Abstract
Objectives: This study investigates the effects of cluster set resistance training (RT) on heart rate variability (HRV) parameters in young, healthy individuals. Methods: This trial was registered in the Brazilian Clinical Trials Registry (ReBEC) under the identification number RBR-9857xj3 on 7 December 2024. Sixteen participants (seven female, 25 ± 2 years old) performed both cluster set and traditional RT protocols with equal relative intensity (85% 10RM), volume load (4 × 10 repetitions), and rest intervals (120 s). Cluster set configuration involved the introduction of a shorter rest interval between a cluster of sets [4 × (2 × 5) with 90 s inter-set rest and 30 s intra-set rest]. HRV parameters (RMSSD, HFnu, SD1, LFnu, LF/HF ratio, and SD2) were assessed before and 30 min post-exercise. The rating of perceived exertion (RPE) was assessed immediately after RT protocols. Results: The traditional RT protocol led to a significant reduction in parasympathetic activity (RMSSD, HFnu, SD1) and an increase in sympathetic activity (LFnu, LF/HF ratio, and SD2) (p < 0.05), whereas the cluster set RT protocol did not alter HRV parameters. Additionally, RPE was significantly higher (p < 0.001) in the traditional RT protocol. Conclusion: This study suggests that the cluster set may have a less pronounced impact on HRV parameters 30 min post-exercise compared to traditional sets. These findings can guide exercise physiologists in designing resistance training programs for clinical populations by prescribing protocols that minimize cardiac autonomic stress.
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide [1]. The autonomic nervous system, through its sympathetic and parasympathetic branches, plays a major role in modulating cardiac function [2]. Oscillations in the time interval between heartbeats (R-R intervals derived from an electrocardiogram) result from complex interactions between sympathetic and parasympathetic influences on the heart [3]. Therefore, heart rate variability (HRV) serves as a non-invasive tool to assess the autonomic regulation of the heart. HRV is considered a reliable marker of autonomic nervous system activity because it reflects the dynamic balance between sympathetic and parasympathetic modulation of the sinoatrial node, providing insights into the heart’s adaptability to various physiological demands [3,4,5]. Reduced HRV (indicative of higher sympathetic activation) is associated with inadequate cardiovascular adaptation and an increased risk of arrhythmias and sudden cardiac death [6,7]. Mechanistically, diminished HRV reflects impaired vagal tone and heightened sympathetic dominance, which may lead to electrical instability in cardiac tissue. This imbalance facilitates the emergence of ventricular arrhythmias through mechanisms such as increased myocardial excitability, shortened refractory periods, and triggered activity, especially under conditions of physiological stress or ischemia [8]. Conversely, higher HRV reflects a healthy state of autonomic control and an adaptive organism [7]. In this context, assessing HRV parameters after exercise is a valuable tool for investigating cardiac stress induced by a physical exercise session, particularly in individuals at risk for CVD [9].
Resistance training (RT), a form of exercise that involves lifting external loads to generate muscle force and hypertrophy, has been shown to improve various health outcomes, including muscle strength, power, endurance, bone mineral density, and cardiovascular adaptation [10,11]. These benefits depend on appropriate intensity (a percentage of maximal capacity) and volume (the number of repetitions performed) [12]. However, high-intensity RT may reduce parasympathetic activity and increase sympathetic activation for up to 30 min post-exercise, potentially elevating the risk of sudden cardiac death [6,13,14]. In this context, recent studies have sought to attenuate parasympathetic withdrawal by modifying RT variables such as set configuration and rest intervals. For example, [14] compared a traditional protocol (3 × 10 with 60 s rest) to a short-repetition protocol (8 × 3 with 120 s rest), aiming to reduce cardiovascular stress and perceived exertion. Their findings show that the short-repetition protocol elicited lower RPE and preserved HRV indices (RMSSD and HFnu) to a greater extent, suggesting that strategies involving longer rest periods may help to maintain parasympathetic activity post-exercise. It is likely that lower hormonal release (cortisol, catecholamines, etc.) and metabolic stress after certain RT set configurations can contribute to mitigating parasympathetic withdrawal after exercise [4,13,15].
A recent alternative gaining attention is cluster set RT, which incorporates short rest intervals between clusters of repetitions within a set, allowing for the maintenance of high volume and intensity [11,16]. This approach is commonly used in athletic settings to enhance performance and/or minimize fatigue accumulation during sessions [16]. It has been shown that performing a cluster set RT configuration induces lower metabolic stress (cortisol and lactate) compared to the traditional RT protocol [15]. Thus, a key hypothesis behind cluster set RT is that distributing rest periods may attenuate the autonomic stress induced by exercise, promoting a better balance between sympathetic and parasympathetic activity and potentially resulting in faster HRV recovery after exercise [15,17]. However, despite the growing interest in this protocol, there is a significant gap in the literature regarding the comparative impact of cluster set versus traditional RT on HRV modulation.
Therefore, this study aims to evaluate the effects of two RT protocols, traditional and cluster set, on HRV parameters in healthy individuals. We hypothesize that cluster set RT will elicit a more favorable autonomic response, with a smaller disturbance in parasympathetic activity compared to traditional RT, thereby promoting a more balanced sympathetic–parasympathetic interaction and reducing the risk of adverse cardiovascular effects.
2. Materials and Methods
2.1. Ethical Aspects
All experimental procedures were conducted after thoroughly explaining the study’s purpose and obtaining written consent from the participants. This study complies with the ethical principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of the Federal University of Rio de Janeiro, Brazil (protocol number: 55184922.5.0000.5699). It was also registered with the Brazilian Clinical Trials Registry (ReBEC) under ID RBR-9857xj3 on 7 December 2024.
2.2. Participants
A priori power analysis for an F test (repeated measures, within–between interaction across two time points) was conducted using G*Power software version 3.0.1 to estimate the required sample size. Based on a statistical power (1 − β) of 0.80, a moderate effect size (f = 0.25), and an overall level of significance of 0.05, at least 17 participants were needed to perform the study. Twenty participants were recruited using flyers, posters, emails, and social media within a university campus community in Brazil. They were invited to the laboratory for eligibility assessment and a detailed explanation of the experimental procedures. Before randomization, four participants dropped out due to personal reasons, resulting in sixteen participants who completed all experimental procedures. Table 1 presents the participants’ baseline characteristics.

Table 1.
Demographic characteristics of the participants.
This study includes young adults aged from 18 to 35 years, in good health, without morbidities such as diabetes, hypertension, obesity, or dyslipidemia. All participants had engaged in an RT program for at least 12 months. Exclusion criteria included smoking, use of psychoactive substances (e.g., caffeine or pre-workout supplements), and anabolic steroid use within six months before the experimental procedures. Additionally, individuals with cardiovascular diseases or osteoarticular injuries that could impair lower-limb RT were excluded.
2.3. Experimental Design
This study was conducted using a randomized, controlled, crossover design from December 2024 to April 2025. After an initial visit for exercise familiarization, participants attended three additional laboratory sessions to complete all experimental procedures. During the first session, anthropometric measurements were obtained, and participants’ 10-repetition maximum (10RM) was determined for the leg press (Movement®, São Paulo, Brazil) and leg extension chair (Movement®, São Paulo, Brazil). The 10RM was established within a maximum of four attempts, with a 5 min rest interval between each attempt to minimize fatigue and ensure accurate load assessment. During the second and third visits, participants performed two different RT protocols (cluster set or traditional), which were expected to induce distinct physiological and psychological stress responses [15]. Blood pressure was assessed before, during, immediately after, and 30 min post-exercise. HRV was continuously monitored before and for 30 min after the resistance training protocols to calculate HRV parameters [18]. Rating of perceived exertion (RPE) was assessed immediately after the RT protocol (Figure 1).
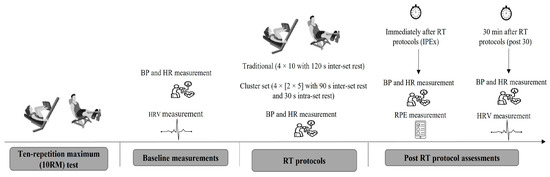
Figure 1.
Schematic representation of the experimental design. Participants performed a ten-repetition maximum (10RM) test, followed by a pre-experiment assessment, which included blood pressure (BP), heart rate (HR), and heart rate variability (HRV) measurements. The resistance training (RT) protocol followed a randomized crossover design, incorporating both traditional and cluster set configurations. BP and HR were assessed during and immediately after exercise (IPEx). Ratings of perceived exertion (RPE) were assessed IPEx. Post-exercise assessments included BP, HR, and HRV measurements.
RT protocols were randomized using a balanced 1:1 model. Randomization was performed by a laboratory staff member, blinded to the participants’ codes, using a random number generator. At least 72 h passed between the second and third visits to ensure adequate muscle recovery, except for female participants, whose assessments were scheduled within the same menstrual cycle phase to maintain consistency. Specifically, female participants were evaluated during the follicular phase to account for potential hormonal influences on exercise performance. All experimental procedures took place in the morning between 9:00 and 11:00 a.m. Participants were instructed to maintain the same diet throughout all visits.
2.4. RT Protocol
During the first laboratory visit, all participants underwent a 10RM test for the leg press and leg extension exercises. Forty-eight hours later, they returned to the laboratory to perform the resistance training protocol (cluster or traditional set) in a randomized order. Each participant served as their control, completing both experimental conditions (crossover design). Participants performed four sets of ten repetitions of the leg press and leg extension chair exercises at 85% of 10RM, using either the traditional (4 × 10 with 120 s inter-set rest) or cluster set (4 × [2 × 5] with 90 s inter-set rest and 30 s intra-set rest) configuration. The total rest time was equalized between conditions [15]. Before the training protocols, participants warmed up on the leg press (the first exercise) using 50% of the workload. Volume load was kept similar between RT protocols, with only the rest interval manipulated between the cluster and traditional protocols. Both RT protocols were performed on the leg press and leg extension machines. The repetition cadence was standardized at 2 s for the eccentric phase and 1 s for the concentric phase. The total duration of each session, including rest intervals and exercise execution, was approximately 20–25 min. No other physical activities were performed during the experimental sessions, and participants remained seated on the equipment and rested during the intervals between sets.
2.5. Heart Rate Variability Measurement
For the evaluation of HRV parameters, participants were placed in a temperature-controlled quiet environment, lying in a supine position on an examination bed while wearing a heart rate monitor strap affixed to the distal third of the sternum. HRV data were recorded over a 15 min resting period using the Polar RS800CX® heart rate monitor, Kempele, na Finlândia a device validated for HRV assessment. The collected data were then transferred to a computer via the Elite HRV® application for further processing. A 5 min segment of HRV recording was selected for analysis using the Kubios® HRV software (version 2.0, University of Kuopio, Kuopio, Finland). This software processed the R-R intervals of the heart rate, extracting key HRV parameters within time-domain, frequency-domain, and non-linear metrics. The percentage of corrected RR intervals was below 2% for all participants, ensuring data quality. The data were detrended with the smooth prior method. The Lambda value was fixed at 500. For time-domain analysis, the root mean square of successive differences between adjacent normal R-R intervals (RMSSD) was considered. In the frequency-domain analysis, low-frequency (LF) indices and high-frequency (HF) indices, expressed in normalized units (nu), along with the low-frequency/high-frequency (LF/HF) ratio, were examined. In addition, the standard deviation of the Poincaré plot width (SD1) and standard deviation of the length of the Poincaré plot (SD2) were adopted as non-linear acquisitions. RMSSD is widely recognized as a sensitive indicator of parasympathetic nervous system activity and has been employed in previous studies [7]. The LF/HF ratio serves as an indicator of sympathovagal balance, where an increase in LFnu and LF/HF ratio suggests a shift towards sympathetic predominance [7]. The selection of HRV parameters in this study (RMSSD, LFnu, HFnu, LF/HF ratio, SD1, and SD2) was based on those most frequently investigated in prior research [4,7,9].
2.6. Blood Pressure Measurement
Systolic and diastolic blood pressure, along with heart rate, were measured at baseline, during, and 30 min after the resistance training (RT) protocols using a validated device (Bp791it, Omron Co., Tokyo, Japan) and an appropriately sized cuff for the upper left arm. All measurements were performed with participants seated [14]. The RT session began with the leg press followed by the leg extension exercise. For both protocols, blood pressure and heart rate were recorded prior to exercise (baseline), during the second (DEx1) and fourth (DEx2) sets of the leg press, during the second (DEx3) and fourth (IPEx) sets of the leg extension, and again 30 min post-exercise.
2.7. Rating of Perceived Exertion
Perceived exertion during resistance training was assessed using a scale where RPE is linked to the number of repetitions in reserve (RIR), as described by [19]. Participants were familiarized with the scale prior to each session, and data were collected exclusively immediately following the RT protocol.
2.8. Statistical Analysis
The normality and homogeneity of variances were assessed using the Shapiro–Wilk and Levene tests, respectively. A two-way repeated measures ANOVA (2 × 2) was used to detect significant differences in HRV parameters, systolic and diastolic blood pressure, and heart rate before and after the resistance training protocols (cluster and traditional sets). The sphericity test was not considered in the ANOVA analysis since the study design involved repeated measures variables with only two levels. When a significant F-value was found, additional post hoc tests with Bonferroni adjustment were performed. To detect significant differences in the RPE after the RT protocols, a dependent-sample t-test was conducted. In addition, an analysis of covariance (ANCOVA) was performed to examine the effect of the RT protocol (cluster vs. traditional) on the outcome measures, with sex included as a covariate. This approach allowed for the adjustment of potential confounding effects of sex on the physiological responses to resistance training. If the ANCOVA revealed a significant sex effect (p < 0.05), it was further explored using the ANOVA. Cohen’s d was used to calculate the effect size of the RT protocol, with thresholds defined as follows: <0.2 being trivial, from 0.2 to <0.5 being small, from 0.5 to <0.8 being moderate, and ≥0.8 being large [20]. All analyses were performed using a commercially available statistical package (IBM SPSS Statistics version 20 for Mac, Chicago, IL, USA), and results are expressed as means ± standard deviation (SD).
3. Results
3.1. HRV Parameters
Table 2 and Figure 2A–F present the HRV parameters of the participants. A significant main effect for time was observed in RMSSD (F(1,30) = 18.86, p < 0.001, partial η2 = 0.401). The post hoc test revealed a significant reduction (p < 0.001, Cohen’s d = 1.14) in RMSSD following the traditional RT protocol compared to baseline values. No significant difference was observed after the cluster RT protocol compared to baseline (p = 0.111, Cohen’s d = 0.25). Although a significant interaction effect (type of exercise protocol during pre- and post-exercise) was found for RMSSD (F(1,30) = 6.65, p = 0.039, partial η2 = 0.135), the post hoc test did not detect a significant change in RMSSD between the traditional and cluster protocols at baseline (p = 0.947) or post-RT (p = 0.157). However, a moderate effect size (Cohen’s d = 0.51) was observed when comparing post-traditional and post-cluster protocols.

Table 2.
Data from heart rate variability (HRV) parameters before (pre) and post-resistance training.
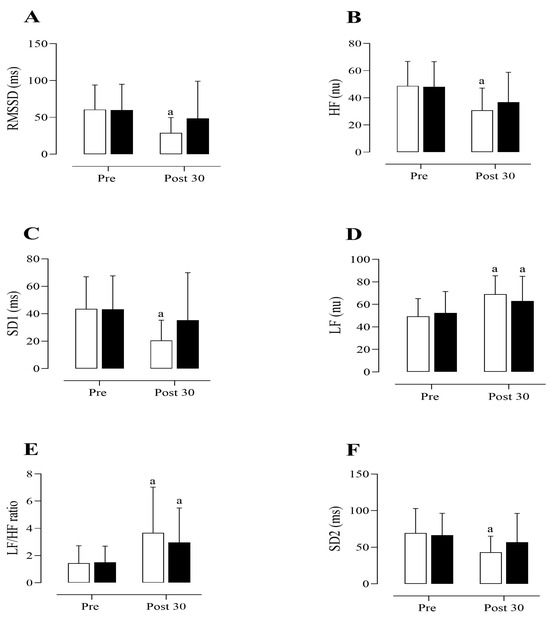
Figure 2.
Heart rate variability (HRV) parameters before (Pre) and 30 min after (Post 30) the resistance training (RT) protocols. (A) Root mean square of successive RR interval differences (RMSSD). (B) High-frequency component (HF). (C) Standard deviation of short-term RR interval variability (SD1) derived from the Poincaré plot. (D) Low-frequency component (LF). (E) Low-frequency to high-frequency ratio (LF/HF). (F) Standard deviation of long-term RR interval variability (SD2) derived from the Poincaré plot. Values are presented as mean ± standard deviation. The symbol “a” indicates a significant difference compared to the pre-exercise values (p < 0.05). LF and HF were expressed in normalized units (nu). White and black bars represent traditional and cluster set RT protocols, respectively.
Regarding HFnu, a significant main effect for time was found (F(1,30) = 25.81, p < 0.001, partial η2 = 0.463). The post hoc analysis revealed a significant reduction in HFnu following the traditional RT protocol compared to baseline (p < 0.001, Cohen’s d = 1.05). Although no significant difference was observed in HFnu at baseline and post-cluster set RT protocol (p = 0.09), a moderate effect size (Cohen’s d = 0.55) was noted. No significant interaction effect was found for HFnu (F(1,30) = 1.33, p = 0.257, partial η2 = 0.043).
A significant main effect for time was observed in the SD1 index (F(1,30) = 17.47, p < 0.001, partial η2 = 0.368). The post hoc test revealed a significant reduction (p < 0.001, Cohen’s d = 1.32) in the SD1 index following the traditional RT protocol compared to baseline. However, no significant effect was found when comparing the post-cluster set RT protocol with baseline values (p = 0.268, Cohen’s d = 0.26). A significant interaction effect was observed (F(1,30) = 6.67, p = 0.015, partial η2 = 0.182); however, the post hoc test did not show a significant difference in the SD1 index between the traditional and cluster set RT protocols at baseline (p = 0.834) or post-exercise (p = 0.145). Nevertheless, a moderate effect size (Cohen’s d = 0.55) was noted when comparing post-traditional and post-cluster set protocols.
A significant main effect for time was observed in LFnu (F(1,30) = 27.06, p < 0.001, partial η2 = 0.474). The post hoc analysis revealed a significant increase in LFnu following both the traditional (p < 0.001, Cohen’s d = 1.23) and cluster set RT (p = 0.016, Cohen’s d = 0.52) protocols compared to baseline. No significant interaction effect was found for LFnu (F(1,30) = 2.51, p = 0.124, partial η2 = 0.077). A significant main effect for time was observed in the LF/HF ratio (F(1,30) = 17.01, p < 0.001, partial η2 = 0.362). The post hoc analysis revealed a significant increase in the LF/HF ratio following both the traditional (p < 0.01, Cohen’s d = 0.88) and cluster set (p = 0.028, Cohen’s d = 0.77) RT protocols compared to baseline. No significant interaction effect was observed for the LF/HF ratio (F(1,30) = 0.74, p = 0.394, partial η2 = 0.024).
A significant main effect of time was observed for the SD2 index (F(1,30) = 16.77, p < 0.001, partial η2 = 0.359). The post hoc analysis indicated a significant decrease in SD2 after the traditional RT protocol compared to baseline (p < 0.001, Cohen’s d = 0.93). There was no significant difference between post-cluster set RT protocol values and baseline (p = 0.130, Cohen’s d = 0.27). Additionally, no significant interaction effect was observed for SD2 (F(1,30) = 3.59, p = 0.068, partial η2 = 0.107).
3.2. Blood Pressure and Heart Rate Data
A significant main effect of time was found for systolic blood pressure (F(5,150) = 49.30, p < 0.001, partial η2 = 0.622). Post hoc analysis showed a significant rise in systolic blood pressure at DEx1, DEx2, DEx3, and IPEx (all p < 0.001) following both traditional and cluster set RT protocols. No significant interaction effect was found for systolic blood pressure (F(5,150) = 0.21, p = 0.959, partial η2 = 0.007) (Figure 3A). No significant main effect for time was observed (F(5,150) = 3.54, p = 0.07, partial η2 = 0.106), nor was there a significant interaction effect (F(5,150) = 0.43, p = 0.70, partial η2 = 0.14) in diastolic blood pressure (Figure 3B).
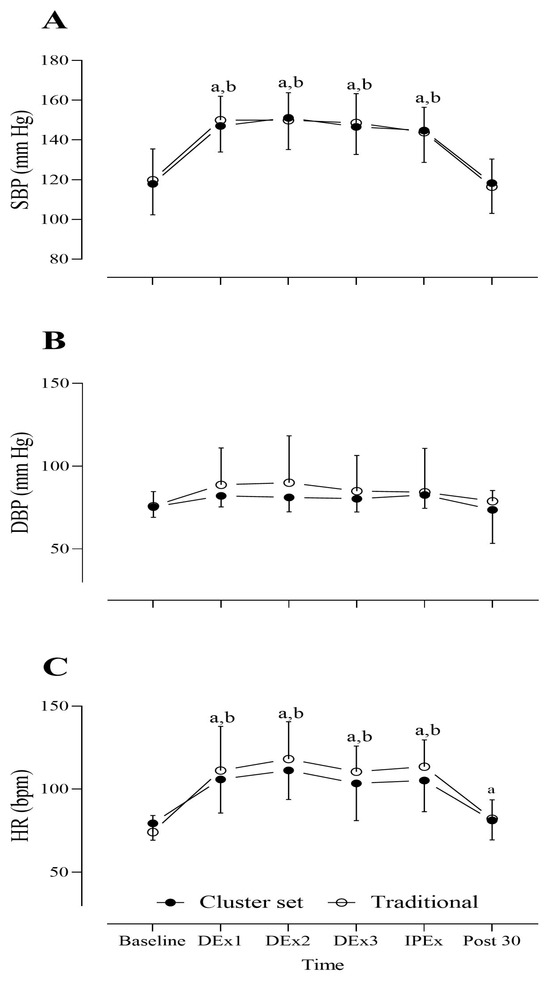
Figure 3.
Data on (A) systolic blood pressure (SBP), (B) diastolic blood pressure (DBP), and (C) heart rate (HR) measured at baseline (before exercise), during exercise (DEx), immediately post-exercise (IPEx), and 30 min post-exercise (post 30) following resistance training (RT) protocols (traditional and cluster set). Values are expressed as mean ± standard deviation. Statistical significance was set at p < 0.05. The symbols “a” and “b” indicate significant differences from baseline values for the traditional and cluster set RT protocols, respectively. Bpm = beats per minute.
A significant main effect for time was found in heart rate (F(5,150) = 77.9, p < 0.001, partial η2 = 0.772). The post hoc test revealed a significant increase in heart rate in DEx1 (p < 0.001), DEx2 (p < 0.001), DEx3 (p < 0.001), IPEx (p < 0.001), and 30 min post-traditional RT protocol. A significant increase in heart rate was also observed in DEx1 (p < 0.001), DEx2 (p < 0.001), DEx3 (p < 0.001), and IPEx (p < 0.001) following the cluster set RT protocol; however, no significant difference in heart rate was found 30 min post-cluster set RT protocol (p = 0.99). No significant interaction effect was observed (F(5,150) = 1.95, p = 0.08, partial η2 = 0.06) (Figure 3C).
3.3. RPE Data
A significantly lower RPE (t(15) = 5.3, p < 0.001) was observed following the cluster set RT protocol (7.4 ± 1.2) compared to the traditional set (9.1 ± 0.6).
3.4. Gender Effects
No significant main effects of time between sexes or interaction effects among sex, time, and group were observed for RMSSD (F(1,28) = 2.07, p = 0.161, partial η2 = 0.069; F(1,28) = 0.115, p = 0.291, partial η2 = 0.040), HFnu (main effect: F(1,28) = 0.08, p = 0.774, partial η2 = 0.003; interaction effect: F(1,28) = 1.15, p = 0.291, partial η2 = 0.040), LFnu (main effect: F(1,28) = 0.47, p = 0.49, partial η2 = 0.003; interaction effect: F(1,28) = 0.16, p = 0.49, partial η2 = 0.028), or LF/HF ratio (main effect: F(1,28) = 0.46, p = 0.49, partial η2 = 0.017; interaction effect: F(1,28) = 0.16, p = 0.68, partial η2 = 0.006).
Similarly, no significant main or interaction effects were found for SD1 (main effect: F(1,28) = 2.58, p = 0.11, partial η2 = 0.08; interaction effect: F(1,28) = 1.09, p = 0.31, partial η2 = 0.037) or SD2 (main effect: F(1,28) = 4.83, p = 0.056, partial η2 = 0.14; interaction effect: F(1,28) = 0.32, p = 0.57, partial η2 = 0.012).
Regarding cardiovascular parameters, no significant main or interaction effects were observed for systolic blood pressure (main effect: F(5,140) = 0.92, p = 0.467, partial η2 = 0.032; interaction effect: F(5,140) = 0.291, p = 0.917, partial η2 = 0.01), diastolic blood pressure (main effect: F(5,140) = 0.83, p = 0.529, partial η2 = 0.029; interaction effect: F(5,140) = 2.56, p = 0.06, partial η2 = 0.08), or heart rate (main effect: F(5,140) = 0.966, p = 0.441, partial η2 = 0.03; interaction effect: F(5,140) = 0.104, p = 0.991, partial η2 = 0.004).
4. Discussion
The present study investigates the effects of cluster set RT configurations on HRV parameters in young, healthy individuals. Our findings support the hypothesis that a single session of the cluster set RT protocol did not alter HRV parameters (parasympathetic and sympathetic activity) assessed 30 min after exercise, whereas the traditional RT protocol reduced parasympathetic activity (RMSSD, HFnu, and SD1) and increased sympathetic activity (LFnu, LF/HF ratio, and SD2). Moreover, although volume load and relative intensity (85% of 10RM) were equal, a higher subjective exertion (RPE) was observed after the traditional RT protocol compared to the cluster set. These results suggest that traditional RT substantially impacts cardiac autonomic modulation more than the cluster set RT protocol, providing relevant insights for exercise prescription, including for cardiopathic patients. Although exploring the mechanisms by which cluster sets mitigate reduced parasympathetic withdrawal after exercise goes beyond the aim of this study, it is possible that lower metabolic stress (e.g., reduced lactate and cortisol concentrations) associated with the cluster set protocol may explain differences in autonomic cardiac modulation [15]. However, future studies are needed to further investigate the underlying mechanisms behind the effects of cluster set configurations on physiological outcomes.
Aligned with our findings, a previous study evaluated the effects of two RT protocols (3 × 10 with a 60 s rest vs. 8 × 3 with a 120 s rest) designed to elicit different levels of RPE on HRV parameters (RMSSD, HFnu, and SD1) in trained individuals [14]. The authors reported a significant reduction in RMSSD and HFnu following the 3 × 10–60 s protocol compared to the 8 × 3–120 s RT protocol. Additionally, they observed significantly higher RPE in the 3 × 10–60 s condition, suggesting that higher-intensity exercise can decrease parasympathetic activity 30 min post-exercise [14]. Another study examined the effects of a high-intensity CrossFit workout (Cindy) and treadmill running, both performed for 20 min, on cardiac autonomic modulation [18]. The researchers observed a significant reduction in RMSSD and HF parameters over 30 min following both exercise modalities. However, the CrossFit workout induced a greater reduction in RMSSD and HF parameters compared to treadmill running, which may be associated with higher RPE, also observed in the study [18].
Additionally, Figueiredo et al. (2015) [21] evaluated the influence of different load intensities (60%, 70%, and 80% of 1RM) on HRV throughout 60 min post-RT in trained individuals. Although the authors did not observe a significant difference between intensities in HRV parameters, the effect size data indicated a large decrease in parasympathetic activity (RMSSD) after the 80% of 1RM session compared to the 60% and 70% of 1RM sessions [20]. Moreover, Nunes et al. (2021) [13] compared HRV parameters after a low-intensity RT with short (3 × 10 repetitions, 50% of 1RM) and long (3 × 20 repetitions, 50% of 1RM) set configurations performed for five upper limb exercises (bench press, bent-over barbell row, barbell front raise, barbell biceps curl, and lying barbell triceps extension). A significant reduction in RMSSD was observed after the long-set configuration compared to baseline, whereas no change was observed after the short-set configuration [13].
Taken together, this body of evidence suggests that RT intensity and volume load play a crucial role in autonomic response [13,20]. A recent systematic review and meta-analysis investigated the effects of a single session of RT on HRV parameters [4]. The authors concluded that RT leads to a withdrawal of cardiac parasympathetic modulation and an increase in cardiac sympathetic modulation, with these changes being more pronounced at higher training volumes. Furthermore, the number of sets, intensity, and rest intervals between sets were identified as key factors influencing HRV parameters [4].
Interestingly, both the cluster set and traditional resistance training (RT) protocols used in this study were matched for relative intensity (85% of 10RM), volume load (four sets of ten repetitions), and inter-set rest intervals (120 s). However, the inclusion of a brief intra-set rest in the cluster set configuration (five repetitions followed by a 30 s rest, then five additional repetitions) did not affect parasympathetic or sympathetic activity 30 min post-exercise. In contrast, the traditional RT protocol led to a reduction in parasympathetic activity. This phenomenon may be explained by the lower RPE and heart rate (30 min post-exercise) induced by the cluster set RT, which could indicate lower metabolic stress during exercise. [15]. Reinforcing this hypothesis, a previous study showed significantly lower cortisol and lactate levels 30 min after a cluster set compared to a traditional set RT protocol, which closely resembled the RT protocol adopted in the present study [15]. Furthermore, [17] also investigated the acute effects of RT set configuration on cardiac autonomic modulation. Despite identical intensity (15RM), total volume (200 repetitions), and resting time between sets, the short set configuration (8 × 5 repetitions with 51 s of rest) attenuated the reduction in parasympathetic activity compared to the long set configuration (4 × 10 repetitions with 2 min rest intervals). Additionally, the short set configuration resulted in lower lactate accumulation and velocity loss during exercise, without significant changes in blood pressure or baroreflex sensitivity, suggesting a more favorable autonomic and metabolic response [17]. Nevertheless, variations in rest-distribution strategies during a five-week resistance training program—comprising either four sets of eight repetitions with 5 min inter-set rest intervals or six sets of two repetitions with 1 min rest between sets and 5 min rest between exercises, both performed at a 10RM load—did not result in significant changes in heart rate parameters [22].
Experimental Considerations
Prolonged sympathetic activation and delayed parasympathetic recovery after RT are phenomena typically observed following high-intensity exercise [4]. Consequently, post-exercise HRV modulation has been associated with an increased risk of acute cardiac events [23,24]. Furthermore, HRV measurements are widely used to monitor exercise recovery, enabling adjustments in acute RT protocols based on cardiac autonomic modulation. This approach may optimize microcycle periodization, enhance training adaptations and performance, and help prevent injuries and overtraining. The cluster set RT protocol is an interesting RT configuration to generate muscle adaptation. For example, a recent study demonstrated that performing leg press and leg extension exercises using either cluster or traditional sets elicited similar increases in muscle thickness and lean tissue mass in resistance-trained individuals, suggesting that cluster sets are an efficient RT methodology for promoting muscle adaptations [11].
It is important to note that, although the present study demonstrates a decline in parasympathetic activity following a single session of traditional set RT, this does not mean that this RT configuration should not be recommended for improving cardiac autonomic modulation. A previous study reported that eight weeks of resistance training enhanced HRV parameters in young female college students [25], showing a significant increase in SDNN and a decrease in the LF/HF ratio. These findings suggest that RT reduces sympathetic activity (as indicated by SDNN) and improves sympathovagal balance (LF/HF ratio).
Additionally, the present study does not reveal a significant effect of gender on HRV parameters following cluster set and traditional RT protocols. A limitation of this study is the small sample size (16 participants); however, the large effect sizes observed reinforce our findings, as effect size calculations (Cohen’s d) are not influenced by sample size. Additionally, although the small sample also limited gender comparisons (seven females and nine males), our findings align with previous evidence from a narrative review [9] and a systematic review and meta-analysis [4], which reported that acute resistance-training-induced changes in HRV parameters are not significantly influenced by gender.
Furthermore, these findings should be interpreted with caution, as they are limited to young, healthy, resistance-trained individuals and may not generalize to older or clinical populations.
5. Conclusions
This study suggests that cluster sets may lead to smaller autonomic perturbations, as reflected by the trends and moderate effect sizes in HRV parameters 30 min post-exercise compared to traditional sets. These findings could help exercise physiologists to assist RT programs for clinical populations, such as individuals with hypertension or who are at risk for cardiovascular disease, by favoring protocols that potentially reduce cardiac autonomic stress. Future research should investigate whether these effects are consistent in clinical or older populations.
Author Contributions
Conceptualization: G.V.d.O. and B.d.S.S.; data Curation: G.V.d.O., B.d.S.S., M.C.G.A., C.E.C., and B.C.d.O.B.; formal analysis: B.d.S.S. and M.C.G.A.; funding acquisition: G.V.d.O.; methodology: G.V.d.O. and B.d.S.S.; project administration: M.C.G.A., C.E.C., and B.d.S.S.; writing—original draft: G.V.d.O. and B.d.S.S.; writing—review and editing: G.V.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil—grant number (E-26/210.754/2024).
Institutional Review Board Statement
All study procedures were performed according to the ethical standards of the Declaration of Helsinki and were approved by the institutional ethics committee of the Federal University of Rio de Janeiro, Brazil (protocol number: 55184922.5.0000.5699 date of approval: 18 March 2024). The trial was registered with the Clinical Trials Registry (ReBEC) under the number RBR-9857xj3.
Informed Consent Statement
All experimental procedures were performed after explaining the nature of the study and obtaining written consent from participants.
Data Availability Statement
The datasets used in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We also acknowledge the financial support from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil.
Conflicts of Interest
The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
References
- GBD 2021 Mortality Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 1681–1729. [Google Scholar]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of different training interventions on heart rate variability and cardiovascular health and risk factors in young and middle-aged adults: A systematic review. Front. Physiol. 2021, 12, 657274. [Google Scholar] [CrossRef]
- Kannankeril, P.J.; Goldberger, J.J. Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2091–H2098. [Google Scholar] [CrossRef]
- Marasingha-Arachchige, S.U.; Rubio-Arias, J.Á.; Alcaraz, P.E.; Chung, L.H. Factors that affect heart rate variability following acute resistance exercise: A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Baggish, A.L.; Monteventi, P.; Schmitt, L.; Stucky, F.; Gremeaux, V. Assessing the clinical reliability of short-term heart rate variability: Insights from controlled dual-environment and dual-position measurements. Sci. Rep. 2025, 15, 5611. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Kelly, E.E.; Collier, S.R.; Fernhall, B. Cardiac autonomic modulation during recovery from acute endurance versus resistance exercise. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.C.; Fedewa, M.V.; MacDonald, H.V.; Holmes, C.J.; Cicone, Z.S.; Plews, D.J.; Esco, M.R. The accuracy of acquiring heart rate variability from portable devices: A systematic review and meta-analysis. Sports Med. 2019, 49, 417–435. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—A review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Figueroa, A. Acute and training effects of resistance exercise on heart rate variability. Clin. Physiol. Funct. Imaging 2016, 36, 179–187. [Google Scholar] [CrossRef]
- Steele, J.; Raubold, K.; Kemmler, W.; Fisher, J.; Gentil, P.; Giessing, J. The effects of 6 months of progressive high-effort resistance training methods upon strength, body composition, function, and wellbeing of elderly adults. Biomed. Res. Int. 2017, 2017, 2541090. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; García-Sillero, M.; Maroto-Izquierdo, S.; Baz-Valle, E.; Bautista-Mayorga, B.; Murri, M.; Schoenfeld, B.J.; Benítez-Porres, J. Cluster sets and traditional sets elicit similar muscular hypertrophy: A volume and effort-matched study in resistance-trained individuals. Eur. J. Appl. Physiol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.G. Quantifying workloads in resistance training: A brief review. Prof. Strength Cond. 2010, 19, 31–40. Available online: https://www.researchgate.net/publication/239731099_Quantifying_Workloads_in_Resistance_Training_A_Brief_Review (accessed on 11 July 2024).
- Nunes, J.H.C.; Locatelli, J.C.; Reck, H.B.; Porto, F.E.; Francisquini Neto, A.; Lopes, W.A. Cardiac autonomic control following resistance exercise with different set configurations in apparently healthy young men: A crossover study. Physiol. Behav. 2021, 230, 113292. [Google Scholar] [CrossRef]
- Gobbo, H.R.; Barbosa, G.M.; de Oliveira, L.C.; de Oliveira, G.V. The effect of different resistance training protocols on cardiac autonomic modulation during exercise recovery: A crossover, randomized, and controlled pilot study. J. Vasc. Dis. 2024, 3, 375–384. [Google Scholar] [CrossRef]
- Oliver, J.M.; Kreutzer, A.; Jenke, S.; Phillips, M.D.; Mitchell, J.B.; Jones, M.T. Acute response to cluster sets in trained and untrained men. Eur. J. Appl. Physiol. 2015, 115, 2383–2393. [Google Scholar] [CrossRef]
- Way, K.L.; Thomas, H.J.; Parker, L.; Maiorana, A.; Keske, M.A.; Scott, D.; Reed, J.L.; Tieng, J.; Hackett, D.; Hawkins, T.; et al. Cluster sets to prescribe interval resistance training: A potential method to optimise resistance training safety, feasibility, and efficacy in cardiac patients. Sports Med. Open 2023, 9, 86. [Google Scholar] [CrossRef]
- Rúa-Alonso, M.; Mayo, X.; Mota, J.; Kingsley, J.D.; Iglesias-Soler, E. A short set configuration attenuates the cardiac parasympathetic withdrawal after a whole-body resistance training session. Eur. J. Appl. Physiol. 2020, 120, 1803–1812. [Google Scholar] [CrossRef]
- Kliszczewicz, B.M.; Esco, M.R.; Quindry, J.C.; Blessing, D.L.; Oliver, G.D.; Taylor, K.J.; Price, B.M. Autonomic responses to an acute bout of high-intensity body weight resistance exercise vs. treadmill running. J. Strength Cond. Res. 2016, 30, 1050–1058. [Google Scholar] [CrossRef]
- Cavarretta, D.J.; Hall, E.E.; Bixby, W.R. The effects of increasing training load on affect and perceived exertion. J. Strength Cond. Res. 2022, 36, 16–21. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using effect size—Or why the P value is not enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Figueiredo, T.; Willardson, J.M.; Miranda, H.; Bentes, C.M.; Reis, V.M.; Simão, R. Influence of load intensity on postexercise hypotension and heart rate variability after a strength training session. J. Strength Cond. Res. 2015, 29, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Rial-Vázquez, J.; Rúa-Alonso, M.; Fariñas, J.; Aracama, A.; Tufano, J.; Iglesias-Soler, E. Heart rate responses and cardiovascular adaptations to resistance training programs differing in set configuration: A randomized controlled trial. Res. Q. Exerc. Sport 2023, 94, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Morshedi-Meibodi, A.; Larson, M.G.; Levy, D.; O’Donnell, C.J.; Vasan, R.S. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). Am. J. Cardiol. 2002, 90, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, H.; Sørensen, K.E.; Bjerregaard, P. Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. Clin. Auton. Res. 1991, 1, 233–237. [Google Scholar] [CrossRef]
- Li, R.; Yan, R.; Cheng, W.; Ren, H. Effect of resistance training on heart rate variability of anxious female college students. Front. Public Health 2022, 10, 1050469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).