Dellon Decompression Using WALANT: A Safe and Effective Approach for Patients with Peripheral Artery Disease
Abstract
1. Introduction
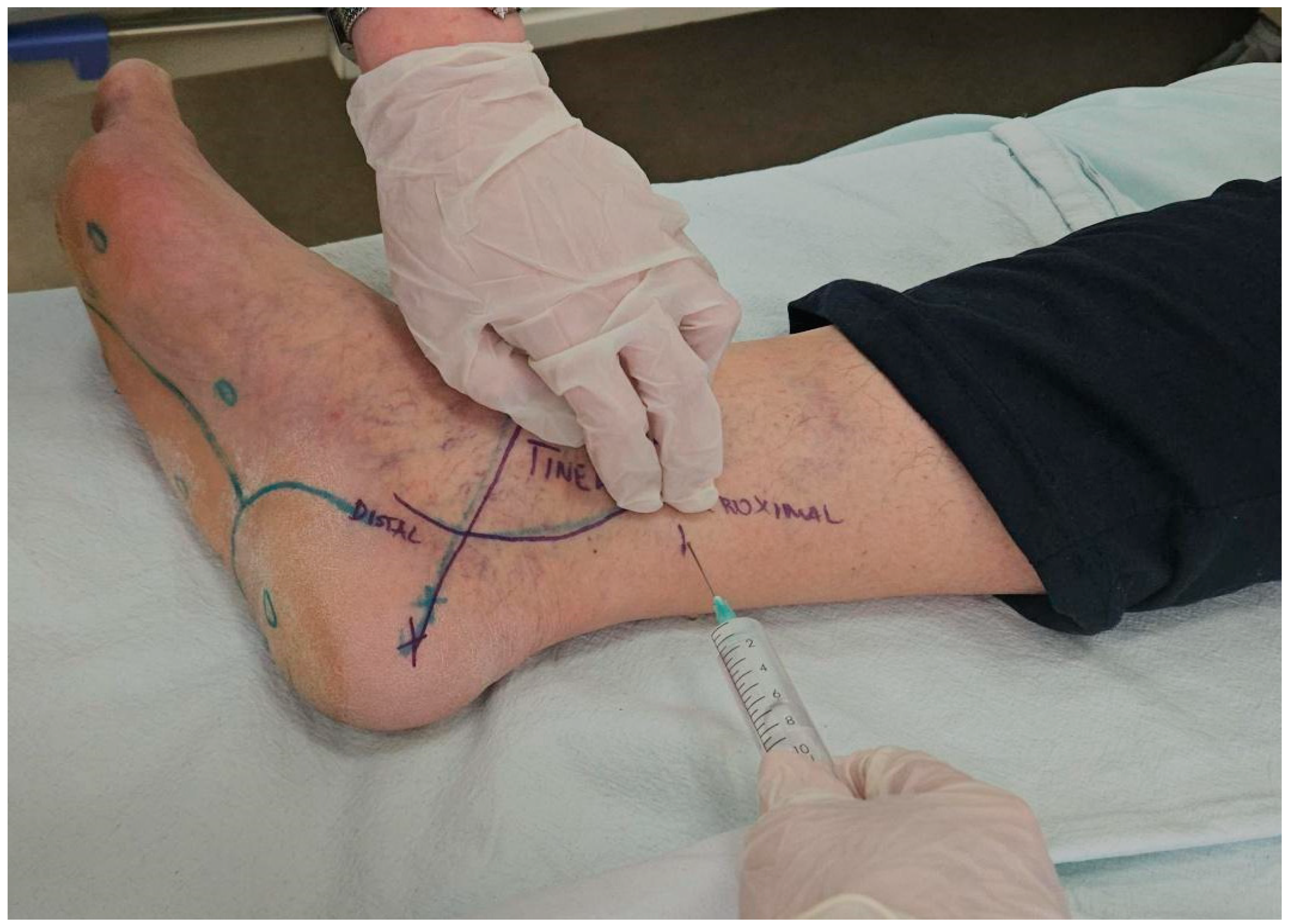
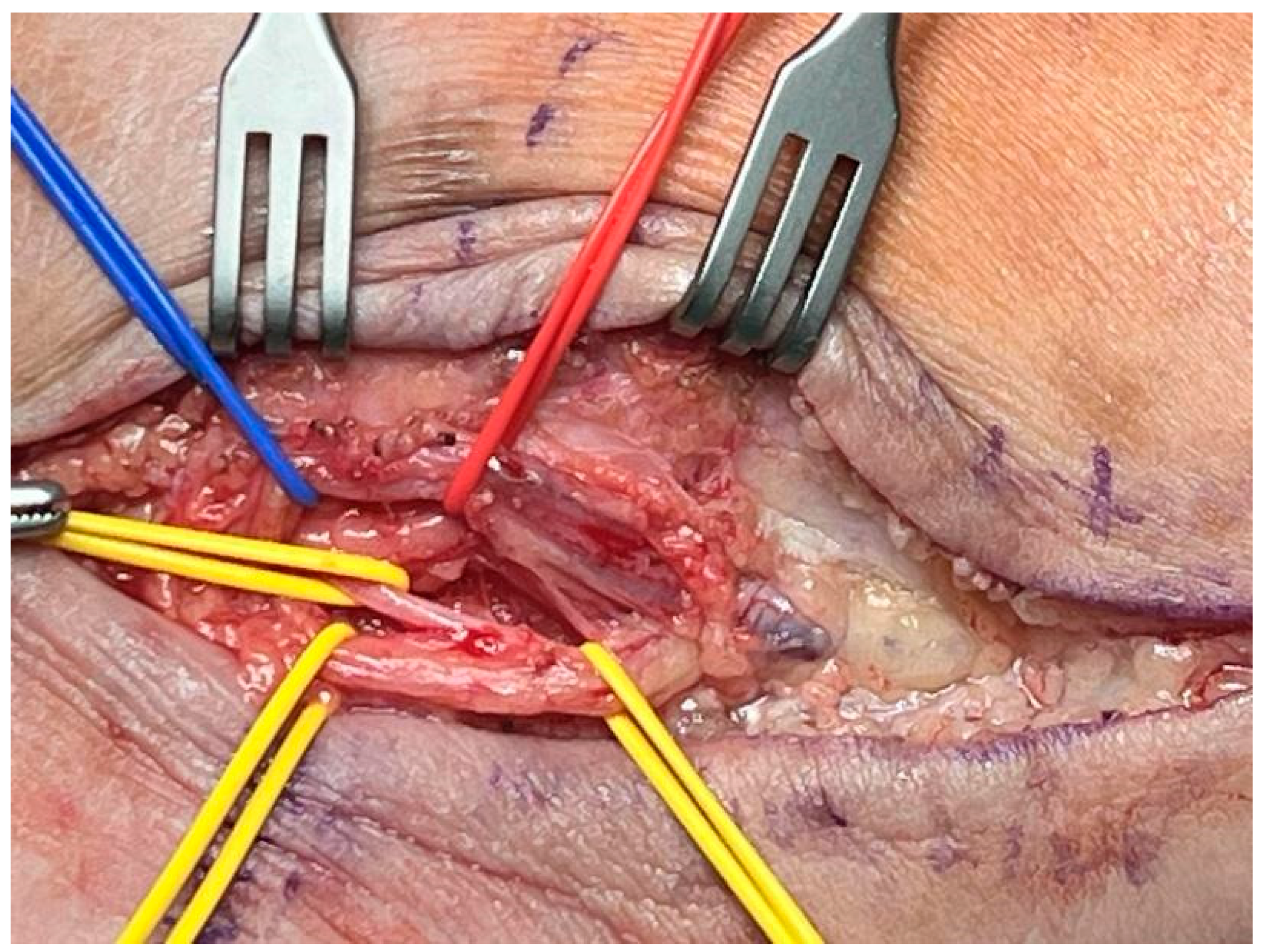
2. Materials and Methods
2.1. Design
2.2. Selection/Exclusion Criteria
2.3. Data Collection
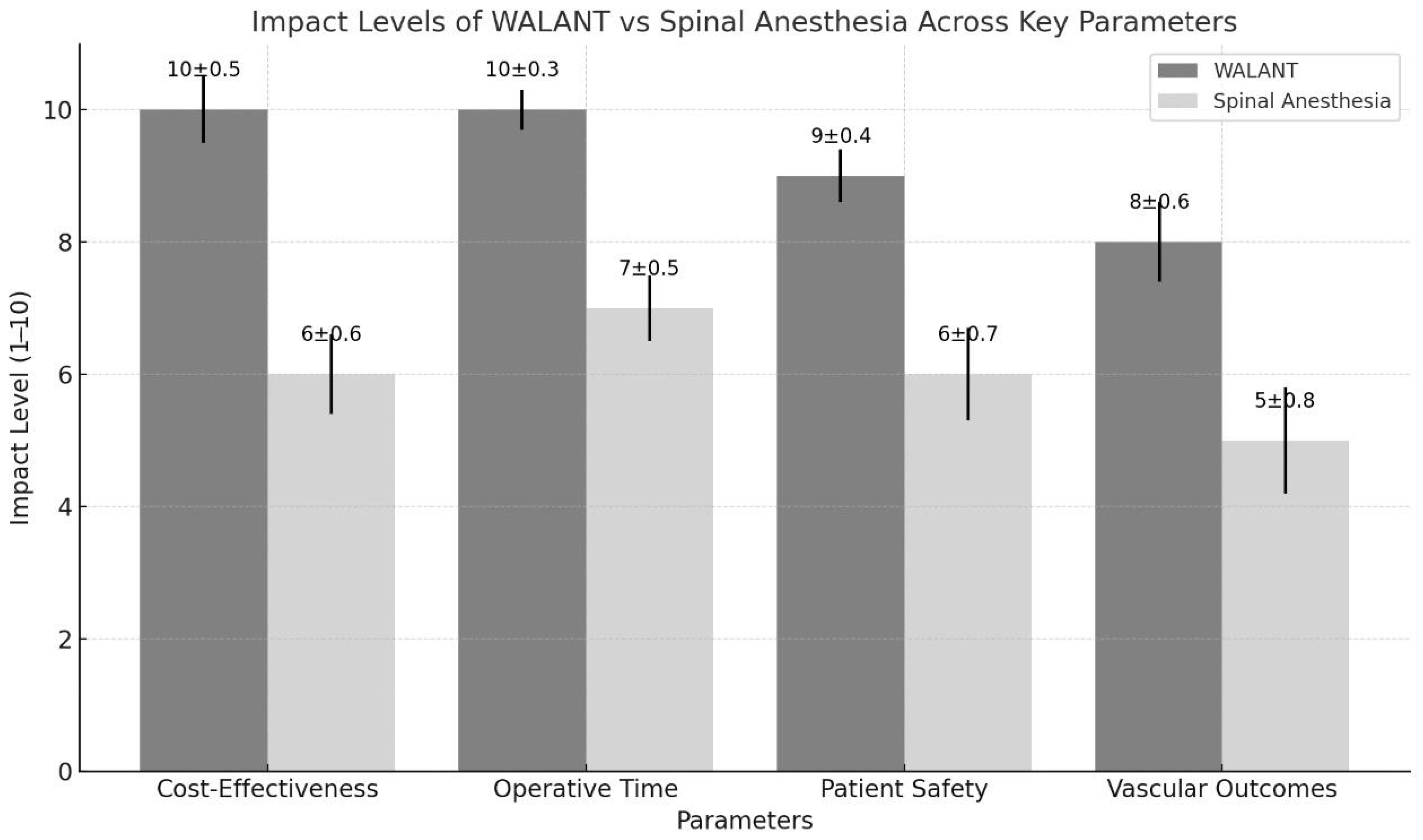
2.4. Impact Level
3. Results
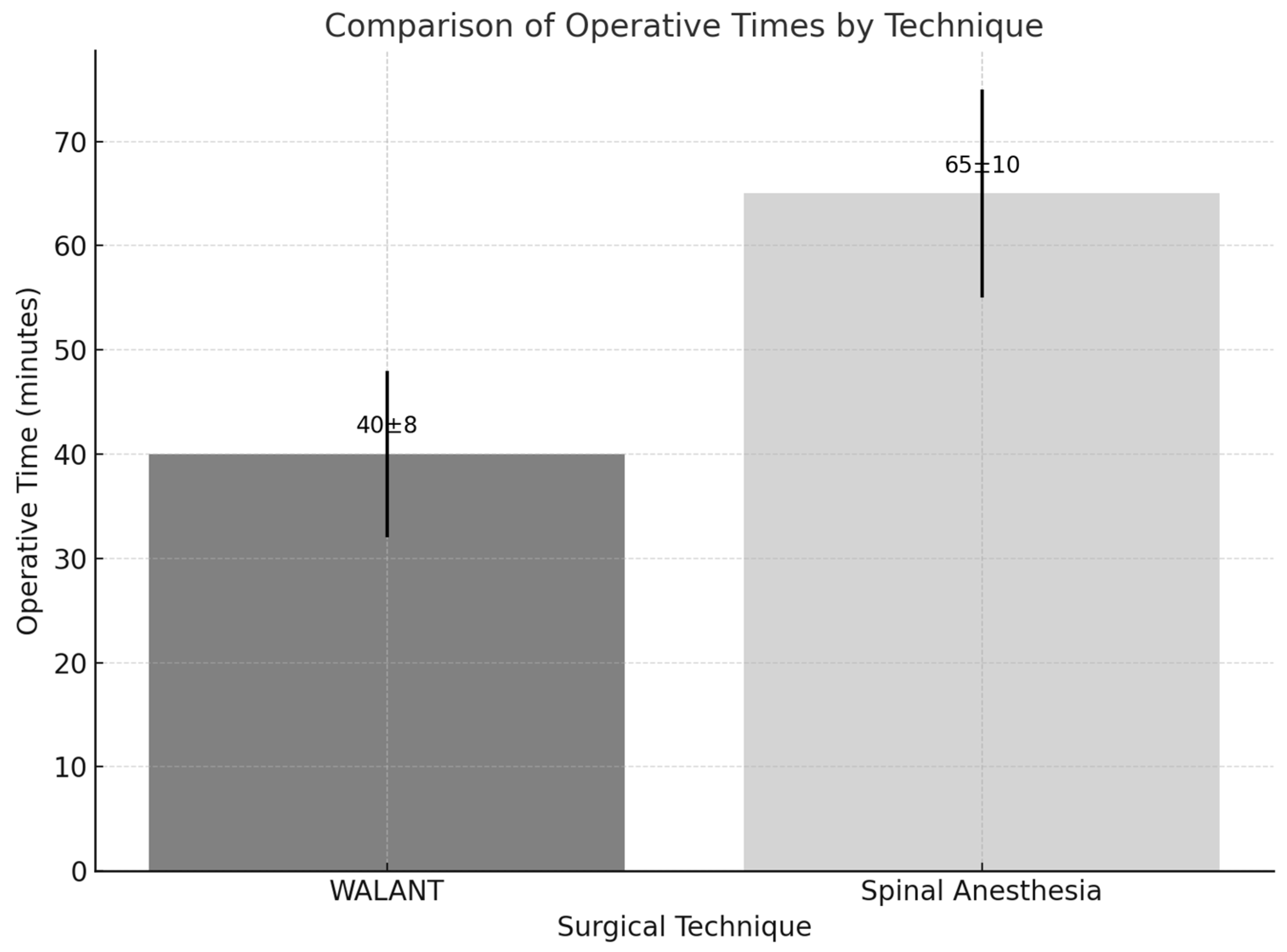
3.1. Operative Efficiency
3.2. Pain Relief and Comfort of the Patient
3.3. Vascular Outcomes
3.4. Safety and Complications
3.5. Functional Outcomes and Patient Satisfaction
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalonde, D. Wide awake local anaesthesia no tourniquet technique (WALANT). BMC Proc. 2015, 9, A81. [Google Scholar] [CrossRef]
- Lalonde, D.H. Conceptual origins, current practice, and views of wide awake hand surgery. J. Hand Surg. (Eur. Vol.) 2017, 42, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.M.; Guerra, S.M.; Koehler, S.M. Current Evidence Involving WALANT Surgery. J. Hand Surg. Glob. Online 2022, 4, 452–455. [Google Scholar] [CrossRef]
- Abdullah, S. WALANT: Past, Present and the Future. J. Hand Surg. Glob. Online 2022, 4, 384. [Google Scholar] [CrossRef]
- Georgieva, G.; Srbov, B.; Nikolovska, B.; Tusheva, S.; Jovanovska, K.; Jovanoski, T.; Dzonov, B.; Gjorgova, S.T.; Pejkova, S. WALANT as an Optimal Approach in Hand Surgery During Pandemics. Prague Med. Rep. 2022, 123, 88–94. [Google Scholar] [CrossRef]
- Masterton, G.; Talwar, C. WALANT in plastic surgery trauma—Why wait? J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1355–1401. [Google Scholar] [CrossRef]
- Bravo, D.; Townsend, C.B.; Tulipan, J.; Ilyas, A.M. Economic and Environmental Impacts of the Wide-Awake, Local Anesthesia, No Tourniquet (WALANT) Technique in Hand Surgery: A Review of the Literature. J. Hand Surg. Glob. Online 2022, 4, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Saghir, N.; Saghir, R.; Young-Sing, Q.; Miranda, B.H. WALANT: A Discussion of Indications, Impact, and Educational Requirements. Arch. Plast. Surg. 2022, 49, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Farooq, R.; Raufi, M.Y.; Soe, B.; Suleman, A.H.; Seraj, S.S.; Arif, A.; Rahman, S.; Bhat, W. WALANT vs. standard anaesthesia in the management of flexor tendon injuries: A systematic review and meta-analysis. J. Hand Microsurg. 2024, 16, 100157. [Google Scholar] [CrossRef]
- Lalonde, D.H.; Wong, A. Dosage of local anesthesia in wide awake hand surgery. J. Hand Surg. Am. 2013, 38, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, D.H. Latest Advances in Wide Awake Hand Surgery. Hand Clin. 2019, 35, 1–6. [Google Scholar] [CrossRef]
- Mayich, D.J. Wide-Awake Local Anesthetic No Tourniquet Surgery of the Foot and Ankle. Orthop. Clin. 2023, 54, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Pinho Teixeira, A.M.; Nunes, C.F.C. Dellon Triple Decompression Under WALANT. Diabetes Complicat. 2023, 7, 1–5. [Google Scholar] [CrossRef]
- Lavigne, F.; Becuwe, L.; Buia, G.; Her, M.; Quesnel, A.; Dubau, B. A prospective comparison of WALANT technique and general anesthesia in forefoot surgery. Orthop. Traumatol. Surg. Res. 2024, 110, 103947. [Google Scholar] [CrossRef] [PubMed]
- Bilgetekin, Y.G.; Kuzucu, Y.; Öztürk, A.; Yüksel, S.; Atilla, H.A.; Ersan, Ö. The use of the wide-awake local anesthesia no tourniquet technique in foot and ankle injuries. Foot Ankle Surg. 2021, 27, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.A.; Younis, Z.; Mannan, M.; Kalim, Z.; Khan, Z.A.; Prabhu, R.M.; Shrivastava, N.; Rashid, N. Wide Awake Local Anaesthesia No Tourniquet Surgery of the Foot and Ankle: A Review of Indications, Technique, Patient Satisfaction, and Complications. Cureus 2024, 16, e74939. [Google Scholar] [CrossRef] [PubMed]
- Aszmann, O.; Tassler, P.L.; Dellon, A.L. Changing the natural history of diabetic neuropathy: Incidence of ulcer/amputation in the contralateral limb of patients with a unilateral nerve decompression procedure. Ann. Plast. Surg. 2004, 53, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L. Treatment of symptomatic diabetic neuropathy by surgical decompression of multiple peripheral nerves. Plast. Reconstr. Surg. 1992, 89, 689–697; discussion 698–699. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L. The Dellon approach to neurolysis in the neuropathy patient with chronic nerve compression. Handchir. Mikrochir. Plast. Chir. 2008, 40, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L.; Mackinnon, S.E. Tibial nerve branching in the tarsal tunnel. Arch. Neurol. 1984, 41, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Maimaijiang, M.; Yushan, M.; Abulaiti, A.; Ren, P.; Yusufu, A. Effectiveness of lower extremity Dellon triple nerve decompression in treatment of early stage diabetic Charcot foot. Zhongguo Xiufu Chongjian Waike Zazhi = Chin. J. Reparative Reconstr. Surg. 2020, 34, 1005–1011. [Google Scholar]
- Fakkel, T.M.; Rinkel, W.D.; Coert, J.H. Does Lower Extremity Nerve Decompression Surgery Improve Quality of Life. Plast. Reconstr. Surg. 2022, 150, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L. Susceptibility of nerve in diabetes to compression implications for pain treatment. Plast. Reconstr. Surg. 2014, 134, 142S–150S. [Google Scholar] [CrossRef]
- Dellon, A.L. Neurosurgical prevention of ulceration and amputation by decompression of lower extremity peripheral nerves in diabetic neuropathy update 2006. In How to Improve the Results of Peripheral Nerve Surgery. Acta Neurochirurgica Supplementum; Springer: Vienna, Austria, 2007; Volume 100, pp. 149–151. [Google Scholar]
- Jensen, J.; Hicks, R.W.; Labovitz, J. Understanding and optimizing tourniquet use during extremity surgery. AORN J. 2019, 109, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Wakai, A.; Winter, D.C.; Street, J.T.; Redmond, P.H. Pneumatic tourniquets in extremity surgery. JAAOS-J. Am. Acad. Orthop. Surg. 2001, 9, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Estebe, J.P.; Davies, J.M.; Richebe, P. The pneumatic tourniquet: Mechanical, ischaemia–reperfusion and systemic effects. Eur. J. Anaesthesiol.|EJA 2011, 28, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.R.; Patel, D.G. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. JBJS 1981, 63, 461–465. [Google Scholar] [CrossRef]
- Pozza, D.H.; Tavares, I.; Cruz, C.D.; Fonseca, S. Spinal Cord Injury and Complications Related to Neuraxial Anaesthesia Procedures: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4665. [Google Scholar] [CrossRef]
- Bina, B.; Hersh, E.V.; Hilario, M.; Alvarez, K.; McLaughlin, B. True Allergy to Amide Local Anesthetics: A Review and Case Presentation. Anesth. Prog. 2018, 65, 119–123. [Google Scholar] [CrossRef]
- Frank, S.G.; Lalonde, D.H. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can. J. Plast. Surg. 2012, 20, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Middlehurst, R.J.; Gibbs, A.; Walton, G. Cardiovascular risk: The safety of local anesthesia, vasoconstrictors, and sedation in heart disease. Anesth. Prog. 1999, 46, 118–123. [Google Scholar]
- Staikou, C.; Chondrogiannis, K.; Mani, A. Perioperative management of hereditary arrhythmogenic syndromes. Br. J. Anaesth. 2012, 108, 730–744. [Google Scholar] [CrossRef]
- Sanatkar, M.; Sadeghi, M.; Esmaeili, N.; Naseri, M.H.; Sadrossadat, H.; Shoroughi, M.; Fathi, H.R.; Ghazizadeh, S. The evaluation of perioperative safety of local anesthesia with lidocaine containing epinephrine in patients with ischemic heart disease. Acta Med. Iran. 2013, 51, 537–542. [Google Scholar]
- Kukreja, P.; Lehtonen, E.; Pinto, M.C.; Patel, H.A.; McKissack, H.M.; Shah, A. Postoperative Tourniquet Pain in Patients Undergoing Foot and Ankle Surgery. Cureus 2018, 10, e3678. [Google Scholar] [CrossRef]
- Kumar, K.; Railton, C.; Tawfic, Q. Tourniquet application during anesthesia: “What we need to know?”. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Horlocker, T.T.; Hebl, J.R.; Gali, B.; Jankowski, C.J.; Burkle, C.M.; Berry, D.J.; Zepeda, F.A.; Stevens, S.R.; Schroeder, D.R. Anesthetic, patient, and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesth. Analg. 2006, 102, 950–955. [Google Scholar] [CrossRef]
- Sharma, J.P.; Salhotra, R. Tourniquets in orthopedic surgery. Indian J. Orthop. 2012, 46, 377–383. [Google Scholar] [CrossRef]
- Concepcion, M.A.; Lambert, D.H.; Welch, K.A.; Covino, B.G. Tourniquet pain during spinal anesthesia: A comparison of plain solutions of tetracaine and bupivacaine. Anesth. Analg. 1988, 67, 828–832. [Google Scholar]
- Kam, P.C.; Kavanagh, R.; Yoong, F.F. The arterial tourniquet: Pathophysiological consequences and anaesthetic implications. Anaesthesia 2001, 56, 534–545, Correction in Anaesthesia 2001, 56, 821. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, D.T.; McClinton, M.A. Upper extremity tourniquet tolerance. J. Hand Surg. Am. 1993, 18, 206–210. [Google Scholar] [CrossRef]
- Firnhaber, J.M.; Powell, C.S. Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 362–369, Correction in Am. Fam. Physician 2019, 100, 74. [Google Scholar] [PubMed]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients with Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, D.; Bell, M.; Benoit, P.; Sparkes, G.; Denkler, K.; Chang, P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: The Dalhousie Project clinical phase. J. Hand Surg. 2005, 30, 1061–1067. [Google Scholar] [CrossRef]
- Wilhelmi, B.J.; Blackwell, S.J.; Miller, J.H.; Mancoll, J.S.; Dardano, T.; Tran, A.; Phillips, L.G. Do not use epinephrine in digital blocks: Myth or truth? Plast. Reconstr. Surg. 2001, 107, 393–397. [Google Scholar] [CrossRef]
- Lanza, F.; Beretz, A.; Stierlé, A.; Hanau, D.; Kubina, M.; Cazenave, J.P. Epinephrine potentiates human platelet activation but is not an aggregating agent. Am. J. Physiol. 1988, 255, H1276–H1288. [Google Scholar] [CrossRef]
- Denkler, K. A comprehensive review of epinephrine in the finger: To do or not to do. Plast. Reconstr. Surg. 2001, 108, 114–124. [Google Scholar] [CrossRef]
- Thomson, C.J.; Lalonde, D.H.; Denkler, K.A.; Feicht, A.J. A critical look at the evidence for and against elective epinephrine use in the finger. Plast. Reconstr. Surg. 2007, 119, 260–266. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tusheva, S.; Georgieva, G.; Srbov, B.; Paljoskovska Jordanova, S.; Jovanovska, K.; Azmanova Mladenovska, S.; Memeti, M.; Aleksovski, D.; Mileska Krzhaloska, B.; Pejkova, S. Dellon Decompression Using WALANT: A Safe and Effective Approach for Patients with Peripheral Artery Disease. J. Vasc. Dis. 2025, 4, 7. https://doi.org/10.3390/jvd4010007
Tusheva S, Georgieva G, Srbov B, Paljoskovska Jordanova S, Jovanovska K, Azmanova Mladenovska S, Memeti M, Aleksovski D, Mileska Krzhaloska B, Pejkova S. Dellon Decompression Using WALANT: A Safe and Effective Approach for Patients with Peripheral Artery Disease. Journal of Vascular Diseases. 2025; 4(1):7. https://doi.org/10.3390/jvd4010007
Chicago/Turabian StyleTusheva, Sofija, Gordana Georgieva, Blagoja Srbov, Savetka Paljoskovska Jordanova, Katerina Jovanovska, Stefania Azmanova Mladenovska, Muamet Memeti, Darko Aleksovski, Biljana Mileska Krzhaloska, and Sofija Pejkova. 2025. "Dellon Decompression Using WALANT: A Safe and Effective Approach for Patients with Peripheral Artery Disease" Journal of Vascular Diseases 4, no. 1: 7. https://doi.org/10.3390/jvd4010007
APA StyleTusheva, S., Georgieva, G., Srbov, B., Paljoskovska Jordanova, S., Jovanovska, K., Azmanova Mladenovska, S., Memeti, M., Aleksovski, D., Mileska Krzhaloska, B., & Pejkova, S. (2025). Dellon Decompression Using WALANT: A Safe and Effective Approach for Patients with Peripheral Artery Disease. Journal of Vascular Diseases, 4(1), 7. https://doi.org/10.3390/jvd4010007






