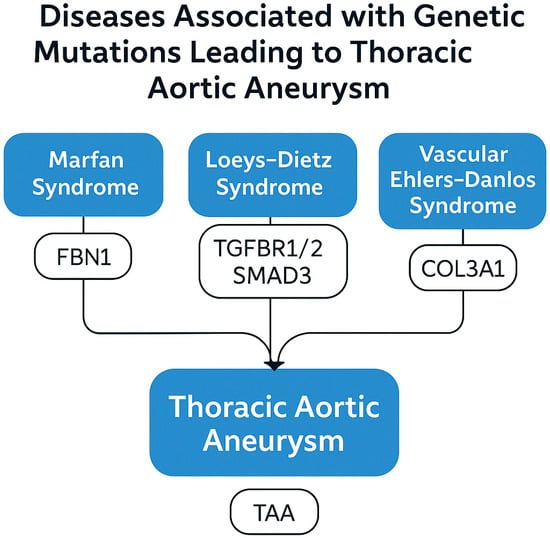
Figure 2.
Genetic syndromes and associated gene mutations contributing to thoracic aortic aneurysm (TAA), including Marfan syndrome (FBN1), Loeys–Dietz syndrome (TGFBR1/2 and SMAD3), and vascular Ehlers–Danlos syndrome (COL3A1).
- Marfan syndrome is caused by mutations in the FBN1 gene that result in a defect in the fibrillin-1 protein. This defect leads to disruption of elastic fiber organization and excessive activation of the TGF-β signaling pathway [42,43]. In addition to the well-established TGF-β pathway genes, variants in smooth muscle contractile apparatus genes such as ACTA2, MYH11, LOX, and PRKG1 have been linked to familial thoracic aortic aneurysms. ACTA2 mutations impair actin polymerization, weakening vessel wall tension and predisposing to dissection. LOX mutations reduce elastin crosslinking, compromising structural integrity.
- Loeys–Dietz syndrome is associated with gene mutations encoding various components of the TGF-β signaling pathway (TGFBR1, TGFBR2, and SMAD3). The risk of aortic dissection is high in this syndrome, and dissection may develop even when dilatation is usually minimal [46,47].
- Ehlers–Danlos syndrome (vascular type) is caused by mutations in the COL3A1 gene that result in a defect in type III collagen synthesis. This condition severely weakens the mechanical strength of the aortic wall and increases the risk of spontaneous rupture [48].
4.2. Genetic Polymorphisms in Sporadic Aortic Aneurysms
Genetic contributions cannot be ruled out in sporadic AAA and TAA cases. Single-nucleotide polymorphisms (SNPs) may increase disease risk by affecting certain genes’ expression levels or functions. For example:
- Some SNPs in the MMP9, IL6, TIMP1, and ACE genes may increase the activity of pathways that facilitate ECM degradation [49,50].
- Genome-wide association studies (GWASs) have identified new risk loci associated with AAA. These studies have shown that genetic variations contribute to aneurysm formation [51].
4.3. Epigenetic Regulations
Epigenetic mechanisms permanently or temporarily regulate gene expression without changing DNA sequences. These processes are crucial in explaining the effects of environmental factors on genetic activity [52].
- DNA Methylation: Hypermethylation in promoter regions can suppress anti-inflammatory genes or genes involved in ECM production [53].
- MicroRNAs (miRNAs): Changes in the expression levels of miRNAs, especially miR-29, miR-195, miR-21, and miR-143/145, have been reported in aneurysmal tissues. The miR-29 family can suppress collagen and elastin synthesis and weaken ECM [27,54,55].
- Histone Modifications: Acetylation or methylation of histone proteins determines whether genes remain on or off by affecting chromatin structure. Increased histone acetylation has been detected in aneurysm tissues, particularly in the promoter regions of genes associated with the inflammatory response [56].
These findings suggest that both inherited and acquired molecular mechanisms contribute to the pathogenesis of aortic aneurysms. Genetic screening, individualized risk analysis, and pharmacological interventions targeting epigenetic targets hold great potential for future diagnostic and therapeutic strategies.
5. Animal Models and Translational Findings
Animal models developed to understand aortic aneurysms at the molecular level play a critical role in elucidating the cellular and biochemical processes involved in pathogenesis [57]. Mouse models, in particular, are frequently preferred in experimental studies due to their suitability for genetic manipulation, short life cycle, and cost-effectiveness [58]. These models offer the opportunity to analyze the disease process’s basic mechanisms and test the effectiveness of new pharmacological targets [59].
5.1. Elastase-Induced Abdominal Aortic Aneurysm Model
In this model, porcine pancreatic elastase (PPE) is infused into the abdominal aorta to break down elastin fibers [35]. After this procedure, the aortic wall weakens rapidly, inflammation begins, and aneurysmal dilatation occurs over time. The main features of the model include increased MMP activity, infiltrating macrophage accumulation, smooth muscle cell apoptosis, and significant oxidative stress [60]. This model is frequently used because it mimics aneurysm development accompanied by ECM destruction and an inflammatory response [61].
5.2. Calcium Chloride (CaCl2) Model
Calcium chloride applied to the perivascular area causes local inflammation and elastin degeneration. As a result of the structural disorders that occur, aortic wall dilation occurs over time [62]. It is more suitable for chronic aneurysm models because it offers a pathology that develops more slowly than the elastase model. Oxidative stress is evident in this model; SMC loss and ECM degradation are observed [31].
5.3. Angiotensin II (Ang II)-Induced Aneurysm Model
This model infuses genetically modified mice (especially ApoE−/− or LDLR−/−) with Angiotensin II subcutaneously. As a result, aneurysms and dissections may develop in both abdominal and thoracic aortic regions [24]. This is characterized by the destruction of elastic lamellae, especially intimal tears and dissection formation in the media. Inflammatory cell infiltration, increased ROS, and atherosclerotic plaque formation are prominent features of this model [63]. It offers significant advantages in modeling aneurysm formation associated with atherosclerosis.
5.4. Genetic Models
Transgenic mouse models carrying single-gene mutations have been used to study inherited aortic diseases. For example, the Fbn1C1039G/+ mouse model mimicking Marfan syndrome is prone to developing spontaneous thoracic aortic aneurysms [41]. Similarly, knockout or overexpression models of the TGFBR1, TGFBR2, SMAD3, and MMP-9 genes have been used to understand the effects of the TGF-β signaling pathway on aneurysms [64,65]. These models are valuable for investigating the disease’s genetic basis and validating potential genetic targets.
5.5. Translational Findings and Clinical Relevance
Animal models have provided evidence for many molecular mechanisms that can be correlated with clinical observations [66]. For example:
- MMP inhibitors (such as doxycycline) have slowed aneurysm development by suppressing MMP activity in experimental models but have had limited success in clinical trials [67].
- Therapeutic agents directed against microRNAs (such as miR-29 antagomirs) have shown positive results by preserving ECM homeostasis in animal models [54].
- Oxidative stress-reducing strategies (such as NADPH oxidase inhibitors) have reduced medial damage by reducing ROS levels [68].
- β-aminopropionitrile (BAPN), an irreversible inhibitor of lysyl oxidase, induces medial degeneration by preventing the crosslinking of collagen and elastin. Administered orally in mice, BAPN leads to weakening of the aortic wall and dissection, especially when combined with Ang II. This model simulates medial degeneration and is increasingly used to study the pathophysiology of thoracic aortic dissection.
However, positive findings obtained in animal models are not always directly transferable to human studies. Genetic, hemodynamic, and environmental differences pose important limitations in terms of translational validity. Therefore, it is of great importance to support preclinical findings with human studies.
6. Molecular Biomarkers and Future Diagnostic–Therapeutic Approaches
Aortic aneurysms are usually among the diseases that are noticed when the risk of rupture increases due to their silent course over a long period of time and the absence of clinical symptoms. This situation makes early diagnosis and risk stratification significantly difficult. In this context, molecular biomarkers, which have become increasingly prominent in recent years, are attracting attention due to their potential to evaluate the presence and progression of aortic aneurysms with non-invasive methods [69,70]. This information obtained at the molecular level offers important opportunities not only in terms of diagnosis but also in terms of monitoring the course of the disease and developing individualized treatment strategies [71].
6.1. Potential Molecular Biomarkers
Matrix metalloproteinases (MMP-2 and MMP-9) have been detected at high levels in aneurysm tissues and peripheral blood and are directly related to the destruction of the extracellular matrix [30,31,61]. The decreased levels of inhibitors such as TIMP-1, which balance this proteolytic activity, indicate that the destruction progresses uncontrolled [13,72]. In evaluating inflammatory processes, cytokines such as IL-6, TNF-α, and CRP have been frequently studied and reported to be found at high levels, especially in cases with rapid progression [73]. In addition, glycoproteins associated with cell migration and inflammation, such as osteopontin, have been detected at significantly increased levels in patients with abdominal aortic aneurysms (AAAs) [74]. MicroRNAs, which have recently gained an important place among molecular biomarkers, play an important role in the development of aneurysms by affecting both ECM integrity and smooth muscle cell functions [75]. MicroRNAs, especially miR-29b, miR-195, miR-21, and miR-143/145, have attracted attention because they affect ECM protein expression, cell proliferation, and inflammatory regulation [27,54,55]. The fact that these molecules can be measured in serum makes them prominent among the non-invasive diagnostic tools of the future [75]. In addition, thrombosis and fibrin degradation products such as D-dimer and structural protein products such as fibronectin, TGF-β1, and elastin peptides indirectly indicate aneurysm-related biochemical changes [76].
6.2. Molecular Targeted Treatment Approaches
The classical approach to the treatment of aortic aneurysms is to repair the pathological region with surgery or endovascular interventions. However, these methods target the anatomical consequences of the disease and do not correct the underlying molecular mechanisms [77]. Therefore, in recent years, molecular treatment strategies targeting the biochemical and cellular basis of the disease have been developed. MMP inhibitors are among the first agents to have been developed in this context, and especially non-selective inhibitors such as doxycycline have been shown to slow aneurysm progression in animal models [67,78]. However, clinical results have been contradictory, and these treatments have not been widely accepted due to systemic side effects. Modulators targeting the TGF-β signaling pathway are promising, especially in hereditary connective tissue diseases [41,42]. Losartan, used in patients with Marfan syndrome, slowed the rate of aortic dilatation by inhibiting TGF-β and paved the way for further studies in this area [41]. In addition, conventional agents such as statins have been reported to provide potential benefits by suppressing inflammation and reducing MMP expression [79]. Biological agents targeting proinflammatory cytokines have also shown positive results, especially in preclinical studies [34]. Therapeutic interventions targeting microRNAs aim to regulate pathological expressions through antagomir and miRNA mimetics, thus restoring ECM homeostasis [52,54]. In addition, considering the role of oxidative stress in the disease process, agents such as NADPH oxidase inhibitors can protect vascular structure by reducing reactive oxygen species [68] (Figure 3).
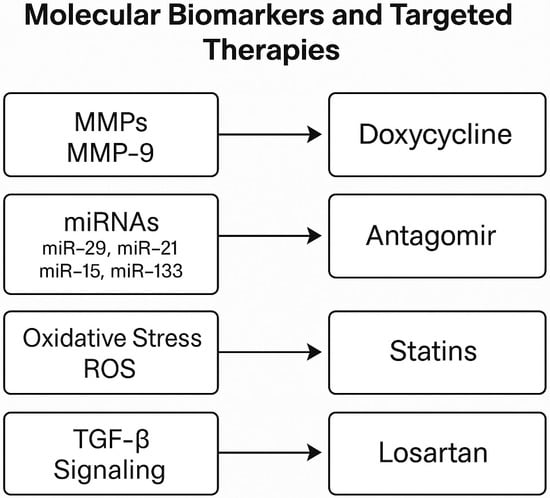
Figure 3.
Selected molecular biomarkers associated with aortic aneurysm pathogenesis and their corresponding targeted therapies. These include MMP-9 (doxycycline), dysregulated microRNAs (antagomirs), oxidative stress (statins), and TGF-β signaling (losartan).
6.3. Future Perspectives
While promising, gene editing technologies (e.g., CRISPR-Cas9) face challenges such as delivery vectors, off-target effects, and ethical concerns. Similarly, RNA-based therapeutics, including siRNAs and miRNA mimics/antagomirs, have shown efficacy in preclinical models but await validation in Phase I/II trials (e.g., NCT04517283). Regulatory pathways and standardization remain critical hurdles.
6.4. Clinical Translation and Ongoing Trials
Recent advances in circulating biomarkers such as miR-29b, MMP-9, and osteopontin have shown potential in stratifying aneurysm rupture risk. Their integration into imaging-based screening protocols is currently under investigation (e.g., ClinicalTrials.gov, NCT04603065). However, none have yet been validated for routine use due to specificity issues. D-dimer and CRP are widely available but not sufficiently specific. Future integration will require biomarker panels validated across aneurysm subtypes.
In the coming period, diagnostic and treatment strategies for aortic aneurysms are expected to be based on individualized approaches largely. Developing biomarker panels with high sensitivity and specificity may enable early detection of the disease [71,76]. Genetic risk scores may contribute to establishing proactive monitoring protocols, especially in cases with familial inheritance [51]. In addition, integrating molecular data with radiological findings using artificial intelligence-based algorithms may allow the development of patient-based risk prediction models [80]. In the long term, introducing innovative approaches such as gene editing technologies and RNA-based therapies into clinical practice may radically change the course of the disease. However, integrating all these developments into clinical practice will be possible through high-quality clinical studies supported by multidisciplinary collaborations.
7. Discussion
The current review highlights the multifactorial and dynamic nature of aortic aneurysm pathogenesis by integrating molecular, genetic, and translational findings. One of the central findings is the pivotal role of extracellular matrix (ECM) degradation mediated by matrix metalloproteinases (MMP-2 and MMP-9), which are consistently elevated in both clinical and experimental studies. However, clinical translation of MMP inhibitors has shown limited efficacy, raising concerns about therapeutic selectivity and side effects. Inflammatory responses, particularly those mediated by IL-6, TNF-α, and the NLRP3 inflammasome, have been implicated as amplifiers of vascular damage. While animal models provide strong evidence supporting these mechanisms, human data remain variable, and inflammation-targeted therapies are still under evaluation in early-phase trials. Similarly, oxidative stress—often driven by NADPH oxidase activation—has been shown to facilitate both SMC apoptosis and MMP upregulation. While promising results have been achieved using antioxidant strategies in preclinical models, their clinical applicability remains uncertain due to heterogeneity in patient populations and underlying risk factors. On the genetic side, rare monogenic mutations, such as those in FBN1, TGFBR1/2, and COL3A1, have been established as links to syndromic thoracic aortic aneurysms. In contrast, common polymorphisms and epigenetic alterations (e.g., miR-29 and DNA methylation) in sporadic aneurysms offer a more nuanced and complex picture. These molecular alterations show promise as biomarkers but require further validation in extensive, prospective human studies.
Despite these advancements, the review is limited by the availability and variability of clinical data. Most molecular insights are derived from animal models, which may not fully replicate the human disease spectrum. Moreover, heterogeneity in aneurysm location (thoracic vs. abdominal), etiology (genetic vs. degenerative), and patient demographics complicate biomarker generalization and therapeutic targeting. In future studies, an integrative multi-omics approach combined with artificial intelligence-based prediction models may offer a more personalized roadmap for both diagnosis and treatment. Longitudinal cohort studies and randomized controlled trials with molecular endpoints are crucial for translating current molecular understanding into clinically actionable strategies.
Thoracic aortic aneurysms (TAAs) and abdominal aortic aneurysms (AAAs) differ significantly in embryologic origin, hemodynamic stress exposure, and molecular drivers. TAAs are often associated with genetic mutations and structural defects, whereas AAAs are more influenced by inflammation, oxidative stress, and atherosclerosis. These differences underscore the need for site-specific biomarkers and therapeutic strategies.
8. Conclusions and Future Perspectives
Aortic aneurysms are clinically heterogeneous but pathophysiologically complex vascular diseases that are usually asymptomatic and have an insidious course. When symptoms occur, they usually lead to fatal complications such as rupture. The formation and progression of this disease are shaped by the interaction of extracellular matrix (ECM) destruction, apoptosis, and phenotypic change of vascular smooth muscle cells (SMCs), activation of chronic inflammatory processes, increased oxidative stress, and various genetic and epigenetic regulations [13,30,31,42,52,73,74]. These multilayered molecular mechanisms cause the aortic wall to weaken over time and lose its elasticity and increase the risk of rupture. Translational and experimental studies conducted in the last decade have revealed the details of these molecular processes and contributed to the identification of new diagnostic and therapeutic targets. In particular, molecules such as matrix metalloproteinases (MMPs), various microRNAs, the transforming growth factor-beta (TGF-β) signaling pathway, inflammatory cytokines, and reactive oxygen species (ROS) stand out as both diagnostic biomarkers and potential treatment targets for the disease [27,41,54,55,70,75]. However, integrating these molecules into clinical use has been limited, with most agents and markers remaining only in the preclinical stage [67,78]. In the future, biomarker panels to be developed in light of this molecular information may both enable early diagnosis of the disease and contribute to an objective assessment of the rate of progression [71,76]. These panels, which can be detected non-invasively and have high specificity, may form the basis of individualized monitoring protocols. In addition, with RNA-based treatment approaches, genetic risk scores, and artificial intelligence-supported decision-making algorithms, patient-based risk prediction can be made more sensitively and targeted [51,80]. In this way, the timing of interventions can be determined more accurately, and the complications of the disease can be reduced. In this review, the molecular dynamics of aortic aneurysms have been comprehensively analyzed in line with the current scientific literature. Many pathogenetic mechanisms, from ECM destruction to cellular stress responses, from genetic mutations to epigenetic regulators, have been discussed; experimental models, molecular biomarkers, and future treatment strategies have been comprehensively evaluated. Given all this information obtained, large-scale, prospective, randomized, and multicenter clinical studies are very important for their integration into clinical practice. In addition, comprehensive databases will be created with the collaboration of multidisciplinary teams, which will contribute to the validation of molecular biomarkers and the creation of new treatment protocols. In this direction, stronger integration of molecular biology with clinical cardiovascular medicine may usher in a new paradigm shift in managing aortic aneurysms.
9. Final Remarks
Aortic aneurysms represent a significant clinical problem due to their asymptomatic course and fatal complications such as sudden rupture. Advances in molecular biology and translational research have revealed complex mechanisms that play a role in the pathogenesis of the disease. These mechanisms include extracellular matrix degradation, inflammation, oxidative stress, and genetic predispositions. Although various promising biomarkers and therapeutic targets have been identified, the integration of these findings into clinical practice is still limited. In the future, the management of aortic aneurysms will progress toward developing biomarker panels with high sensitivity and specificity, creating personalized treatment algorithms, and applying targeted molecular therapies. Integrating clinical data with molecular information and technological innovations such as artificial intelligence will pave the way for personalized medicine in managing vascular diseases. Large-scale, multicenter clinical studies and interdisciplinary collaborations are critical to achieving these goals.
Author Contributions
İ.A. and R.A. defined the review scope, context, and purpose of this study. İ.A., R.A., H.İ.Ö., F.Ö., and M.Ö. provided clinical perspectives and expertise for this study, conducted the literature review, and drafted the manuscript. İ.A. and F.Ö. conceived and crafted the illustrative figures. All authors read and performed a critical review of the manuscript. R.A. and M.Ö. performed the cleaning and organization of the manuscript. İ.A. and R.A. provided conceptualization, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The review was based on publicly available academic literature databases.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Starnes, B.W. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Di Bartolomeo, R.; Eggebrecht, H.; Vrints, C.J. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Golledge, J.; Thanigaimani, S.; Powell, J.T.; Tsao, P.S. Pathogenesis and management of abdominal aortic aneurysm. Eur. Heart J. 2023, 44, 2682–2697. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Primers 2018, 4, 34. [Google Scholar] [CrossRef]
- Golledge, J.; Norman, P.E. Pathophysiology of abdominal aortic aneurysm relevant to improvements in patients’ management. Curr. Opin. Cardiol. 2009, 24, 532–538. [Google Scholar] [CrossRef]
- Thompson, R.W.; Geraghty, P.J.; Lee, J.K. Abdominal aortic aneurysms: Basic mechanisms and clinical implications. Curr. Probl. Surg. 2002, 39, 110–230. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef]
- Mills, A.C.; Sandhu, H.K.; Ikeno, Y.; Tanaka, A. Heritable thoracic aortic disease: A literature review on genetic aortopathies and current surgical management. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 293–304. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Woo, Y.J. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.H.; Chung, S.R.; Park, P.W.; Park, T.K.; Kang, I.S.; Sung, K. Impact of early diagnosis on surgical outcomes in patients with Loeys-Dietz syndrome. Front. Cardiovasc. Med. 2024, 11, 1429222. [Google Scholar] [CrossRef] [PubMed]
- Ganizada, B.H.; Veltrop, R.J.A.; Akbulut, A.C.; Koenen, R.R.; Accord, R.; Lorusso, R.; Schurgers, L.J. Unveiling cellular and molecular aspects of ascending thoracic aortic aneurysms and dissections. Basic. Res. Cardiol. 2024, 119, 371–395. [Google Scholar] [CrossRef]
- Golledge, J.; Tsao, P.S.; Dalman, R.L.; Norman, P.E. Circulating biomarkers in abdominal aortic aneurysm: Current status and prospects. Circulation 2008, 118, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.A.; Mulvihill, J.J.; Barrett, H.E.; Kavanagh, E.G.; Walsh, M.T.; McGloughlin, T.M.; Doyle, B.J. Determining the influence of calcification on the failure properties of abdominal aortic aneurysm tissue. J. Mech. Behav. Biomed. Mater. 2015, 42, 154–167. [Google Scholar] [CrossRef]
- Wolinsky, H.; Glagov, S. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 1967, 20, 99–111. [Google Scholar] [CrossRef]
- Davis, F.M.; Rateri, D.L.; Daugherty, A. Abdominal aortic aneurysm: Novel mechanisms and therapies. Curr. Opin. Cardiol. 2015, 30, 566–573. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Riley, J.T.; Dardik, A. Cells in focus: Endothelial cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Wassef, M.; Baxter, B.T.; Chisholm, R.L.; Fillinger, M.F.; Heinecke, J.; Zarins, C.K. Pathogenesis of abdominal aortic aneurysms: A multidisciplinary research program supported by the NHLBI. J. Vasc. Surg. 2001, 34, 730–738. [Google Scholar] [CrossRef]
- Touat, Z.; Ollivier, V.; Dai, J.; Huisse, M.G.; Bezeaud, A.; Sebbag, U.; Michel, J.B. Renewal of mural thrombus releases plasma markers and is involved in AAA evolution. Am. J. Pathol. 2006, 168, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Lee, M.R.; Park, J.G. Aortic aneurysms: Current pathogenesis and therapeutic targets. Exp. Mol. Med. 2023, 55, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in ApoE-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef]
- Michel, J.B.; Jondeau, G.; Milewicz, D.M. From genetics to response to injury: Vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. Cardiovasc. Res. 2018, 114, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Basalyga, D.M.; Simionescu, D.T.; Xiong, W.; Baxter, B.T.; Starcher, B.C.; Vyavahare, N.R. Elastin degradation and calcification in an abdominal aorta injury model: Role of matrix metalloproteinases. Circulation 2004, 110, 3480–3487. [Google Scholar] [CrossRef]
- Jones, J.A.; Stroud, R.E.; O’Quinn, E.C.; Black, L.E.; Barth, J.L.; Elefteriades, J.A.; Ikonomidis, J.S. Selective microRNA suppression in human thoracic aneurysms: Relationship of miR-29a to aortic size and proteolytic induction. Circulation 2011, 124, 1460–1469. [Google Scholar] [CrossRef]
- Thompson, R.W.; Parks, W.C. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann. New York Acad. Sci. 1996, 800, 157–174. [Google Scholar] [CrossRef]
- Lu, H.; Rateri, D.L.; Bruemmer, D.; Cassis, L.A.; Daugherty, A. Novel mechanisms of abdominal aortic aneurysms. Curr. Atheroscler. Rep. 2012, 14, 402–412. [Google Scholar] [CrossRef]
- Curci, J.A.; Liao, S.; Huffman, M.D.; Shapiro, S.D.; Thompson, R.W. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Investig. 1998, 102, 1900–1910. [Google Scholar] [CrossRef]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhao, S.; Luo, S.; Chen, C.; Liu, X.; Wu, X.; Ji, Y. SLC44A2 regulates vascular smooth muscle cell phenotypic switching and aortic aneurysm. J. Clin. Investig. 2024, 134, e173690. [Google Scholar] [CrossRef]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Anidjar, S.; Salzmann, J.L.; Gentric, D.; Lagneau, P.; Camilleri, J.P.; Michel, J.B. Elastase-induced experimental aneurysms in rats. Circulation 1990, 82, 973–981. [Google Scholar] [CrossRef]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Takahashi, M. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 127–136. [Google Scholar] [CrossRef]
- Huang, J.; Liu, H.; Liu, Z.; Wang, Z.; Xu, H.; Li, Z.; Fu, Y. Inhibition of aortic CX3CR1+ macrophages mitigates thoracic aortic aneurysm progression in Marfan syndrome in mice. J. Clin. Investig. 2025, 135, e178198. [Google Scholar] [CrossRef]
- Sylvester, A.L.; Zhang, D.X.; Ran, S.; Zinkevich, N.S. Inhibiting NADPH Oxidases to Target Vascular and Other Pathologies: An Update on Recent Experimental and Clinical Studies. Biomolecules 2022, 12, 823. [Google Scholar] [CrossRef]
- Jie, H.; Zhang, J.; Wu, S.; Yu, L.; Li, S.; Dong, B.; Yan, F. Interplay between energy metabolism and NADPH oxidase-mediated pathophysiology in cardiovascular diseases. Front. Pharmacol. 2025, 15, 1503824. [Google Scholar] [CrossRef]
- Ailawadi, G.; Eliason, J.L.; Upchurch, G.R., Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J. Vasc. Surg. 2003, 38, 584–588. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Dietz, H.C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Dietz, H.C. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Dietz, H.C. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011, 473, 308–316. [Google Scholar] [CrossRef]
- Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: A paradox in pathogenesis. J. Vasc. Res. 2009, 46, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Regalado, E.S. Use of genetics for personalized management of heritable thoracic aortic disease: How do we get there? J. Thorac. Cardiovasc. Surg. 2015, 149 (Suppl. S2), S3–S5. [Google Scholar] [CrossRef] [PubMed]
- MacCarrick, G.; Black, J.H.; Bowdin, S.; El-Hamamsy, I.; Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Dietz, H.C. Loeys-Dietz syndrome: A primer for diagnosis and management. Genet. Med. 2014, 16, 576–587. [Google Scholar] [CrossRef]
- Takeda, N.; Komuro, I. Genetic basis of hereditary thoracic aortic aneurysms and dissections. J. Cardiol. 2018, 71, 345–351. [Google Scholar] [CrossRef]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Wheeldon, N. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef]
- Duellman, T.; Warren, C.L.; Peissig, P.; Wynn, M.; Yang, J. Matrix metalloproteinase-9 genotype as a potential genetic marker for abdominal aortic aneurysm. Circ. Cardiovasc. Genet. 2012, 5, 529–537. [Google Scholar] [CrossRef]
- Klarin, D.; Verma, S.S.; Judy, R.; Dikilitas, O.; Wolford, B.N.; Paranjpe, I.; Tsao, P.S. Genetic Architecture of Abdominal Aortic Aneurysm in the Million Veteran Program. Circulation 2020, 142, 1633–1646. [Google Scholar] [CrossRef]
- Bown, M.J.; Jones, G.T.; Harrison, S.C.; Wright, B.J.; Bumpstead, S.; Baas, A.F.; Samani, N.J. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am. J. Hum. Genet. 2011, 89, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Spin, J.M.; Raaz, U.; Eken, S.M.; Toh, R.; Azuma, J.; Tsao, P.S. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 2014, 5, 5214. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shan, D.; He, L.; Yang, S.; Feng, Y.; Zhang, Y.; Yu, J. Anemoside B4 attenuates abdominal aortic aneurysm by limiting smooth muscle cell transdifferentiation and its mediated inflammation. Front. Immunol. 2024, 15, 1412022. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Tsao, P.S. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Investig. 2012, 122, 497–506. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2009, 104, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef]
- Daugherty, A.; Cassis, L.A. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 429–434. [Google Scholar] [CrossRef]
- Yin, L.; Kent, E.W.; Wang, B. Progress in murine models of ruptured abdominal aortic aneurysm. Front. Cardiovasc. Med. 2022, 9, 950018. [Google Scholar] [CrossRef]
- Tian, K.; Malik, F.; Zhao, S. Animal models for abdominal aortic aneurysms: Where we are and where we need to go. Animal Model. Exp. Med. 2025, 8, 573–577. [Google Scholar] [CrossRef]
- Atkinson, G.; Bianco, R.; Di Gregoli, K.; Johnson, J.L. The contribution of matrix metalloproteinases and their inhibitors to the development, progression, and rupture of abdominal aortic aneurysms. Front. Cardiovasc. Med. 2023, 10, 1248561. [Google Scholar] [CrossRef]
- Freestone, T.; Turner, R.J.; Coady, A.; Higman, D.J.; Greenhalgh, R.M.; Powell, J.T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1145–1151. [Google Scholar] [CrossRef]
- Chiou, A.C.; Chiu, B.; Pearce, W.H. Murine aortic aneurysm produced by periarterial application of calcium chloride. J. Surg. Res. 2001, 99, 371–376. [Google Scholar] [CrossRef]
- Rateri, D.L.; Davis, F.M.; Balakrishnan, A.; Howatt, D.A.; Moorleghen, J.J.; O’Connor, W.N.; Daugherty, A. Angiotensin II induces region-specific medial disruption during development of ascending aortic aneurysms. Am. J. Pathol. 2014, 184, 2586–2595. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Schepers, D.; Bolar, N.A.; Doyle, J.J.; Gallo, E.; Fert-Bober, J.; Loeys, B.L. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet. 2012, 44, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Norman, P.E. Atherosclerosis and abdominal aortic aneurysm: Cause, response, or common risk factors? Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Curci, J.A. Digging in the “soil” of the aorta to understand the growth of abdominal aortic aneurysms. J. Vasc. Surg. 2009, 49, 260–263. [Google Scholar] [CrossRef]
- Gavrila, D.; Li, W.G.; McCormick, M.L.; Thomas, M.; Daugherty, A.; Cassis, L.A.; Weintraub, N.L. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E–deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1671–1677. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2021, 11, 609161. [Google Scholar] [CrossRef]
- MA3RS Study Investigators. Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture, and Need for Surgical Repair. Circulation 2017, 136, 787–797. [Google Scholar] [CrossRef]
- Takagi, H.; Manabe, H.; Kawai, N.; Goto, S.; Umemoto, T. Plasma fibrinogen and D-dimer concentrations are associated with the presence of abdominal aortic aneurysm: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef]
- Golledge, J.; Muller, J.; Daugherty, A.; Norman, P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2605–2613. [Google Scholar] [CrossRef]
- Lei, C.; Kan, H.; Xian, X.; Chen, W.; Xiang, W.; Song, X.; Zheng, Y. FAM3A reshapes VSMC fate specification in abdominal aortic aneurysm by regulating KLF4 ubiquitination. Nat. Commun. 2023, 14, 5360. [Google Scholar] [CrossRef]
- Khan, H.; Abu-Raisi, M.; Feasson, M.; Shaikh, F.; Saposnik, G.; Mamdani, M.; Qadura, M. Current Prognostic Biomarkers for Abdominal Aortic Aneurysm: A Comprehensive Scoping Review of the Literature. Biomolecules 2024, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Salata, K.; Syed, M.; Hussain, M.A.; de Mestral, C.; Greco, E.; Mamdani, M.; Al-Omran, M. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008657. [Google Scholar] [CrossRef]
- Baxter, B.T.; Matsumura, J.; Curci, J.A.; McBride, R.; Larson, L.; Blackwelder, W. Effect of Doxycycline on Aneurysm Growth Among Patients with Small Infrarenal Abdominal Aortic Aneurysms: A Randomized Clinical Trial. JAMA 2020, 323, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Pramana, K.A.A.P.; Pintaningrum, Y.; Rahmat, B. The effects of statin therapy on aneurysm size, growth rate, and matrix metalloproteinases-9 levels in patients with aortic aneurysm: A systematic review and meta-analysis. Egypt. Heart J. 2023, 75, 88. [Google Scholar] [CrossRef]
- Mangum, K.D.; Farber, M.A. Genetic and epigenetic regulation of abdominal aortic aneurysms. Clin. Genet. 2020, 97, 815–826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).