Biochemical Insights and Clinical Applications of Ischemia-Modified Albumin in Ischemic Conditions
Abstract
1. Introduction
2. Atherosclerotic Coronary Artery Disease (ACAD)
2.1. Global Prevalence
2.2. Local Prevalence
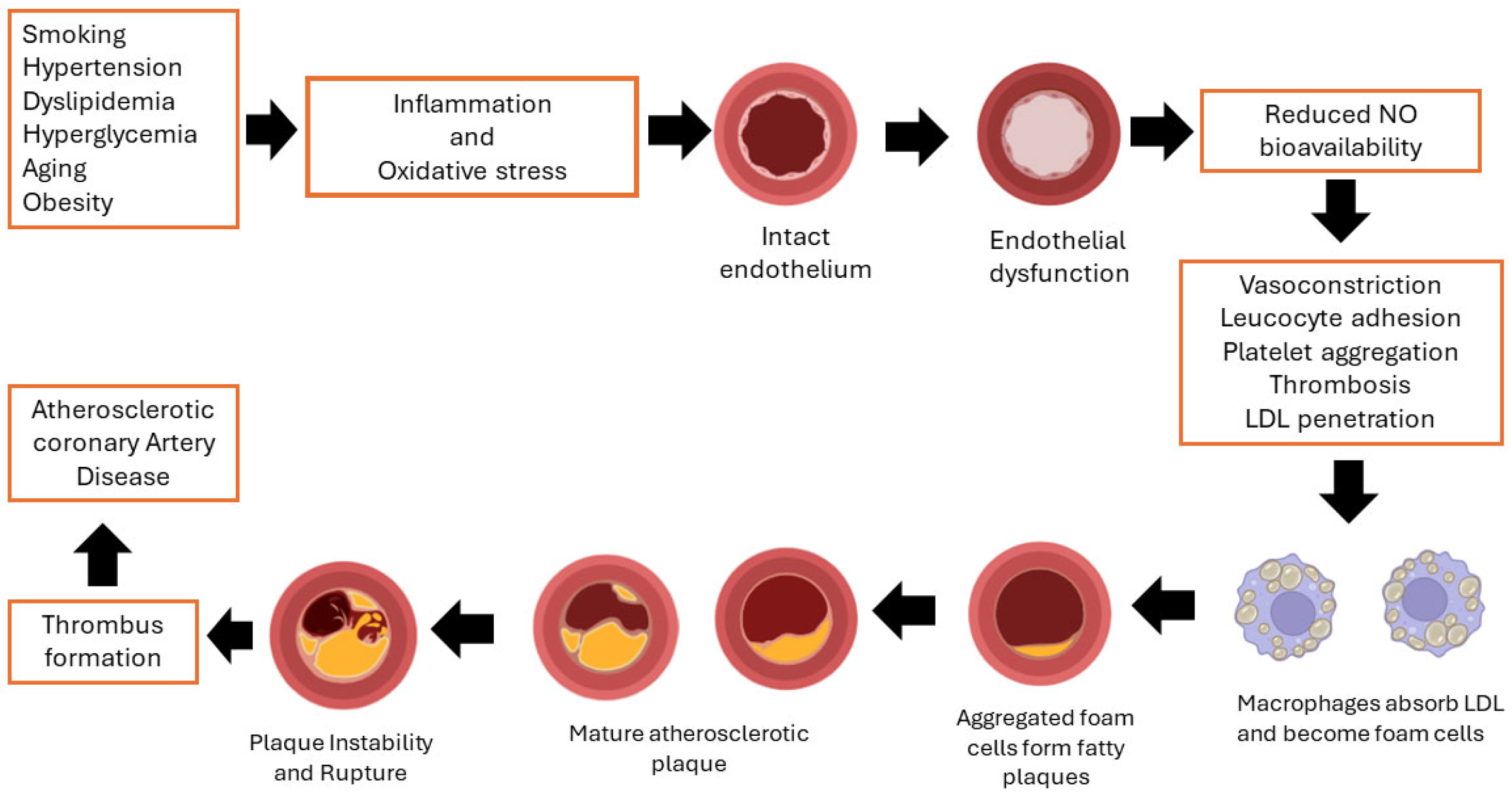
2.3. Pathophysiology of CAD
2.4. Diagnostic Tests of CAD
2.5. Use of Biomarkers in the Diagnosis of CAD
3. Ischemia Modified Albumin (IMA) as a Biomarker of Myocardial Ischemia
3.1. Human Serum Albumin
3.2. Formation of IMA
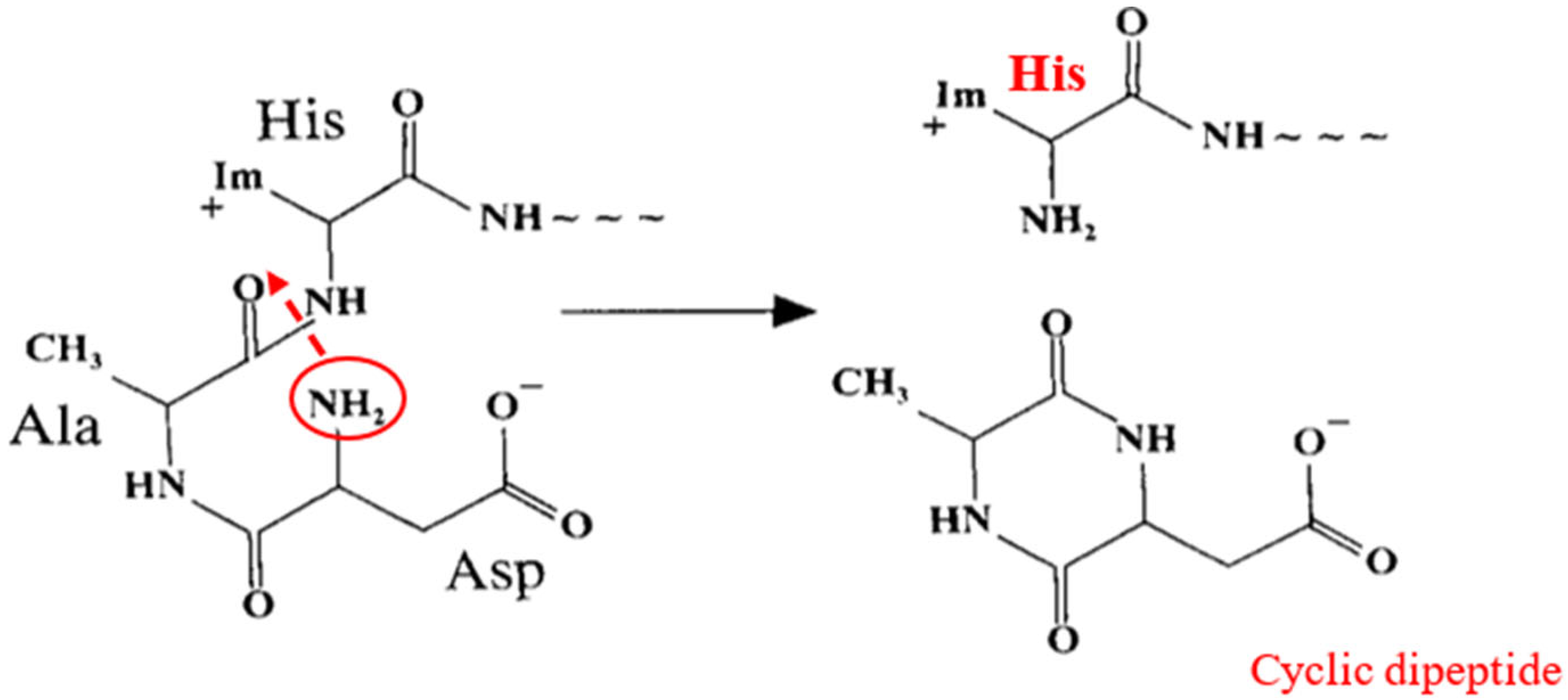
3.2.1. First Model/Hypothesis—Auto Degradation of NTS
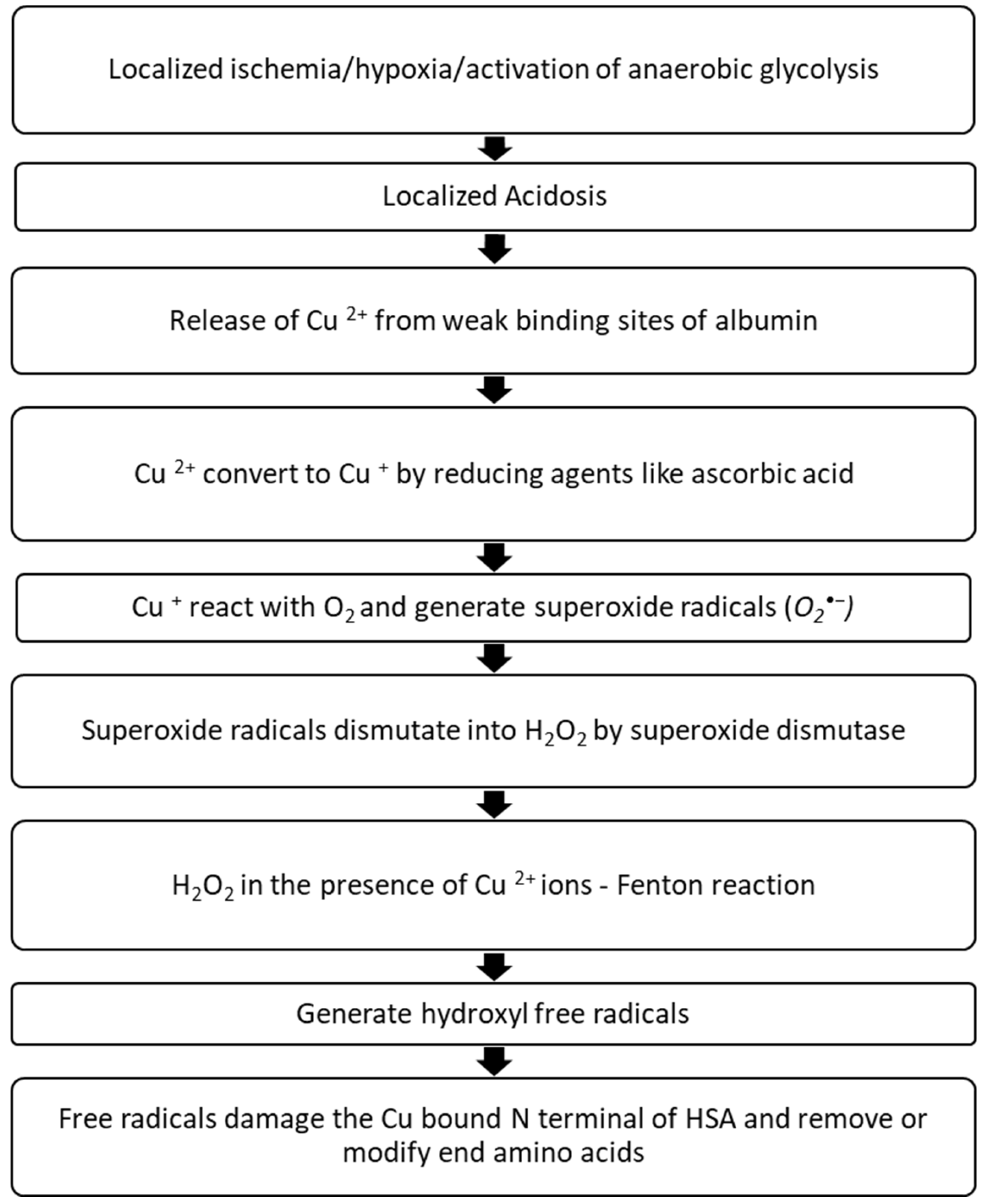
3.2.2. The Second Model—N-Terminal Site Modification by Free Radicals
3.2.3. 3rd Hypothesis—Acetylation of the N-Terminus
3.2.4. Other Hypotheses—Inhibition of Cobalt Binding to HSA by Fatty Acids
4. Detection Methods of IMA
4.1. Albumin Cobalt Binding (ACB) Assay
4.1.1. Further Investigations on ACB Assay
4.1.2. Automation of ACB Assay
4.2. Cobalt Albumin Binding (CAB) Assay
The Cobalt (II) Principal Binding Site Is Not the N-Terminus?
4.3. Albumin Copper Binding (ACuB) Assay
4.4. Nickel-Albumin Binding Assay
4.5. Quantum Dot Coupled X-ray Fluorescence Spectroscopy (Q-XFR)
4.6. Immunological Methods
Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Liquid Crystal Biosensor for IMA
4.8. Surface Plasmon Resonance (SPR) Immunosensor for IMA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CDC. Centers for Disease Control and Prevention. Heart Disease Facts|cdc.gov. 2022. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 21 February 2023).
- Overview—Cardiovascular Disability—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK209971/#ddd00024 (accessed on 8 April 2023).
- Nelson, A.J.; Ardissino, M.; Psaltis, P.J. Current approach to the diagnosis of atherosclerotic coronary artery disease: More questions than answers. Ther. Adv. Chronic Dis. 2019, 10, 2040622319884819. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, J.B.; Samarutilake, G.D.N.; Zacky, M.H.M.; De Silva, P.V.; Karunanayake, A.; Weerasooriya, M.A. Prevalence of coronary artery disease in a semi urban population in Southern Sri Lanka. Ceylon Med. J. 2017, 62, 34. [Google Scholar] [CrossRef]
- Stark, B.; Johnson, C.; Roth, G.A. Global prevalence of coronary artery disease: An update from the global burden of disease study. J. Am. Coll. Cardiol. 2024, 83, 2320. [Google Scholar] [CrossRef]
- World Heart Federation, World Heart Report 2023: Full Report. Available online: https://world-heart-federation.org/resource/world-heart-report-2023/ (accessed on 1 June 2024).
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Ministry of Health MS Unit. Annual Health Statistics 2019 Sri Lanka. 2021. Available online: https://www.health.gov.lk/wp-content/uploads/2022/12/AHB-2019-1.pdf (accessed on 9 June 2024).
- Cardiovascular Disease Burden in Sri Lanka|Cardiovascular|RemediumOne. Available online: https://www.remediumone.com/therapeutic-expertise/therapeutic-areas/cardiovascular/cardiovascular-disease-burden-in-sri-lanka/ (accessed on 1 June 2024).
- Senaviratna, N. Cardiovascular disease burden in a country: In the context of Sri Lanka. Eur. J. Med. Health Res. 2023, 1, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Coronary Heart Disease—What is Coronary Heart Disease?|NHLBI, NIH. 2022. Available online: https://www.nhlbi.nih.gov/health/coronary-heart-disease (accessed on 21 February 2023).
- Hamm, C.W. Cardiac Biomarkers for Rapid Evaluation of Chest Pain. Circulation 2001, 104, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Roy, D.; Gaze, D.C.; Collinson, P.O.; Kaski, J.-C. Role of “Ischemia Modified Albumin”, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg. Med. J. 2004, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Khan, M. Cardiac Biomarkers: What Is and What Can Be. Indian J. Cardiovasc. Dis. Women WINCARS 2018, 3, 240–244. [Google Scholar] [CrossRef]
- Mishra, B.; Pandey, S.; Niraula, S.R.; Rai, B.K.; Karki, P.; Baral, N.; Lamsal, M. Utility of Ischemia Modified Albumin as an Early Marker for Diagnosis of Acute Coronary Syndrome. J. Nepal Health Res. Counc. 2018, 16, 16–21. [Google Scholar] [CrossRef]
- Oran, I.; Oran, B. Ischemia-Modified Albumin as a Marker of Acute Coronary Syndrome: The Case for Revising the Concept of “N-Terminal Modification” to “Fatty Acid Occupation” of Albumin. Dis. Markers 2017, 2017, 5692583. [Google Scholar] [CrossRef]
- Coronary Artery Disease—Symptoms and Causes—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/coronary-artery-disease/symptoms-causes/syc-20350613 (accessed on 8 April 2023).
- University of Ottawa Heart Institute. Coronary Artery Disease (Atherosclerosis). Available online: https://www.ottawaheart.ca/heart-condition/coronary-artery-disease-atherosclerosis (accessed on 8 April 2023).
- WHO 2021 Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 6 March 2023).
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014; 280p, Available online: https://apps.who.int/iris/handle/10665/148114 (accessed on 1 June 2024).
- Ministry of Health Sri Lanka. Performance and Progress Report—Ministry of Health Sri Lanka. p. 104. Available online: https://www.health.gov.lk (accessed on 1 June 2024).
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Shahjehan, R.D.; Bhutta, B.S. Coronary Artery Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK564304/ (accessed on 3 June 2024).
- Bhakthavatsala Reddy, C.; Cyriac, C.; Desle, H.B. Role of “Ischemia Modified Albumin” (IMA) in acute coronary syndromes. Indian Heart J. 2014, 66, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Battistoni, A.; Rubattu, S. Natriuretic peptides in heart failure: Current achievements and future perspectives. Int. J. Cardiol. 2019, 281, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Gaze, D.C. Ischemia Modified Albumin: A Novel Biomarker for the Detection of Cardiac Ischemia. Drug Metab. Pharmacokinet. 2009, 24, 333–341. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Lau, E.; Rao, N.; Bampos, N.; Winkler, J.; Curtis, C. Reduction in the cobalt binding capacity of human albumin with myocardial ischemia. Ann. Emerg. Med. 1999, 34, S56. [Google Scholar] [CrossRef]
- Yücel, D. Ischemia—Modified albumin by albumin cobalt binding test: A false myth or reality. Turk. J. Biochem. 2023, 48, 1–4. [Google Scholar] [CrossRef]
- Palomeras Soler, E.; Casado Ruiz, V. Epidemiology and Risk Factors of Cerebral Ischemia and Ischemic Heart Diseases: Similarities and Differences. Curr. Cardiol. Rev. 2010, 6, 138–149. [Google Scholar] [CrossRef]
- George, M.G. Risk Factors for Ischemic Stroke in Younger Adults. Stroke 2020, 51, 729–735. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Cengiz, H.; Dagdeviren, H.; Kanawati, A.; Suzen Çaypinar, S.; Yesil, A.; Ekin, M.; Yasar, L. Ischemia-modified albumin as an oxidative stress biomarker in early pregnancy loss. J. Matern. Fetal Neonatal Med. 2015, 29, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Amuzescu, A.; Matei, C.; Georgescu, S.R. Ischemia-Modified Albumin—A Potential New Marker of Oxidative Stress in Dermatological Diseases. Medicina 2022, 58, 669. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Y.; Tian, L.; Wang, Z. Correlation between ischemia-modified albumin level and coronary collateral circulation. BMC Cardiovasc. Disord. 2020, 20, 326. [Google Scholar] [CrossRef]
- Karaşin, S.S.; Çift, T. The Role of Ischemia-modified Albumin as a Biomarker in Preeclampsia. Rev. Bras. Ginecol. E Obstetrícia 2020, 42, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Savci, U.; Senel, E.; Oztekin, A.; Sungur, M.; Erel, O.; Neselioglu, S. Ischemia-modified albumin as a possible marker of oxidative stress in patients with telogen effluvium, An. Bras. Dermatol. 2020, 95, 447–451. [Google Scholar] [CrossRef]
- Soliman, H.; Okda, M.; El-Kholy, M.; El-Kabany, R. Ischemia-modified albumin for evaluating severity and predicting prognosis in patients with acute cerebrovascular disease. Menoufia Med. J. 2020, 33, 1072–1076. [Google Scholar]
- Lee, E.; Eom, J.-E.; Jeon, K.-H.; Kim, T.H.; Kim, E.; Jhon, G.-J.; Kwon, Y. Evaluation of albumin structural modifications through cobalt-albumin binding (CAB) assay. J. Pharm. Biomed. Anal. 2014, 91, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Christenson, R.H.; Duh, S.H.; Sanhai, W.R.; Wu, A.H.B.; Holtman, V.; Painter, P.; Branham, E.; Apple, F.S.; Murakami, M.; Morris, D.L. Characteristics of an Albumin Cobalt Binding Test for Assessment of Acute Coronary Syndrome Patients: A Multicenter Study. Clin. Chem. 2001, 47, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, S.; Ducassé, J.; Cournot, M.; Maupas-Schwalm, F.; Elbaz, M.; Baixas, C.; Juchet, H.; Lang, T.; Lauque, D. Clinical Assessment of Ischemia-modified Albumin and Heart Fatty Acid-binding Protein in the Early Diagnosis of Non-ST-elevation Acute Coronary Syndrome in the Emergency Department. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2010, 17, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, P.; Borra, S.K.; Yeruva, R.K.R.; Victor, D.; Babu, S.; Cherian, K.M. Estimation of Ischemia Modified Albumin (IMA) Levels in Patients with Acute Coronary Syndrome. Indian J. Clin. Biochem. 2014, 29, 367–371. [Google Scholar] [CrossRef]
- Pan, S.; Tong, C.; Lin, Q.; Yao, C.; Zhao, J.; Deng, Z. Ischemia-modified albumin measured with ultra-filtration assay in early diagnosis of acute coronary syndrome. World J. Emerg. Med. 2010, 1, 37–40. [Google Scholar] [PubMed]
- Friedrichs, B. Th. Peters. Jr.: All about Albumin. Biochemistry, Genetics, and Medical Applications. XX and 432 pages, numerous figures and tables. Academic Press, Inc., San Diego, California, 1996. Price: 85.00 US $. Food Nahr. 1997, 41, 382. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatol. Baltim. Md 1988, 8, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, S.; Lachowicz, J.I.; Nowakowski, M.E.; Jaremko, M.; Jaremko, Ł. Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem. 2019, 198, 110716. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Guizado, T.R.C. Analysis of the structure and dynamics of human serum albumin. J. Mol. Model. 2014, 20, 2450. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Chan, B.; Dodsworth, N.; Woodrow, J.; Tucker, A.; Harris, R. Site-specific N-terminal Auto-degradation of Human Serum Albumin. Eur. J. Biochem. 1995, 227, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Chevion, M. Site-specific modification of albumin by free radicals. Reaction with copper(II) and ascorbate. Biochem. J. 1986, 236, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Quiles, J.; Gaze, D.C.; Collinson, P.; Kaski, J.C.; Baxter, G.F. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart 2006, 92, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Curtis, G.; Rao, N.; Bampos, N.; Lau, E. Characterization of the Co(2+) and Ni(2+) binding amino-acid residues of the N-terminus of human albumin. An insight into the mechanism of a new assay for myocardial ischemia. Eur. J. Biochem. 2001, 268, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.T.; Blindauer, C.A. Allosteric inhibition of cobalt binding to albumin by fatty acids: Implications for the detection of myocardial ischemia. J. Med. Chem. 2012, 55, 4425–4430. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Babu, M.R.; Bitla, A.R. Automation of Ischemia Modified Albumin on Beckman Analyser. Natl. J. Lab. Med. 2017, 6, 9–14. [Google Scholar]
- Mothes, E.; Faller, P. Evidence that the Principal Co II -Binding Site in Human Serum Albumin Is Not at the N-Terminus: Implication on the Albumin Cobalt Binding Test for Detecting Myocardial Ischemia. Biochemistry 2007, 46, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, M.; Wszelaka-Rylik, M.; Poznański, J.; Bal, W. Spectroscopic and thermodynamic determination of three distinct binding sites for Co(II) ions in human serum albumin☆. J. Inorg. Biochem. 2009, 103, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Eom, J.-E.; Lee, E.; Jeon, K.-H.; Sim, J.; Suh, M.; Jhon, G.-J.; Kwon, Y. Development of an Albumin Copper Binding (ACuB) Assay to Detect Ischemia Modified Albumin. Anal. Sci. 2014, 30, 985–990. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.H.; Santos Hausen, B.d.; da Silva, D.B.; Becker, A.M.; Anraku de Campos, M.M.; Medeiros Frescura Duarte, M.M.; Moresco, R.N. Characteristics of a nickel–albumin binding assay for assessment of myocardial ischaemia. Biomarkers 2010, 15, 353–357. [Google Scholar] [CrossRef]
- da Silva, S.H.; da Silva Pereira, R.; dos Santos Hausen, B.; Signor, C.; Gomes, P.; de Campos MM, A.; Moresco, R.N. Assessment of the nickel-albumin binding assay for diagnosis of acute coronary syndrome. Clin. Chem. Lab. Med. 2011, 49, 541–546. [Google Scholar] [CrossRef]
- Hossain, M.; Su, M. Nanoparticle location and material dependent dose enhancement in X-ray radiation therapy. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 23047–23052. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Wang, C.; Su, M. Multiplexed biomarker detection using x-ray fluorescence of composition-encoded nanoparticles. Appl. Phys. Lett. 2010, 97, 263704. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.; Jiang, T.; Zhang, B.; Huang, J.; Liao, P.; Fu, W. Interference-free determination of ischemia-modified albumin using quantum dot coupled X-ray fluorescence spectroscopy. Biosens. Bioelectron. 2014, 51, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.J.; Seo, M.-H.; Kim, H.-S. Insignificant role of the N-terminal cobalt-binding site of albumin in the assessment of acute coronary syndrome: Discrepancy between the albumin cobalt-binding assay and N-terminal-targeted immunoassay. Biomarkers 2012, 17, 394–401. [Google Scholar] [CrossRef]
- Ahmad, A.; Manjrekar, P.; Yadav, C.; Agarwal, A.; Srikantiah, R.M.; Hegde, A. Evaluation of Ischemia-Modified Albumin, Malondialdehyde, and Advanced Oxidative Protein Products as Markers of Vascular Injury in Diabetic Nephropathy. Biomark. Insights 2016, 11, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef]
- Ceriello, A. Acute hyperglycaemia: A “new” risk factor during myocardial infarction. Eur. Heart J. 2005, 26, 328–331. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.-A.; Ul-Malik, R.A.; Kadir, I.T.A.A. Measurement of Adjusted Ischemia-Modified Albumin Marker in Early Pregnancy Loss. Asian J. Pharm. Clin. Res. 2020, 13, 33–36. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Salvagno, G.L.; Guidi, G.C. Standardization of ischemia-modified albumin testing: Adjustment for serum albumin. Clin. Chem. Lab. Med. 2007, 45, 261–262. [Google Scholar] [CrossRef]
- Ding, M.; Li, M.; Yang, H. Clinical diagnostic value of combined detection of IMA, D-D and MCP-1 in acute myocardial infarction. Exp. Ther. Med. 2021, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, T.; Fu, Y.; Wang, Z.; Leeson, M.S.; Jiang, J.; Liu, T. Liquid Crystal Biosensors: Principles, Structure and Applications. Biosensors 2022, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-W.; Hisamoto, H.; Chen, C.-H. Quantitative analysis of liquid crystal-based immunoassay using rectangular capillaries as sensing platform. Opt. Express 2019, 27, 17080–17090. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lei, H.; Luo, S.; Tang, P.; Peng, X.; Wang, X. Liquid crystal biosensor for detecting ischemia modified albumin. Res. Chem. Intermed. 2017, 43, 353–360. [Google Scholar] [CrossRef][Green Version]
- Englebienne, P.; Hoonacker, A.V.; Verhas, M. Surface plasmon resonance: Principles, methods and applications in biomedical sciences. J. Spectrosc. 2003, 17, 255–273. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Yang, M.; Chen, M.-M.; Chen, L.-C.; Xiong, X.-L. A gold nanoparticles enhanced surface plasmon resonance immunosensor for highly sensitive detection of ischemia-modified albumin. Sensors 2013, 13, 12794–12803. [Google Scholar] [CrossRef] [PubMed]


| Method | Principle | Advantages | Limitations |
|---|---|---|---|
| Albumin Cobalt Binding (ACB) Test [29,30,42,43,58,60,69] | This is the most widely used traditional method. It relies on the decreased binding capacity of cobalt to the N-terminus of albumin due to ischemic modifications. | Simple, cost-effective, and can be performed with standard laboratory equipment. | Results can vary due to factors like albumin concentration and the presence of other interfering substances. Sensitivity and specificity can be lower compared to newer methods. |
| Cobalt Albumin Binding (CAB) assay [42] | An improved version of the ACB assay. | This assay reduces the impact of pH fluctuations on the binding of cobalt ions to albumin by optimizing pH conditions and using NaH2PO4 buffer at pH 7.4. The assay requires smaller volumes of specimens and reagents, making it more efficient and cost-effective. | Lack of standard Human Serum Albumin (HSA) as a control. Effect of pH on cobalt-DTT Binding: Although pH optimization has been addressed, cobalt binding to DTT decreases significantly with pH reduction. |
| Albumin Copper Binding (ACuB) assay [63] | It relies on the decreased binding capacity of copper to the N-terminus of albumin due to ischemic modifications. | Cu2+ ions, which have a higher binding affinity for the N-terminus of HSA, enhance the assay’s sensitivity. Lucifer yellow is a copper-selective agent that doesn’t denature protein structures and releases metal ions from binding sites like DTT. Direct fluorescence measurements can be obtained. | Environmental factors such as pH changes and the presence of competing ions could still impact the binding efficiency. Fluorescence measurement requires specific equipment and dyes. |
| Nickel-Albumin Binding assay [64,65] | It relies on the decreased binding capacity of nickel to the N-terminus of albumin due to ischemic modifications. | Using nickel ions, which have a higher affinity for binding to the N-terminus of HSA than cobalt, enhances detection sensitivity. | Using DTT as a colorizing agent can denature protein structures and release metal ions from binding sites, potentially affecting the accuracy and reliability of the assay results. Requires precise control of reaction conditions While Ni2+ ions have a relatively high binding affinity, they are not as strong as Cu2+, which may limit the assay’s sensitivity compared to the Albumin Copper Binding (ACuB) assay. |
| Quantum Dot coupled X-ray Fluorescence Spectroscopy (Q-XFR) [66,67,68] | Primary X-ray irradiates the target element, and that interaction emits a characteristic secondary X-ray, which an X-ray fluorescence detector will detect. This characteristic secondary X-ray is unique to a particular aspect of interest. The XRF peak’s intensity is related to its number of elements. | Higher specificity (95.9%) compared to the ACB assay (82.9%) This method effectively removes interferences from excessively high or low albumin levels by calculating the difference between total HSA and intact HSA levels. Rapid and accurate detection | Specialized X-ray fluorescence spectroscopy equipment is required, and specialized training and expertise are needed to operate it. Complexity of sample preparation |
| Enzyme-Linked Immunosorbent Assay (ELISA) [69,70,72,73,74,75,76] | Specific antibodies bind the target antigen and detect the presence and quantity of antigens binding. | Precise detection of IMA using antibodies, targeting the specific protein modification associated with ischemia. Provides quantitative measurement of IMA levels Adjusting IMA values based on serum albumin levels can minimize false positives and negatives, ensuring more accurate results. | ELISA results may vary based on the timing of sample collection The procedure involves multiple steps. Despite high specificity, there is always a risk of non-specific binding, which can affect the accuracy of the assay results. |
| Liquid crystal biosensor [77,78,79] | Liquid crystals are phase transition metals that exist between liquid and crystal states. They have both liquid and crystal properties. They respond readily to external stimuli such as changes in surface properties (binding specific biomolecules onto the surface), temperature, pressure, and electromagnetic field. These stimuli will alter the orientation of the LC and change the color/brightness of the sensor. | High sensitivity and specificity The method is simple and provides quick results. Changes in the LC orientation result in optical signal changes observable through a polarizing microscope, allowing for immediate visual detection of IMA levels. LC biosensors can detect various biomolecules, including proteins, antibodies, and cells, making them versatile tools for different diagnostic applications. | Need for specialized equipment External stimuli sensitivity must be controlled |
| Surface Plasmon Resonance (SPR) immunosensor for IMA [81] | SPR is an optical-electrical phenomenon that occurs when a photon of incoming light strikes a metal surface. A portion of the light energy interacts with the metal surface, and the electrons in the metal surface move due to excitation. Electron movements are known as plasmons, traveling parallel to the metal surface. The plasmon oscillation, in turn, generates an electric field from the metal surface. This allows for more precise measurements of molecule adsorption on the metal surface and subsequent interactions with specific ligands. | High sensitivity in detecting IMA, particularly when enhanced with colloidal gold nanoparticles Real-time, in situ monitoring of biomolecular interactions Label-free detection Low interference Versatility and biocompatibility Rapid and simple | SPR systems require sophisticated and expensive equipment, which may limit their availability in resource-constrained settings. Specialized training is required to operate the instruments. Over time, the metal surface can become fouled with non-specific proteins or other contaminants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senadeera, N.N.; Ranaweera, C.B.; Perera, I.C.; Kottahachchi, D.U. Biochemical Insights and Clinical Applications of Ischemia-Modified Albumin in Ischemic Conditions. J. Vasc. Dis. 2024, 3, 245-266. https://doi.org/10.3390/jvd3030020
Senadeera NN, Ranaweera CB, Perera IC, Kottahachchi DU. Biochemical Insights and Clinical Applications of Ischemia-Modified Albumin in Ischemic Conditions. Journal of Vascular Diseases. 2024; 3(3):245-266. https://doi.org/10.3390/jvd3030020
Chicago/Turabian StyleSenadeera, Nimesha N., Chathuranga B. Ranaweera, Inoka C. Perera, and Darshana U. Kottahachchi. 2024. "Biochemical Insights and Clinical Applications of Ischemia-Modified Albumin in Ischemic Conditions" Journal of Vascular Diseases 3, no. 3: 245-266. https://doi.org/10.3390/jvd3030020
APA StyleSenadeera, N. N., Ranaweera, C. B., Perera, I. C., & Kottahachchi, D. U. (2024). Biochemical Insights and Clinical Applications of Ischemia-Modified Albumin in Ischemic Conditions. Journal of Vascular Diseases, 3(3), 245-266. https://doi.org/10.3390/jvd3030020





