Bilateral Carotid Calcification Correlates with Regional Cerebral Glucose Metabolism: Insights from PET/CT Imaging of Patients with Cardiovascular Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient Evaluation
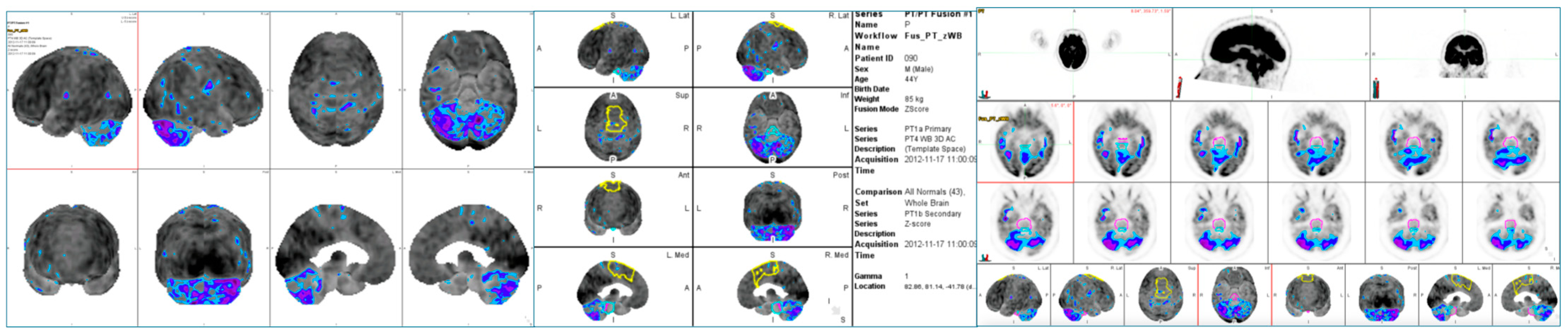
2.3. Brain PET/CT Acquisition Protocol
2.4. Brain PET/CT Data Analysis
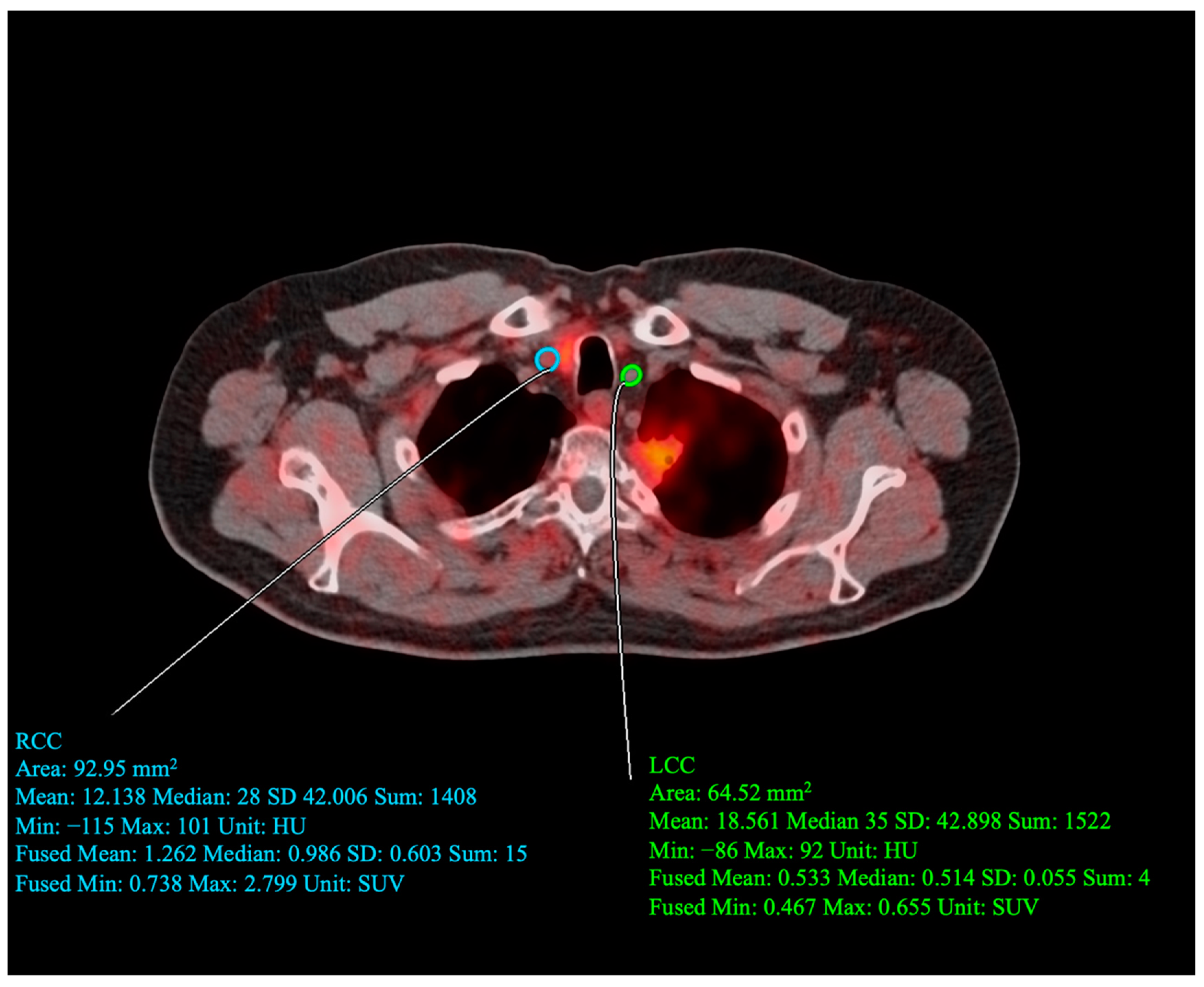
2.5. Carotid PET/CT Acquisition Protocol
2.6. Carotid PET/CT Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Correlations between Brain Metabolic Activity and Cardiovascular Risk
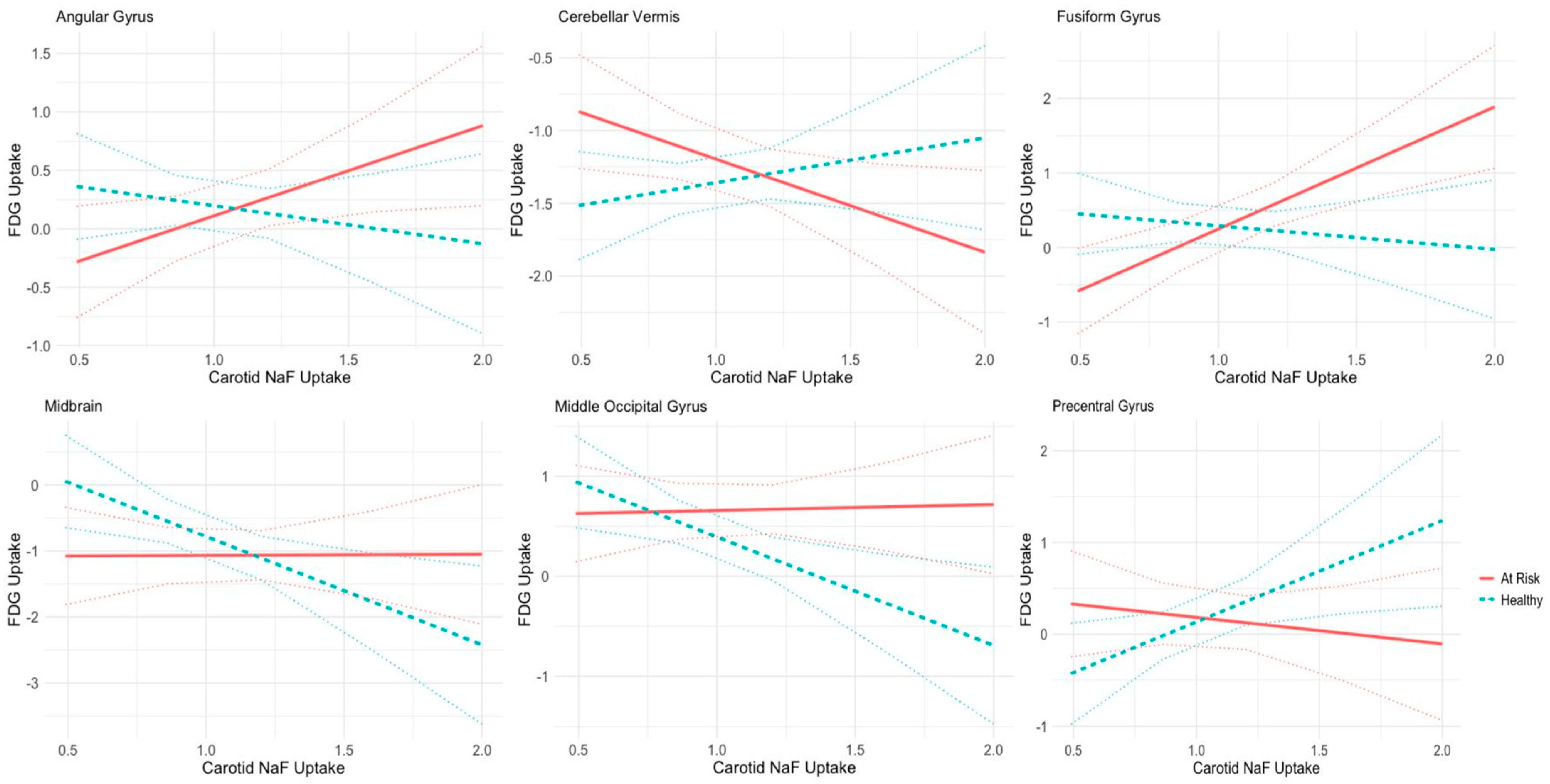
3.3. Correlations between Regional Cerebral Metabolism and Average Bilateral 18F-NaF PET Uptake in Carotid Arteries
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18F-FDG | 18F-fluorodeoxyglucose; |
| A1c | Glycated hemoglobin; |
| AD | Alzheimer’s disease; |
| ACA | Anterior cerebral artery; |
| ACE | Angiotensin-converting enzyme; |
| ARBs | Angiotensin receptor blockers; |
| BMI | Body mass index; |
| CAMONA | Cardiovascular molecular calcification assessed by 18F-NaF PET-CT |
| CHADS-VaSc | Congestive heart failure, hypertension, age, diabetes, stroke/transient ischemic attack, vascular disease, and sex category; |
| CVD | Cardiovascular disease; |
| FDG | Fluorodeoxyglucose; |
| FRS | Framingham risk score; |
| NaF | Sodium fluoride; |
| PET-CT | Positron emission tomography–computed tomography; |
| SCORE | Systematic coronary risk evaluation; |
| SUV | Standard uptake value; |
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 2 May 2023).
- Bytyçi, I.; Shenouda, R.; Wester, P.; Henein, M.Y. Carotid Atherosclerosis in Predicting Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e224–e237. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. Cardiovascular Risk Factors Promote Brain Hypoperfusion Leading to Cognitive Decline and Dementia. Cardiovasc. Psychiatry Neurol. 2012, 2012, 367516. [Google Scholar] [CrossRef]
- Wang, A.; Liu, X.; Chen, G.; Hao, H.; Wang, Y.; Wang, Y. Association between Carotid Plaque and Cognitive Impairment in Chinese Stroke Population: The SOS-Stroke Study. Sci. Rep. 2017, 7, 3066. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease: A clinical review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J. Vascular Cognitive Impairment. Contin. Lifelong Learn. Neurol. 2019, 25, 147. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Teune, L.K.; Bartels, A.L.; de Jong, B.M.; Willemsen, A.T.; Eshuis, S.A.; de Vries, J.J.; van Oostrom, J.C.; Leenders, K.L. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov. Disord. 2010, 25, 2395–2404. [Google Scholar] [CrossRef]
- Marcus, C.; Mena, E.; Subramaniam, R.M. Brain PET in the Diagnosis of Alzheimer’s Disease. Clin. Nucl. Med. 2014, 39, e413–e426. [Google Scholar] [CrossRef]
- Minoshima, S.; Cross, D.; Thientunyakit, T.; Foster, N.L.; Drzezga, A. 18F-FDG PET Imaging in Neurodegenerative Dementing Disorders: Insights into Subtype Classification, Emerging Disease Categories, and Mixed Dementia with Copathologies. J. Nucl. Med. 2022, 63 (Suppl. 1), 2S–12S. [Google Scholar] [CrossRef]
- Mayer, M.; Borja, A.J.; Hancin, E.C.; Auslander, T.; Revheim, M.E.; Moghbel, M.C.; Werner, T.J.; Alavi, A.; Rajapakse, C.S. Imaging Atherosclerosis by PET, with Emphasis on the Role of FDG and NaF as Potential Biomarkers for This Disorder. Front. Physiol. 2020, 11, 511391. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2020.511391 (accessed on 14 May 2023). [CrossRef]
- McKenney-Drake, M.L.; Moghbel, M.C.; Paydary, K.; Alloosh, M.; Houshmand, S.; Moe, S.; Salavati, A.; Sturek, J.M.; Territo, P.R.; Weaver, C.; et al. 18F-NaF and 18F-FDG as molecular probes in the evaluation of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2190–2200. [Google Scholar] [CrossRef]
- Rojulpote, C.; Patil, S.; Gonuguntla, K.; Karambelkar, P.; Bravo, P.E.; Seraj, S.M.; Asadollahi, S.; Raynor, W.Y.; Bhattaru, A.; Borja, A.J.; et al. NaF-PET/CT global assessment in detecting and quantifying subclinical cardiac atherosclerosis and its association with blood pressure in non-dyslipidemic individuals. Am. J. Cardiovasc. Dis. 2020, 10, 101–107. [Google Scholar] [PubMed]
- Teichner, E.M.; You, J.C.; Hriso, C.; Wintering, N.A.; Zabrecky, G.P.; Alavi, A.; Bazzan, A.J.; Monti, D.A.; Newberg, A.B. Alterations in cerebral glucose metabolism as measured by 18F-fluorodeoxyglucose-PET in patients with persistent postconcussion syndrome. Nucl. Med. Commun. 2021, 42, 772–781. [Google Scholar] [CrossRef]
- Partovi, S.; Yuh, R.; Pirozzi, S.; Lu, Z.; Couturier, S.; Grosse, U.; Schluchter, M.D.; Nelson, A.; Jones, R.; O’Donnell, J.K.; et al. Diagnostic performance of an automated analysis software for the diagnosis of Alzheimer’s dementia with 18F FDG PET. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 12–23, Published 15 January 2017. [Google Scholar]
- Blomberg, B.A.; Thomassen, A.; Takx, R.A.P.; Hildebrandt, M.G.; Simonsen, J.A.; Buch-Olsen, K.M.; Diederichsen, A.C.; Mickley, H.; Alavi, A.; Høilund-Carlsen, P.F. Delayed 18F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: Results from the CAMONA study. J. Nucl. Cardiol. 2014, 21, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.A.; de Jong, P.A.; Thomassen, A.; Lam, M.G.; Vach, W.; Olsen, M.H.; Mali, W.P.; Narula, J.; Alavi, A.; Høilund-Carlsen, P.F. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: Results of the CAMONA study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. J. Prev. Cardiol. 2022, 75, 429. [Google Scholar] [CrossRef]
- Williams, P.L. Gray’s Anatomy; Churchill Livingstone: Edinburgh, UK, 1980. [Google Scholar]
- Rehman, A.; Al Khalili, Y. Neuroanatomy, Occipital Lobe. [Updated 24 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544320 (accessed on 28 July 2023).
- Kuybu, O.; Tadi, P.; Dossani, R.H. Posterior Cerebral Artery Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Marinković, S.V.; Milisavljević, M.M.; Vucković, V.D. Microvascular anatomy of the uncus and the parahippocampal gyrus. Neurosurgery 1991, 29, 805–814. [Google Scholar] [CrossRef]
- Rahman, M.; Tadi, P. Neuroanatomy, Pons. [Updated 30 January 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560589/ (accessed on 2 August 2023).
- DiGuiseppi, J.; Tadi, P. Neuroanatomy, Postcentral Gyrus. [Updated 24 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549825/ (accessed on 8 August 2023).
- Nestor, P.G.; Nakamura, M.; Niznikiewicz, M.; Thompson, E.; Levitt, J.J.; Choate, V.; Shenton, M.E.; McCarley, R.W. In search of the functional neuroanatomy of sociality: MRI subdivisions of orbital frontal cortex and social cognition. Soc. Cogn. Affect. Neurosci. 2013, 8, 460–467. [Google Scholar] [CrossRef]
- Sutoko, S.; Atsumori, H.; Obata, A.; Funane, T.; Kandori, A.; Shimonaga, K.; Hama, S.; Yamawaki, S.; Tsuji, T. Lesions in the right Rolandic operculum are associated with self-rating affective and apathetic depressive symptoms for post-stroke patients. Sci. Rep. 2020, 10, 20264. [Google Scholar] [CrossRef]
- Kim, M.S.; Tak, H.J.; Son, S.M. Recovery of cerebellar peduncle injury in a patient with a cerebellar tumor: Validation by diffusion tensor tractography. Neural. Regen. Res. 2014, 9, 1929–1932. [Google Scholar] [CrossRef]
- Ishibashi, K.; Onishi, A.; Fujiwara, Y.; Oda, K.; Ishiwata, K.; Ishii, K. Longitudinal effects of aging on 18F-FDG distribution in cognitively normal elderly individuals. Sci. Rep. 2018, 8, 11557. [Google Scholar] [CrossRef]
- Terribilli, D.; Schaufelberger, M.S.; Duran, F.L.; Zanetti, M.V.; Curiati, P.K.; Menezes, P.R.; Scazufca, M.; Amaro, E., Jr. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol. Aging 2011, 32, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, M.; Doron, K. Cerebellum: Connections and Functions. Cerebellum 2008, 7, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S. Compensatory Mechanisms in the Aging Motor System. Ageing Res. Rev. 2006, 5, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Northington, F.J.; Chavez-Valdez, R.; Martin, L.J. Neuronal Cell Death in Neonatal Hypoxia-Ischemia. Ann. Neurol. 2011, 69, 743–758. [Google Scholar] [CrossRef]
- ElAli, A.; Thériault, P.; Préfontaine, P.; Rivest, S. Mild chronic cerebral hypoperfusion induces neurovascular dysfunction, triggering peripheral beta-amyloid brain entry and aggregation. Acta Neuropathol. Commun. 2013, 1, 75. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef]
- Machulda, M.M.; Hagen, C.E.; Wiste, H.J.; Mielke, M.M.; Knopman, D.S.; Roberts, R.O.; Vemuri, P.; Lowe, V.J.; Jack, C.R., Jr.; Petersen, R.C. Practice effects and longitudinal cognitive change in clinically normal older adults differ by Alzheimer imaging biomarker status. Clin. Neuropsychol. 2017, 31, 99–117. [Google Scholar] [CrossRef]
- Costantini, L.C.; Barr, L.J.; Vogel, J.L.; Henderson, S.T. Hypometabolism as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008, 9, S16. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Asthana, S.; Schellenberg, G.; Baker, L.; Cherrier, M.; Boyt, A.A.; Martins, R.N.; Raskind, M.; Peskind, E.; Plymate, S. Insulin Effects on Glucose Metabolism, Memory, and Plasma Amyloid Precursor Protein in Alzheimer’s Disease Differ According to Apolipoprotein-E Genotype. Ann. N. Y. Acad. Sci. 2000, 903, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Xu, H.; Dintica, C.S.; Pan, K.Y.; Qi, X.; Buchman, A.S.; Bennett, D.A.; Xu, W. Associations Between Cardiovascular Risk, Structural Brain Changes, and Cognitive Decline. J. Am. Coll. Cardiol. 2020, 75, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Song, R.; Xu, H.; Shang, Y.; Qi, X.; Buchman, A.S.; Bennett, D.A.; Xu, W. Association of Cardiovascular Risk Burden with Risk and Progression of Disability: Mediating Role of Cardiovascular Disease and Cognitive Decline. J. Am. Heart Assoc. 2020, 9, e017346. Available online: https://www.ahajournals.org/doi/full/10.1161/JAHA.120.017346?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org (accessed on 2 May 2023). [CrossRef] [PubMed]
- Wang, Z.; Cui, K.; Song, R.; Li, X.; Qi, X.; Buchman, A.S.; Bennett, D.A.; Xu, W. Influence of Cardiovascular Risk Burden on Motor Function Among Older Adults: Mediating Role of Cardiovascular Diseases Accumulation and Cognitive Decline. Front. Med. 2022, 9, 856260. Available online: https://www.frontiersin.org/articles/10.3389/fmed.2022.856260 (accessed on 2 May 2023). [CrossRef] [PubMed]
- Di Fusco, S.A.; Arca, M.; Scicchitano, P.; Alonzo, A.; Perone, F.; Gulizia, M.M.; Gabrielli, D.; Oliva, F.; Imperoli, G.; Colivicchi, F. Lipoprotein(a): A risk factor for atherosclerosis and an emerging therapeutic target. Heart 2023, 109, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Qizilbash, N.; Gregson, J.; Douglas, I.; Johnson, M.; Pearce, N.; Evans, S.; Pocock, S. Blood cholesterol and risk of dementia in more than 1·8 million people over two decades: A retrospective cohort study. Lancet Healthy Longev. 2021, 2, e498–e506. [Google Scholar] [CrossRef]
- Varamini, B.; Sikalidis, A.K.; Bradford, K.L. Resveratrol increases cerebral glycogen synthase kinase phosphorylation as well as protein levels of drebrin and transthyretin in mice: An exploratory study. Int. J. Food Sci. Nutr. 2014, 65, 89–96. [Google Scholar] [CrossRef]
- Debette, S.; Seshadri, S.; Beiser, A.; Au, R.; Himali, J.J.; Palumbo, C.; Wolf, P.A.; DeCarli, C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011, 77, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Wendell, C.R.; Waldstein, S.R.; Ferrucci, L.; O’Brien, R.J.; Strait, J.B.; Zonderman, A.B. Carotid Atherosclerosis and Prospective Risk of Dementia. Stroke 2012, 43, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, X.; Moeini, M.; Sakadžić, S.; Thorin, E.; Lesage, F. Atherosclerosis is associated with a decrease in cerebral microvascular blood flow and tissue oxygenation. PLoS ONE 2019, 14, e0221547. [Google Scholar] [CrossRef]
- Aparicio, H.J.; Petrea, R.E.; Massaro, J.M.; Manning, W.J.; Oyama-Manabe, N.; Beiser, A.S.; Kase, C.S.; D’Agostino, R.B.; Wolf, P.A.; Vasan, R.S.; et al. Association of descending thoracic aortic plaque with brain atrophy and white matter hyperintensities: The Framingham Heart Study. Atherosclerosis 2017, 265, 305–311. [Google Scholar] [CrossRef]
- Schmidt, R.; Seiler, S.; Loitfelder, M. Longitudinal change of small-vessel disease-related brain abnormalities. J. Cereb. Blood Flow Metab. 2016, 36, 26–39. [Google Scholar] [CrossRef]
- van Dijk, E.J.; Prins, N.D.; Vrooman, H.A.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 2008, 39, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
| Healthy | At-Risk | p-Value | |
|---|---|---|---|
| Count | n = 66 | n = 38 | |
| Demographics | |||
| Female, n (%) | 32 (48.4) | 19 (50.0) | 1.0 |
| Age, years | 43.9 ± 13.5 | 56.1 ± 11.8 | <0.01 |
| Body mass index | 26.5 ± 4.1 | 26.8 ± 4.4 | 0.32 |
| Comorbidities | |||
| Smoking history, n (%) | 33 (50.0) | 18 (47.3) | 0.84 |
| Family history of coronary artery disease, n (%) | 16 (24.2) | 9 (23.6) | 1.0 |
| Arterial hypertension, n (%) | 30 (45.4) | 16 (42.1) | 0.83 |
| Hypercholesterolemia, n (%) | 13 (19.6) | 5 (13.1) | 0.43 |
| Hypertriglyceridemia, n (%) | 10 (15.1) | 5 (13.1) | 1.0 |
| Atrial fibrillation, n (%) | 8 (12.1) | 1 (2.6) | 0.15 |
| Heart valve disease, n (%) | 4 (6.0) | 1 (2.6) | 0.65 |
| Peripheral artery disease, n (%) | 3 (4.5) | 1 (2.6) | 1.0 |
| History of cerebrovascular disease, n (%) | 2 (3.0) | 0 (0.0) | 0.53 |
| Laboratory tests | |||
| Total cholesterol, mmol/L | 5.2 ± 0.9 | 4.9 ± 0.8 | 0.08 |
| HDL cholesterol, mmol/L | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.56 |
| LDL cholesterol, mmol/L | 3.2 ± 0.8 | 3.0 ± 0.7 | 0.22 |
| Triglycerides, mmol/L | 1.1 ± 0.6 | 1.1 ± 0.8 | 0.22 |
| HbA1c, mmol/L | 35.2 ± 4.2 | 35.3 ± 6.0 | 0.46 |
| C-reactive protein, mg/L | 2.5 ± 3.1 | 2.5 ± 4.1 | 0.89 |
| White blood cell count, 109 cells/L | 6.0 ± 2.0 | 6.4 ± 2.1 | 0.07 |
| Fibrinogen, μmol/L | 9.4 ± 1.8 | 11.4 ± 11.8 | 0.37 |
| Creatinine, μmol/L | 79.0 ± 18.3 | 81.5 ± 13.9 | 0.13 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 81.4 ± 15.6 | 80.3 ± 13.2 | 0.63 |
| Medications | |||
| Aspirin, n (%) | 8 (12.1) | 1 (2.6) | 0.15 |
| Beta blockers, n (%) | 7 (10.6) | 2 (5.2) | 0.48 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, n (%) | 11 (16.6) | 2 (5.2) | 0.12 |
| Lipid-lowering medication, n (%) | 10 (15.1) | 4 (10.5) | 0.56 |
| Risk profile | |||
| 10-year Framingham risk, % (25–75th percentile) | 3.9 (1.6–8.2) | 8.5 (5.8–16.8) | <0.01 |
| CHA2DS2-VASc score, % (25–75th percentile) | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 0.58 |
| Level of physical activity, % (25–75th percentile) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 0.99 |
| Chronic Kidney Disease: no data; Diabetes: all negative | |||
| Cardiovascular Risk (FRS) | Age | |||||
|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |
| Angular Gyrus | −0.014 | 0.011 | 0.221 | −0.015 | 0.006 | 0.018 |
| Basis Pontis | 0.025 | 0.012 | 0.038 | 0.029 | 0.006 | <0.001 |
| Cerebellar Vermis | 0.011 | 0.009 | 0.217 | 0.014 | 0.005 | 0.007 |
| Cuneus | −0.030 | 0.014 | 0.035 | 0.023 | 0.008 | 0.004 |
| Fusiform Gyrus | 0.019 | 0.013 | 0.165 | 0.024 | 0.007 | 0.001 |
| Midbrain | 0.001 | 0.017 | 0.919 | 0.044 | 0.010 | <0.001 |
| Middle Occipital Gyrus | −0.032 | 0.011 | 0.005 | 0.015 | 0.006 | 0.021 |
| Parahippocampal Gyrus | −0.034 | 0.014 | 0.020 | 0.035 | 0.008 | <0.001 |
| Pons | 0.034 | 0.013 | 0.008 | 0.022 | 0.007 | 0.002 |
| Pontine Tegmentum | 0.053 | 0.015 | <0.001 | 0.013 | 0.008 | 0.129 |
| Postcentral Gyrus | 0.028 | 0.012 | 0.022 | −0.006 | 0.006 | 0.361 |
| Posterior Cingulate Gyrus | −0.032 | 0.015 | 0.044 | 0.006 | 0.008 | 0.463 |
| Posterior Orbital Gyrus | 0.038 | 0.013 | 0.003 | −0.028 | 0.007 | <0.001 |
| Precentral Gyrus | 0.015 | 0.014 | 0.278 | −0.023 | 0.007 | 0.002 |
| Precuneus | −0.036 | 0.014 | 0.014 | 0.013 | 0.008 | 0.101 |
| Primary Visual Cortex | −0.025 | 0.012 | 0.037 | 0.025 | 0.006 | <0.001 |
| Rolandic Operculum | 0.031 | 0.014 | 0.031 | −0.010 | 0.008 | 0.189 |
| Superior Cerebellar Peduncle | 0.031 | 0.011 | 0.005 | −0.002 | 0.006 | 0.745 |
| Superior Occipital Gyrus | −0.038 | 0.013 | 0.004 | 0.035 | 0.007 | <0.001 |
| Carotid Uptake | Health Status Interaction Effects | Laterality Interaction Effects | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | β | SE | p-Value | |
| Angular Gyrus | 0.571 | 0.414 | 0.169 | −1.09 | 0.512 | 0.033 | 0.401 | 0.506 | 0.428 |
| Cerebellar Hemisphere | −0.818 | 0.486 | 0.023 | 0.661 | 0.442 | 0.136 | −0.033 | 0.437 | 0.938 |
| Cerebellar Vermis | −0.584 | 0.339 | 0.086 | 0.947 | 0.420 | 0.025 | −0.110 | 0.415 | 0.789 |
| Cerebellum | −0.825 | 0.354 | 0.021 | 0.747 | 0.438 | 0.090 | −0.045 | 0.433 | 0.916 |
| Cingulate Gyrus | −1.232 | 0.540 | 0.023 | 0.133 | 0.669 | 0.841 | 0.378 | 0.661 | 0.567 |
| Fusiform Gyrus | 1.660 | 0.498 | 0.001 | −1.954 | 0.617 | 0.001 | −0.045 | 0.609 | 0.941 |
| Globus Pallidus | 1.505 | 0.571 | 0.009 | −0.803 | 0.706 | 0.256 | −0.449 | 0.698 | 0.520 |
| Medulla | −0.888 | 0.405 | 0.029 | 0.524 | 0.501 | 0.297 | 0.381 | 0.495 | 0.442 |
| Midbrain | −0.009 | 0.641 | 0.987 | −1.657 | 0.793 | 0.037 | 0.052 | 0.783 | 0.946 |
| Middle Occipital Gyrus | 0.068 | 0.419 | 0.871 | −1.142 | 0.519 | 0.028 | −0.016 | 0.512 | 0.973 |
| Posterior Cingulate Gyrus | −1.253 | 0.567 | 0.028 | −0.927 | 0.702 | 0.188 | 0.412 | 0.694 | 0.552 |
| Precentral Gyrus | −0.234 | 0.501 | 0.640 | 1.393 | 0.620 | 0.025 | −0.109 | 0.613 | 0.859 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teichner, E.M.; Subtirelu, R.C.; Patil, S.; Al-Daoud, O.; Parikh, C.; Nguyen, L.; Atary, J.; Newberg, A.; Høilund-Carlsen, P.F.; Alavi, A. Bilateral Carotid Calcification Correlates with Regional Cerebral Glucose Metabolism: Insights from PET/CT Imaging of Patients with Cardiovascular Risk Factors. J. Vasc. Dis. 2024, 3, 112-126. https://doi.org/10.3390/jvd3020010
Teichner EM, Subtirelu RC, Patil S, Al-Daoud O, Parikh C, Nguyen L, Atary J, Newberg A, Høilund-Carlsen PF, Alavi A. Bilateral Carotid Calcification Correlates with Regional Cerebral Glucose Metabolism: Insights from PET/CT Imaging of Patients with Cardiovascular Risk Factors. Journal of Vascular Diseases. 2024; 3(2):112-126. https://doi.org/10.3390/jvd3020010
Chicago/Turabian StyleTeichner, Eric M., Robert C. Subtirelu, Shiv Patil, Omar Al-Daoud, Chitra Parikh, Linh Nguyen, Jordan Atary, Andrew Newberg, Poul Flemming Høilund-Carlsen, and Abass Alavi. 2024. "Bilateral Carotid Calcification Correlates with Regional Cerebral Glucose Metabolism: Insights from PET/CT Imaging of Patients with Cardiovascular Risk Factors" Journal of Vascular Diseases 3, no. 2: 112-126. https://doi.org/10.3390/jvd3020010
APA StyleTeichner, E. M., Subtirelu, R. C., Patil, S., Al-Daoud, O., Parikh, C., Nguyen, L., Atary, J., Newberg, A., Høilund-Carlsen, P. F., & Alavi, A. (2024). Bilateral Carotid Calcification Correlates with Regional Cerebral Glucose Metabolism: Insights from PET/CT Imaging of Patients with Cardiovascular Risk Factors. Journal of Vascular Diseases, 3(2), 112-126. https://doi.org/10.3390/jvd3020010





