1. Introduction
The assessment of aortic elastic properties stands at the intersection of cardiovascular research, diagnosis, and risk stratification. The aorta, a vital conduit of the circulatory system, is heavily burdened in maintaining pulsatile blood flow, buffering cardiac pulsations, and reducing afterload on the left ventricle. During each cardiac cycle, the left ventricle (LV) ejects blood into the aorta, generating a high-pressure wave. However, the pulse wave is not directly transmitted to the smaller vasculature but is initially transformed in the aorta. The elastic properties of the large arteries enable the expansion of the vessel in order to accommodate the stroke volume and attenuate e the high-pressure pulsations. This aortic distention protects the smaller arteries and capillaries from the pulsatile pressure that may injure their fragile structure.
Therefore, disturbances in aortic elastic properties, characterized by stiffness, compliance, and distensibility changes, have been considered as important factors in the pathogenesis of numerous cardiovascular pathologies [
1,
2]. These comprise various disorders such as arterial hypertension, atherosclerosis, acute and chronic aortic syndromes, and valvular heart diseases [
1]. If the aortic elasticity is compromised, it loses its ability to diminish the high pulsatile pressure developed in the left ventricle, therefore exposing the cardiovascular system to heightened stress.
As we delve into the intricate dynamics of arterial mechanics, we were interested in unraveling the significance of aortic elastic properties in cardiovascular risk stratification.
In the recent two decades, considerable advancements in imaging techniques and measurement tools have refined our understanding of aortic elastic properties. However, translating these findings into clinical practice remains a complex challenge. The intricate interplay between genetics, aging, atherosclerosis, hypertension, and other cardiovascular risk factors complicates the assessment of aortic elasticity. Yet, it is precisely within these challenges that opportunities for improved risk stratification emerge [
3]. The impairment in aortic elasticity may potentially serve as an early warning system, offering clinicians and researchers the ability to discern the subtle signs of impending cardiovascular issues.
While assessing aortic elastic properties offers valuable insights into cardiovascular risk, its integration into routine clinical practice necessitates overcoming several challenges. These include standardizing measurement techniques, establishing normative values across diverse populations, and determining the clinical relevance of altered aortic elasticity [
4].
This is a narrative review that endeavors to carry out the following: (a) Provide an overview of the fundamental concepts related to aortic elastic properties. (b) Explore the evolving methodologies for assessing aortic stiffness and compliance. (c) Discuss the clinical implications of altered aortic elasticity in cardiovascular risk stratification, based on the published data. By comprehensively examining the published scientific papers, we seek to bridge the gap between research and clinical utility, offering clinicians and researchers a deeper understanding of aortic elastic properties and their significance in cardiovascular health and risk assessment.
2. Searching Strategy
Two independent reviewers (N.M. and Ts.V.) systematically searched PubMed, MEDLINE, and SCOPUS.
Studies were included in this narrative review according to the following eligibility criteria: (1) studies comprising of patients with any form of cardiovascular disease, hypertension, atherosclerosis, or cerebrovascular disease and (2) studies valuating aortic elastic properties, including aortic compliance, stiffness, or distensibility.
The main search was performed with the following Medical Subject Headings (Mesh) and free-text terms:
(“Aortic Elastic Properties” OR “Aortic Stiffness” OR “Aortic Compliance” OR “Aortic Distensibility”) AND (“Cardiovascular Risk” OR “Cardiovascular Disease” OR “Atherosclerosis” OR “Hypertension”] OR/AND (“Coronary Artery Disease”) AND (“Cardiovascular Risk Stratification” OR “Risk Assessment” OR “Risk Factors”). We conducted a modified narrative review according to recent recommendations [
5]. The search was performed before 20 September 2023, and all relevant data were derived from research articles, reviews, case reports, etc. In
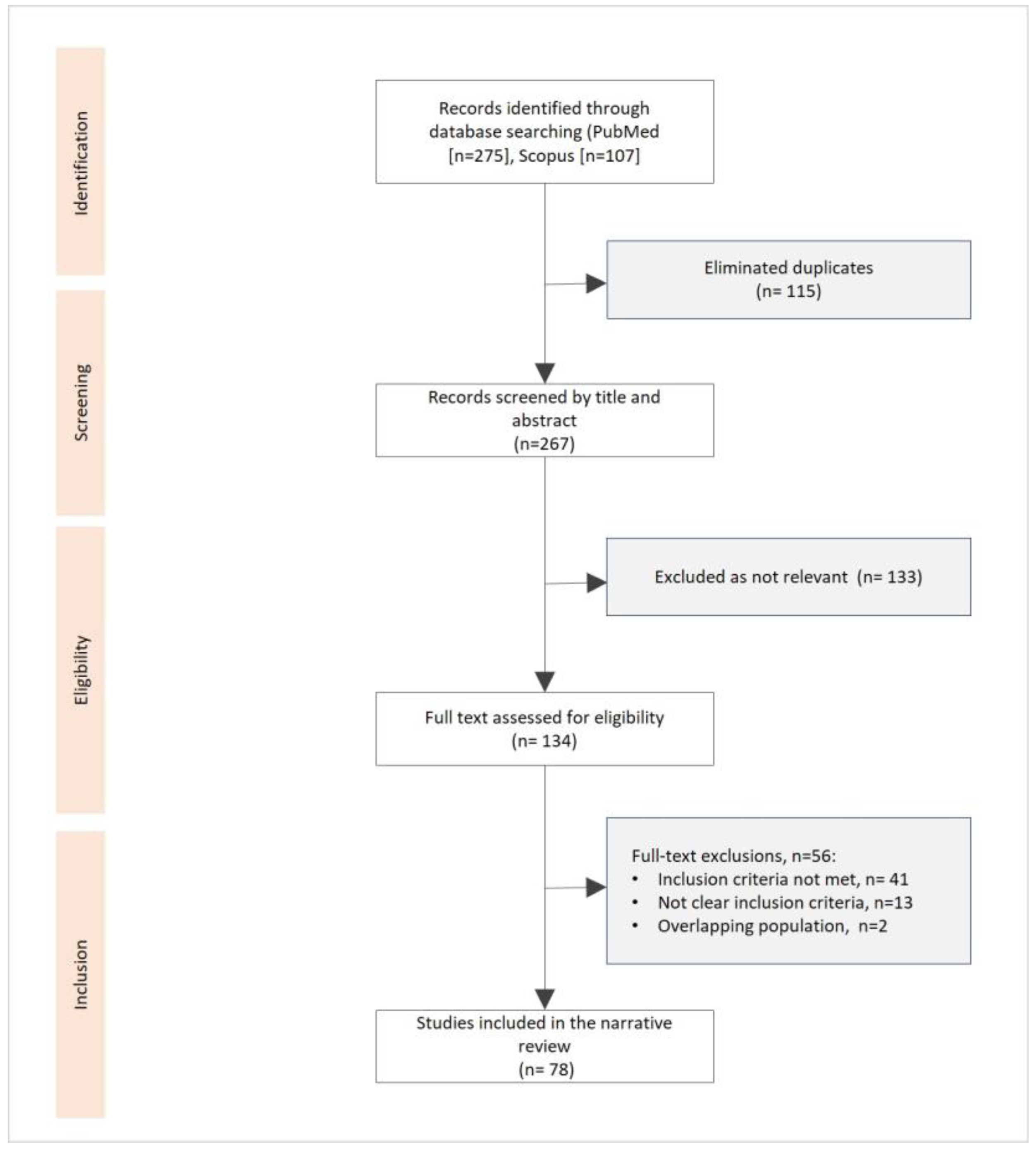
Figure 1, we present a flowchart for the studies identified, screened, evaluated, and included in our narrative review.
3. Physiology of Large Arteries
One of the main characteristics of the elastic arteries is that their medial layer is rich in collagen and elastin filaments. This quality enables the stretch in response to ventricular contractions [
6]. It has been revealed that the thoracic aorta contains up to 40% elastic filaments. However, the amount of elastin decreases with the reduction of the vessel size in peripheral circulation [
7]. Reduced elasticity has been proved in the segments of the aorta from the proximal to the distal vessel parts [
8]. The elastic arteries during systole store almost half of the LV stroke volume. In contrast, in diastole, the aortic wall elastic forces release this residual volume to the periphery vessels continuously, ensuring a stable peripheral circulation. This hemodynamic process has been described as the Windkessel effect [
9]. During LV systole, the heart ejects about 60–100 mL of blood into the aorta and arteries, with relatively 50% of the stroke volume being forwarded directly to peripheral circulation. However, the aorta acts as an elastic reservoir, storing the other 50% of the stroke volume [
7]. With the fall in aortic pressure in diastole, the aorta recoils gradually, and the stored blood volume is ejected into peripheral circulation. Therefore, pressure and blood flow are maintained throughout diastole, and a relatively continuous peripheral flow is provided despite the pulsatile myocardial contractions. Thus, the elastic properties of the large arteries enable the sustained pressure in the arteries despite the alternating left ventricular ejection and the pulsatile blood flow. Apart from peripheral circulation, this physiologic process also affects the heart, by reducing the LV afterload, which leads to an improvement in coronary blood flow and left ventricular relaxation [
10]. One other mechanism necessary for maintaining adequate diastolic pressure is that, usually, in a healthy organism, the pulse wave velocity in the large arteries is rather slow. The reflection of this signal in peripheral circulation, and the return of the wave to the ascending aorta forms the dicrotic wave during early diastole [
11].
The Windkessel effect depends on the elastic properties of the aorta [
9]. The physics definition of elastic materials are substances that readopt their initial shape after the application of an external force. During a myocardial contraction, the ejected stroke volume kinetic energy is first converted into potential energy within the ballooned aortic wall [
9]. Afterwards, the stored potential energy is transformed into kinetic energy during diastole when the aorta gradually recoils [
10]. Therefore, despite the diastolic cessation of the heart’s contraction, the amount of blood within the peripheral arteries does not reach a diastolic halt, and blood pressure does not decrease to null.
4. Concept of Elasticity, Stiffness, and Compliance
The aortic elastic properties include both the ability to distend due to the increased pressure and the ability to recoil gradually to the initial vessel size as the blood pressure reduces in diastole. The vessels’ elastic resistance (elastance) explains the resistance in which the large arteries push against their distention in a situation where an additional volume is ejected and when the intraarterial pressure rises.
Furthermore, the elastic modulus (Young’s modulus), E, is used in physics to define the elastic resistance of materials [
12]. E is quantified as the association between the pressure applied and the distension achieved. To determine E of the aorta, the thickness of the arterial wall needs to be considered. In humans, we can derive the aortic E’ from the absolute change in pulse pressure, calculated as the difference between systolic and diastolic blood pressure (AP), linked to the related alterations in volume (AV): E’ = AP/AV [
6]. Compliance (C) can be considered as the volume distensibility of a vessel, and is the reciprocal of elastic resistance: C = 1/E’ = AV/AP. The elasticity of the vessel wall is impaired when the intravascular pressure is increased and/or when the arterial stiffness intensifies with the aging of the individual [
13].
5. Degenerative Changes in Large Arteries
A change in the aortic elastic properties is registered with human aging [
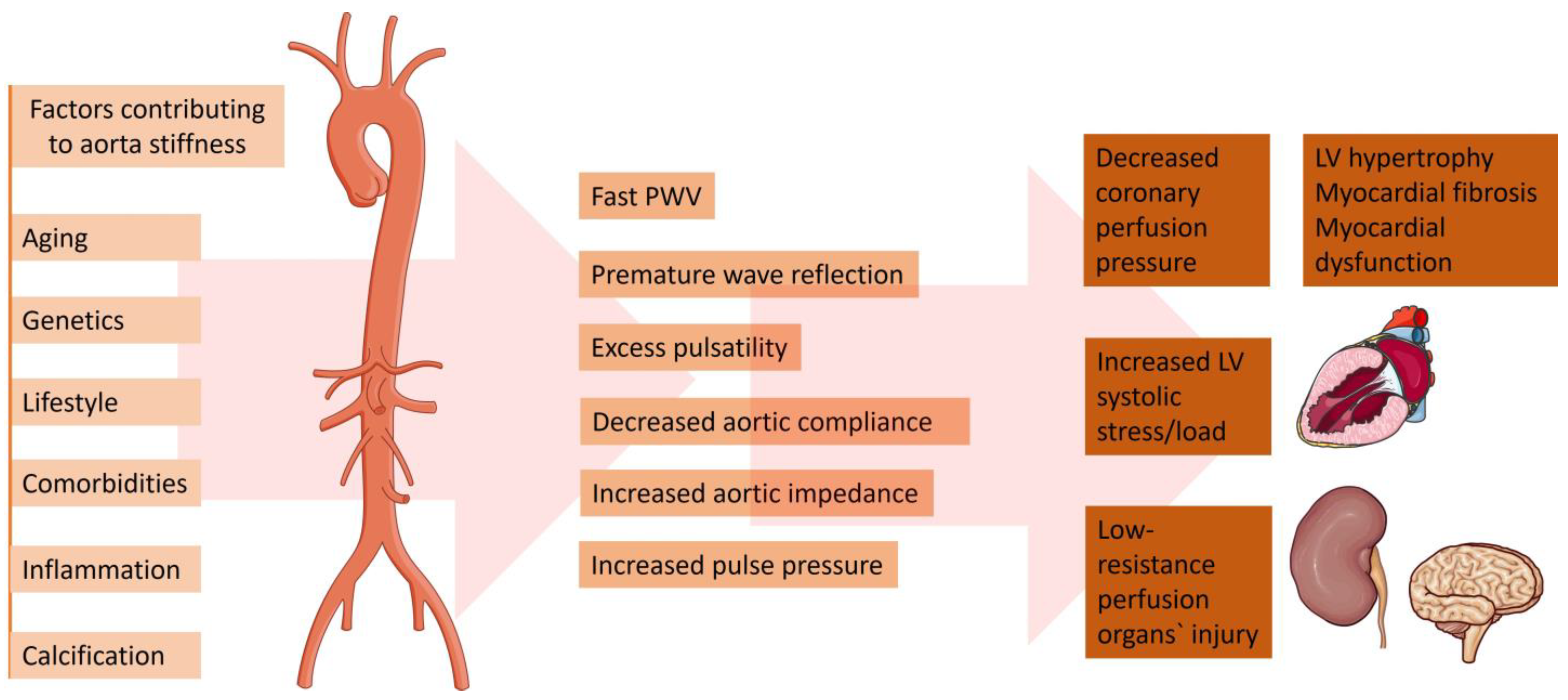
14]. Being the largest artery in the organism, the aorta is the most susceptible to pathological stiffening after being cumulative exposed to CV risk factors (
Figure 2). The ascending aorta has been proven to be the most elastic segment of the vessel. Therefore, it displays the earliest alterations with aging [
15]. The main determinant for the increasing aortic stiffness is the medial layer of the aortic wall [
16]. This vascular damage provokes vessel wall inflammation, followed by elastin degeneration and collagen deposition. Genetics play a significant role in the reduction of aortic elasticity. Previously published data revealed that gene modifications in the matrix metalloprotein-9 gene are independent predictors of reduced aortic elasticity [
17]. Furthermore, inherited elastic tissue diseases, such as Marfan and Ehlers–Danlos syndrome, are related to increased aortic wall stiffness from early age [
18]. On the other hand, there is an abundance of data demonstrating the relation between diabetes and vessel stiffness [
19,
20]. Patients with reduced kidney function are characterized with dysregulated mineral metabolism. An enhanced inflammatory biomarkers’ synthesis—such as C-reactive protein, tumor necrosis factor (TNF)α, and interleukin (IL-)6—has been registered [
21]. Moreover, patients with chronic kidney disease are more prone to electrolyte disbalance, which could lead to increased arterial stress [
22]. On the other hand, in the case of chronic aortic regurgitation, arterial compliance and distensibility are aggravated. The latter phenomenon is probably a result of a compensatory mechanism to counteract the extensive stroke of the heart [
23]. The absence of elevated arterial compliance is linked to rapid hemodynamic deterioration and disease progression [
24].
6. Methods for Evaluation Arterial Elastic Properties
Several different methods to assess arterial compliance have been documented [
25,
26,
27,
28].
6.1. Pulse Wave Velocity
Evaluation of arterial pulsed wave velocity is one of the most widespread approaches for the noninvasive evaluation of arterial stiffness. Oscillometric pulse wave velocity (o-PWV) emerges as an attractive, operator-independent, and non-invasive technique for assessing arterial stiffness. It leverages the measurement of arterial pressure oscillations to offer valuable insights into arterial rigidity, contributing a vital component to cardiovascular risk assessment.
After it has been proven as a simple and accurate method, to predict adverse CV outcomes [
29,
30,
31], the latest clinical recommendations have included the measurement of PWV as the gold standard method for screening and CV risk stratification in patients with arterial hypertension [
32]. Pulse wave velocity may be obtained by acquiring the transit time of the pulse from the pressure waveforms at two different sites of a current vascular segment. Carotid–femoral pulse wave velocity (CFPWV) represents a global estimate of the arterial stiffness through the entire aorta and, as such, it is a currently widely accepted method for evaluating aortic elasticity [
33]. This method is easily applied because of the superficial site of the common carotid and femoral arteries. However, the measurement of arterial pulse waveforms may be technically cumbersome in patients who are overweight. Another technique for assessing arterial elasticity is the cardiac–ankle vascular index (CAVI). To determine the CAVI, phonocardiography data together with brachial and ankle pulse waveforms are required [
34]. The heart-to-ankle transit time is determined as the period between the pulse onset at the heart and the upstroke of the ankle pulse waveform. A probable pitfall is the insertion of a long muscular arterial segment (femoral to ankle), which may lead to inaccuracies. Although easily applicable and reproducible, the method of PWV is a surrogate marker, which integrates an estimate of aortic elasticity along the entire vessel length. Therefore, it does not offer data on segmental aortic stiffness. Previous data have revealed that separate vessel segments have different compliance and do not stiffen uniformly. Hence, the difference in the elastic properties of the distinct vessel segments may have an important impact on the overall cardiovascular risk [
35].
6.2. Echocardiography
Transthoracic echocardiography is an omnipresent method, widely applicable in current clinical practice. Aortic elasticity may be determined by obtaining vessel diameters or cross-sectional area in systole and diastole [
35]. On the other hand, transesophageal echocardiography (TOE) is a more specialized imaging modality requiring more experience. Aortic measurements obtained from TOE may be more accurate due to the proximity of the esophagus and the aorta [
36].
6.3. Magnetic Resonance Imaging
With the technology advent in recent years, cardiac magnetic resonance imaging has enabled us to obtain accurate vessel pulse wave velocity. The method allows us to acquire wave velocity simultaneously at two or more arterial locations. Furthermore, the distance between the two vessel segments can be measured with high accuracy without any approximation [
37].
6.4. Computed Tomography Angiography
In recent decades, the improvement of technology has made the acquisition of computed tomography cardiac images possible with high temporal resolution [
36,
37,
38]. Previously published data revealed that arterial elasticity measurements acquired using electrocardiographically gated CT were feasible in phantom, porcine specimens, and aortic models of polydimethylsiloxane [
39,
40]. Advantages of computed tomography over other imaging modalities are its high spatial resolution and capability to characterize atherosclerotic plaque. Furthermore, CT allows us to compare regional aortic elasticity [
41]. A previous study revealed a negative association between age and ascending aortic elasticity by using electrocardiographically (ECG) gated dual-source (DS) CT [
42]. It is worth mentioning that the protocols for the evaluation of aortic elastic properties used in the various studies with CT are different and not standardized. In some studies, to calculate the aortic distensibility, a cross-sectional area (CSA) was measured by paralleling the body axis [
43,
44,
45,
46]. On the other hand, others obtained CSA perpendicular to the aortic center line [
42]. To the best of our knowledge, there is no comparison of the different methods to understand which technique provides the most accurate measurements,
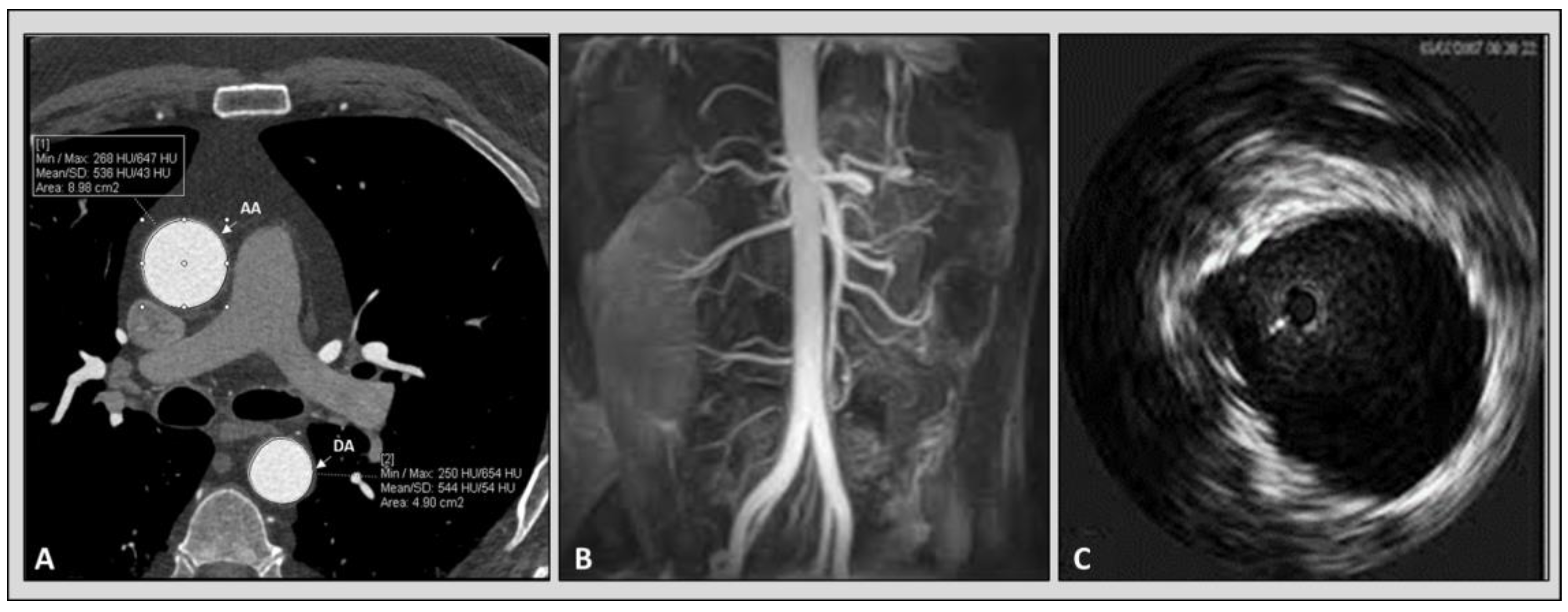
Figure 3.
6.5. Intravascular Ultrasound Imaging of the Aorta
The intravascular ultrasound (IVUS) was used for the first time in 1990 by Weintraub to evaluate a patient with aortic dissection [
47]. Afterwards, there have been a few reports and published studies considering the use of IVUS for the imaging of aortic pathology [
48,
49,
50]. In 1985, Hughes et al. published data about an in vivo experiment with dogs, analyzing the application of intravascular ultrasonic catheter with a simultaneous evaluation of intra-aortic pressure [
51]. This study revealed that the acquisition of arterial dimensions with this ultrasonic system may lay the foundations of a new method for aortic elastic properties evaluation. Almost 10 years later, another group performed a study assessing the aortic distensibility in six patients using intravascular ultrasound imaging [
52]. It was shown that IVUS may provide an accurate in vivo evaluation of human aortic compliance. In a small study including 12 patients with enlarged ascending aorta who underwent both CT angiography and IVUS, we did not find a significant difference between the measurements and aortic elastic parameters among the two modalities [
53].
7. Hemodynamic Effects of Increased Aortic Stiffness
As previously discussed, an elastic aorta in a young, healthy person can effectively shield excess pulsatility triggered by intermittent left ventricular ejection and exhibit a slow PWV [
54]. In this normal hemodynamic condition, the reflected pulse waves arrive at the left ventricle in its diastole. This leads to an increase in coronary perfusion pressure but does not cause a systolic overload [
55]. On the other hand, with the gradual reduction in aortic elasticity, there is an elevation in forward wave amplitude and PWV. This leads to an earlier arrival of the reflected waves—in systole instead of diastole. This, in turn, leads to systolic pressure augmentation which may aggravate left ventricular oxygen demand and reduce the coronary perfusion pressure [
56]. Therefore, aortic stiffening plays a crucial role in a vicious cycle of hemodynamic consequences, provoking arteria hypertension, myocardial remodeling and heart failure [
35]. Notably, the reduction in aortic compliance may be damaging to other tissues and organs, not just the heart. Transmission of pulsatile energy with excessive shear forces into smaller vessels may damage organs in low resistance beds (i.e., brain, kidney) [
23].
8. Aortic Elasticity and Clinical Consequences
8.1. Aortic Elasticity and Arterial Hypertension
Data revealed that a reduced aortic elasticity increases systolic blood pressure (SBP) and simultaneously results in a reduction in the diastolic blood pressure (DBP), therefore causing a wide pulse pressure [
57]. Prior studies demonstrated that aortic compliance declines in patients with hypertension [
58,
59]. Importantly, in patients with hypertension, the main structural alteration of the vessel wall is the hypertrophy of the arterial tunica media [
60]. This medial hypertrophy is a result of the accumulation of extracellular matrix of the media and even the adventitia. This pathophysiologic process decreases the elasticity and distensibility independently of the BP level [
61]. Thus, an increase in aortic stiffness and a reduction in aortic distensibility may be an early predictor for coronary atherosclerosis and show end-organ injury in patients with hypertension. A study found that and the arterial distensibility is decreased in patients with a hypertensive response to treadmill exercise testing compared to those with a normal BP response to exercise and that patients suggestive of a hypertensive response to treadmill exercise testing have higher left ventricular mass (LVM) when compared to those with a normal BP response to exercise [
62]. Furthermore, LVM was inversely associated with aortic distensibility but was positively correlated with aortic stiffness in patients, indicating a hypertensive response to exercise. An extended follow-up study showed that increased stiffness for a period longer than 5 years is more marked in patients receiving anti-hypertension treatment compared to normotensives patients [
63]. Three factors were linked to a faster progression of aortic stiffness in hypertensive patients on therapy: not well-controlled BP values, elevated HR, and increased serum creatinine [
63].
8.2. Aortic Elasticity and Atherosclerosis
Arterial distensibility can be used as an essential marker of coronary artery disease risk in humans. The aorta elastic properties, carotid intimal media thickness, and endothelial functions can be impacted by risk factors for coronary artery disease (CAD), such as hypercholesterolemia, hypertension, diabetes mellitus, sex, age, and smoking. A reduction of aortic elastic properties may be related to the presence of atherosclerosis and coronary artery disease [
64]. It has been demonstrated that decreased aortic distensibility, which indicates a damage to the aortic elastic structure, is associated with CAD [
65,
66]. Recent data showed that LDL oxidative modification and vascular wall remodeling provoked by hyperglycemia may play a critical role in decreasing arterial distensibility. Furthermore, the aortic distensibility reduced with cumulative cardiovascular risk factors [
67,
68,
69]. A study evaluating aortic elastic properties with coronary computed tomography (CTA) measured aortic distensibility, and the results revealed that distensibility decreased proportionally with the severity of coronary artery disease and the calcium score independently of the cardiovascular risk factor [
43]. Additionally, aortic distensibility improved the ability to detect a high calcium score [
43].
8.3. Aortic Elasticity and Valve Pathology
Previously published data, analyzing the aortic elastic assets in an experimental model of aortic regurgitation reveals that augmented distensibility in the aorta leads to a decrease in its attribute impedance after the incident of valvar regurgitation [
51]. Therefore, the higher elasticity of the aorta is accepted as an adaptation process to the acute expansion of total systolic volume. This hemodynamic adaptation in patients with chronic aortic regurgitation was described by Devlin et al. [
23,
70], who reported a reduced arterial distensibility in patients with decreased LV contractility and preserved ejection fraction (EF). On the other hand, patients with impaired contractility and reduced LV EF had an increase in the LV afterload. Therefore, this maladaptation mechanism may contribute to increasing the dysfunction of the LV in chronic aortic regurgitation [
24]. Another study has revealed that coronary flow reserve and aortic distensibility in the descending aorta are impaired in patients with aortic stenosis [
71]. Meanwhile, Leung et al. revealed that aortic distensibility has a straightforward effect on resting and hyperemic coronary blood flow, and, hence, the aortic stress in aortic stenosis could be an important factor determining coronary perfusion [
72].
9. Aortic Stress and Cardiovascular Outcomes
Aortic elastic properties may have a critical influence on cardiovascular health. Aortic stress is increasingly used as a risk stratification tool. In recent years, the European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension proposed the evaluation of arterial PWV [
32]. A large body of evidence documented the predictive value of artery stress for prognosing cardiovascular events [
73,
74,
75,
76]. Arterial stiffness has been proven as an important predictor of mortality [
63], coronary artery disease, and stroke in the general population [
77]. A previously published study evaluated the predictive value of arterial elastic properties on coronary heart disease in more than a thousand patients with arterial hypertension. The risk evaluation of coronary heart disease was performed using the Framingham risk score considering gender, age, smoking, BP, diabetes, cholesterol, etc. After performing multi-regression analysis, PWV remained significantly associated with coronary events after a correction of the Framingham score. This study revealed that reduced aortic elasticity is an independent predictor of primary coronary events in patients with essential hypertension [
61]. Moreover, reduced arterial distensibility has been correlated with higher morbidity and both all-cause and CV mortality in patients with hypertension [
74,
78,
79]. The authors observed that factors such as advanced age, male sex, higher BMI, diabetes mellitus, lower HDL, higher mean BP, and heart rate correlated with a lower PWV ratio. A meta-analysis that included more than 17,000 patients from prospective studies revealed that enhanced arterial stiffness strongly predicts coronary heart disease, stroke, and overall CV disease [
31]. Furthermore, a meta-regression analysis was used to determine the influence of constant study moderators such as age, duration of follow-up, and baseline aortic PWV on the overall heterogeneity. The compelling predictor of the degree of the log RR for outcomes in subjects with high aortic PWV was age at enrollment. However, there were variations in the study subjects. For example, age was contrariwise correlated to the predictive character of high aortic PWV for CV mortality only in patients with ESRD, suggesting that aortic PWV is a stronger prognostic factor in younger patients with ESRD. On the contrary, no relationship between age and aortic PWV was found in patients with hypertension and the general population. This suggested that stiffness preserves its prognostic value independently of age. No reliable robust link between the predictive capacity of high aortic PWV and the duration of follow-up or the value of aortic PWV at enrollment. Haluska et al. assessed the relationship between arterial distensibility and long-term clinical outcomes of 719 patients. They found that impaired arterial distensibility alone correlated with poor outcomes in patients with variable cardiovascular risk. Moreover, arterial distensibility could detect patients at risk of future adverse events (i.e., fatal and non-fatal) [
80].
The predictive worth of arterial stiffness relies on its pathophysiological importance for arterial and overall CV performance [
81,
82,
83]. Large artery stiffening expands left ventricular afterload [
10] and correlates with hypertrophy of the left ventricle [
61] and weakened coronary perfusion [
84,
85]. Thus, the coronary perfusion and/or myocardial demand equilibrium is dysregulated. Moreover, the stiffening of large arteries is involved in the pathogenesis of hypertension [
81]. This implies that arterial stiffness may depend not only on a genetic background [
86] but also on the cumulative harm of CV risk factors that act on the arterial wall over time. In contrast, the person’s risk factors can vary over time; thus, when recorded at the time of risk evaluation, the CV risk factors may not display their actual effects on the arterial wall. Nevertheless, aortic PWV may correspond to a surrogate endpoint, indicating in which patients the traditional CV risk factors convert into true risk.
10. Clinical Implications
Research on aortic elasticity has several important applications in clinical practice, particularly in the field of cardiovascular medicine. Firstly, research on aortic elastic properties provides valuable insights into an individual’s cardiovascular risk profile. As previously discussed, changes in aortic stiffness and compliance are associated with an increased risk of cardiovascular events. Clinicians can use this information to identify patients at a higher risk and tailor preventive measures and treatment strategies accordingly. Aortic properties can be integrated into risk stratification models for cardiovascular diseases. These models provide a more accurate assessment of an individual’s risk, enabling more targeted interventions and monitoring. Aortic elasticity research allows for the development of more personalized treatment plans. Clinicians can use aortic properties to tailor medication regimens and lifestyle recommendations to individual patients, improving the effectiveness of treatment and reducing adverse effects. Research in this area can help assess overall arterial health beyond the aorta. Measurements of aortic elasticity can be indicative of the health of other arteries in the body, providing a more comprehensive view of a patient’s vascular health. Furthermore, research on aortic elasticity is essential for the early detection of aortic aneurysms, which can be life-threatening if left untreated. Monitoring aortic distensibility can help identify patients at risk and guide the timing of surgical interventions. In summary, aortic elasticity research plays a critical role in improving cardiovascular risk assessment, guiding treatment decisions, and tailoring interventions to individual patients. It offers a more comprehensive view of vascular health and has the potential to enhance clinical practice in the field of cardiovascular medicine.
11. Limitations
Our narrative review on aortic elasticity is a valuable endeavor for synthesizing the existing knowledge and providing insights into this important topic. However, such a review may have certain limitations that should be acknowledged. As a narrative review, our study is prone to selection and publication bias. The articles and studies included in this review may not be entirely representative of the entire body of literature on aortic elastic properties. Furthermore, published studies may not represent the full spectrum of research conducted. Negative or null findings are often underreported, potentially skewing the overall understanding of aortic elastic properties. Moreover, this review’s quality depends on the quality of the studies it includes. If there are many low-quality studies that were selected from the literature, this could affect the overall reliability of this review. Lastly, the findings from this narrative review may be based on a synthesis of existing evidence and may not be as generalizable as those from a systematic review or meta-analysis. Studies on aortic elastic properties may not always account for all potential confounding variables, which can affect the validity of their findings.
12. Conclusions
In conclusion, this narrative review has highlighted the critical role of aortic elastic properties in cardiovascular risk stratification. Aortic stiffness, compliance, and distensibility are valuable biomarkers for assessing vascular health and predicting adverse cardiovascular outcomes. The associations between altered aortic elasticity and conditions such as hypertension, atherosclerosis, and coronary artery disease underscore their clinical relevance. The exploration of potential mechanisms and clinical implications outlines the need for further research to establish precise links between aortic elasticity and cardiovascular risk. Furthermore, the integration of advanced imaging, genetic factors, and personalized medicine is suggested to refine risk assessment strategies and enhance patient care.
Despite substantial progress in understanding these associations, several avenues for future research and clinical applications remain unexplored. A further investigation is needed to elucidate the precise mechanisms linking aortic elastic properties to cardiovascular risk, potentially paving the way for more targeted interventions. Integrating advanced imaging techniques, genetic factors, and personalized medicine approaches can enhance risk assessment and inform tailored preventive strategies. Overall, continued research in this field promises to refine our ability to stratify cardiovascular risk and ultimately improve patient care and outcomes.
Author Contributions
Conceptualization, N.M. and D.V.; methodology, N.M.; software, T.V. (Tsvetelina Velikova); validation, N.M., T.V. (Toni Velikov) and D.V.; formal analysis, N.M.; investigation, N.M.; resources, T.V. (Toni Velikov); data curation, T.V. (Tsvetelina Velikova); writing—original draft preparation, N.M. and T.V. (Tsvetelina Velikova); writing—review and editing, T.V. (Toni Velikov) and D.V.; visualization, N.M. and T.V. (Tsvetelina Velikova); supervision T.V. (Tsvetelina Velikova), D.V.; project administration, D.V.; funding acquisition, T.V. (Tsvetelina Velikova). All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the European Union-NextGenerationEU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patient(s) for the acquisition of the images contained within this study. However, no identifying information is presented in the used images.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
NM reports receiving speaker fees from Abbott, TEVA, Gedeon Richter, and Berlin Chemie. The other authors have nothing to disclose. The authors declare no conflicts of interest.
References
- El-Naggar, H.M.; Anwar, H.S.; Helmy, H.; Demitry, S.R. Aortic Elasticity Indices as Predictors of Coronary Artery Disease Severity Assessed by SYNTAX Score. J. Cardiovasc. Echography 2021, 31, 234–241. [Google Scholar] [CrossRef]
- Cengiz Elçioglu, B.; Kılıç, A.; Baydar, O.; Şahin, Ş.T.; Çelik, H.G.; Aytekin, V.; Aytekin, S. Evaluation of Aortic Elasticity Parameters Measured by Transthoracic Echocardiography in a Normotensive Population: A Single-Center Study. Normotansif Bir Popülasyonda Aortik Elastikiyet Parametrelerinin Transtorasik Ekokardiyografi ile Değerlendirilmesi: Tek Merkezli Bir Çalışma. Turk Kardiyol. Dern. Ars. Turk Kardiyol. Derneginin Yayin. Organidir 2023, 51, 369–377. [Google Scholar] [CrossRef]
- An, D.-W.; Hansen, T.W.; Aparicio, L.S.; Chori, B.; Huang, Q.-F.; Wei, F.-F.; Cheng, Y.-B.; Yu, Y.-L.; Sheng, C.-S.; Gilis-Malinowska, N.; et al. Derivation of an Outcome-Driven Threshold for Aortic Pulse Wave Velocity: An Individual-Participant Meta-Analysis. Hypertension 2023, 80, 1949–1959. [Google Scholar] [CrossRef]
- Cheng, K.-S.; Baker, C.; Hamilton, G.; Hoeks, A.; Seifalian, A. Arterial elastic properties and cardiovascular risk/event. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 383–397. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef]
- Kassab, G.S. Biomechanics of the cardiovascular system: The aorta as an illustratory example. J. R. Soc. Interface 2006, 3, 719–740. [Google Scholar] [CrossRef]
- Bader, H. biochemistry. Importance of the gerontology of elastic arteries in the development of essential hypertension. Clin. Physiol. Biochem. 1983, 1, 36–56. [Google Scholar]
- Mohiaddin, R.H.; Underwood, S.R.; Bogren, H.G.; Firmin, D.N.; Klipstein, R.H.; Rees, R.S.; Longmore, D.B. Regional aortic compliance studied by magnetic resonance imaging: The effects of age, training, and coronary artery disease. Heart 1989, 62, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef]
- Nichols, W.; O’Rourke, M.; Kenney, W.L. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 3rd. ed.; LWW: Philadelphia, PA, USA, 1991. [Google Scholar]
- O’Rourke, M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990, 15, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Rowe, R.C.; York, P. The relationship between Young’s modulus of elasticity of organic solids and their molecular structure. Powder Technol. 1991, 65, 139–146. [Google Scholar] [CrossRef]
- Stratos, C.; Stefanadis, C.; Kallikazaros, I.; Boudoulas, H.; Toutouzas, P. Ascending aorta distensibility abnormalities in hypertensive patients and response to nifedipine administration. Am. J. Med. 1992, 93, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Angoff, R.; Mosarla, R.C.; Tsao, C.W. Aortic stiffness: Epidemiology, risk factors, and relevant biomarkers. Front. Cardiovasc. Med. 2021, 8, 709396. [Google Scholar] [CrossRef] [PubMed]
- Redheuil, A.; Yu, W.C.; Wu, C.O.; Mousseaux, E.; De Cesare, A.; Yan, R.; Kachenoura, N.; Bluemke, D.; Lima, J.A. Reduced ascending aortic strain and distensibility: Earliest manifestations of vascular aging in humans. Hypertension 2010, 55, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef]
- Yasmin; McEniery, C.M.; O’Shaughnessy, K.M.; Harnett, P.; Arshad, A.; Wallace, S.; Maki-Petaja, K.; McDonnell, B.; Ashby, M.J.; Brown, J.; et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1799–1805. [Google Scholar] [CrossRef]
- Harada, K.; Yasuoka, K.; Shimada, Y. Usefulness of tissue doppler imaging for assessing aortic wall stiffness in children with the marfan syndrome. Am. J. Cardiol. 2004, 93, 1072–1075. [Google Scholar] [CrossRef]
- Lee, J.M.; Shirodaria, C.; E Jackson, C.; Robson, M.D.; Antoniades, C.; Francis, J.M.; Wiesmann, F.; Channon, K.M.; Neubauer, S.; Choudhury, R.P. Multi-modal magnetic resonance imaging quantifies atherosclerosis and vascular dysfunction in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2007, 4, 44–48. [Google Scholar] [CrossRef]
- Stacey, R.B.; Bertoni, A.G.; Eng, J.; Bluemke, D.A.; Hundley, W.G.; Herrington, D. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension 2010, 55, 26–32. [Google Scholar] [CrossRef]
- Safar, M.E.; London, G.M.; Plante, G.E. Arterial stiffness and kidney function. Hypertension 2004, 43, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Mark, P.B.; Johnston, N.; Foster, J.; Connell, J.M.; Dargie, H.; Jardine, A.; Padmanabhan, N. Aortic stiffness and diastolic flow abnormalities in end-stage renal disease assessed by magnetic resonance imaging. Nephron Clin. Prac. 2008, 109, c1–c8. [Google Scholar] [CrossRef]
- Kopel, L.; Tarasoutchi, F.; Medeiros, C.; Carvalho, R.T.; Grinberg, M.; Lage, S.G. Arterial distensibility as a possible compensatory mechanism in chronic aortic regurgitation. Arq. Bras. Cardiol. 2001, 77, 262–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, R.A.; McDonald, R.W.; Bristow, J.; Cheitlin, M.; Nauman, D.; Massie, B.; Greenberg, B. Correlates of aortic distensibility in chronic aortic regurgitation and relation to progression to surgery. J. Am. Coll. Cardiol. 1992, 19, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Roccabianca, S.; Figueroa, C.; Tellides, G.; Humphrey, J. Quantification of regional differences in aortic stiffness in the aging human. J. Mech. Behav. Biomed. Mater. 2014, 29, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; De Backer, J.; Devos, D.; Rabben, S.I.; Gillebert, T.C.; Van Bortel, L.M.; De Sutter, J.; De Paepe, A.; Verdonck, P.R. Aortic reflection coefficients and their association with global indexes of wave reflection in healthy controls and patients with Marfan’s syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H2385–H2392. [Google Scholar] [CrossRef]
- Lind, L.; Fors, N.; Hall, J.; Marttala, K.; Stenborg, A. A comparison of three different methods to determine arterial compliance in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. J. Hypertens. 2006, 24, 1075–1082. [Google Scholar] [CrossRef]
- Tanriverdi, H.; Evrengul, H.; Kara, C.O.; Kuru, O.; Tanriverdi, S.; Ozkurt, S.; Kaftan, A.; Kilic, M. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: Noninvasive indicators of atherosclerosis. Respiration 2006, 73, 741–750. [Google Scholar] [CrossRef]
- Jae, S.Y.; Heffernan, K.S.; Park, J.B.; Kurl, S.; Kunutsor, S.K.; Kim, J.-Y.; A Laukkanen, J. Association between estimated pulse wave velocity and the risk of cardiovascular outcomes in men. Eur. J. Prev. Cardiol. 2021, 28, e25–e27. [Google Scholar] [CrossRef]
- Garcia-Carretero, R.; Vigil-Medina, L.; Barquero-Perez, O.; Ramos-Lopez, J. Pulse wave velocity and machine learning to predict cardiovascular outcomes in prediabetic and diabetic populations. J. Med. Syst. 2020, 44, 16. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Park, J.B.; Sharman, J.E.; Li, Y.; Munakata, M.; Shirai, K.; Chen, C.-H.; Jae, S.Y.; Tomiyama, H.; Kosuge, H.; Bruno, R.M.; et al. Expert Consensus on the Clinical Use of Pulse Wave Velocity in Asia. Pulse 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamamoto, T.; Takahara, A.; Shirai, K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: Theory and applications. J. Hypertens. 2015, 33, 1742–1757. [Google Scholar] [CrossRef]
- Nelson, A.J.; Worthley, S.G.; Cameron, J.D.; Willoughby, S.R.; Piantadosi, C.; Carbone, A.; Dundon, B.K.; Leung, M.C.; A Hope, S.; Meredith, I.T.; et al. Cardiovascular magnetic resonance-derived aortic distensibility: Validation and observed regional differences in the elderly. J. Hypertens. 2009, 27, 535–542. [Google Scholar] [CrossRef] [PubMed]
- SCOT-Heart Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef]
- Taguchi, K.; Anno, H. High temporal resolution for multislice helical computed tomography. Med. Phys. 2000, 27, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Soschynski, M.; Hagen, F.; Baumann, S.; Hagar, M.T.; Weiss, J.; Krauss, T.; Schlett, C.L.; Mühlen, C.v.Z.; Bamberg, F.; Nikolaou, K.; et al. High temporal resolution dual-source photon-counting CT for coronary artery disease: Initial multicenter clinical Experience. J. Clin. Med. 2022, 11, 6003. [Google Scholar] [CrossRef]
- Ganten, M.-K.; Krautter, U.; von Tengg-Kobligk, H.; Böckler, D.; Schumacher, H.; Stiller, W.; Delorme, S.; Kauczor, H.-U.; Kauffmann, G.W.; Bock, M. Quantification of aortic distensibility in abdominal aortic aneurysm using ECG-gated multi-detector computed tomography. Eur. Radiol. 2008, 18, 966–973. [Google Scholar] [CrossRef]
- Medynsky, A.; Sherebrin, M.; Rankin, R.; Holdsworth, D.; Roach, M. (Eds.) An in vitro time study of distensibility in porcine aortas using high resolution X-ray CT. In Proceedings of the 1996 Fifteenth Southern Biomedical Engineering Conference, Dayton, OH, USA, 29–31 March 1996; IEEE: Piscataway, NJ, USA, 1996. [Google Scholar]
- Carrascosa, P.; Capuñay, C.; Deviggiano, A.; Rodríguez-Granillo, G.A.; Sagarduy, M.I.; Cortines, P.; Carrascosa, J.; Parodi, J.C. Thoracic aorta cardiac-cycle related dynamic changes assessed with a 256-slice CT scanner. Cardiovasc. Diagn. Ther. 2013, 3, 125–128. [Google Scholar]
- Li, N.; Beck, T.; Chen, J.; Biermann, C.; Guo, L.; Sun, H.; Gao, F.; Liu, C. Assessment of thoracic aortic elasticity: A preliminary study using electrocardiographically gated dual-source CT. Eur. Radiol. 2011, 21, 1564–1572. [Google Scholar] [CrossRef]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Flores, F.; Azmoon, S.; Ismaeel, H.; Shavelle, D.; Mao, S.S.; Ebrahimi, R.; Budoff, M.J. Impaired aortic distensibility measured by computed tomography is associated with the severity of coronary artery disease. Int. J. Cardiovasc. Imaging 2011, 27, 459–469. [Google Scholar] [CrossRef][Green Version]
- Ganten, M.; Krautter, U.; Hosch, W.; Hansmann, J.; von Tengg-Kobligk, H.; Delorme, S.; Kauczor, H.-U.; Kauffmann, G.W.; Bock, M. Age related changes of human aortic distensibility: Evaluation with ECG-gated CT. Eur. Radiol. 2007, 17, 701–708. [Google Scholar] [CrossRef]
- Okuyama, T.; Ehara, S.; Shirai, N.; Sugioka, K.; Yamashita, H.; Kataoka, T.; Naruko, T.; Itoh, T.; Otani, K.; Matsuoka, T.; et al. Assessment of aortic atheromatous plaque and stiffness by 64-slice computed tomography is useful for identifying patients with coronary artery disease. Circ. J. 2008, 72, 2021–2027. [Google Scholar] [CrossRef]
- Jang, S.; Yong, H.S.; Doo, K.W.; Kang, E.-Y.; Woo, O.H.; Choi, E.J. Relation of aortic calcification, wall thickness, and distensibility with severity of coronary artery disease: Evaluation with coronary CT angiography. Acta Radiol. 2012, 53, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.R.; Schwartz, S.L.; Pandian, N.G.; E Katz, S.; Kwon, O.J.; Millan, V.; Bojar, R. Evaluation of acute aortic dissection by intravascular ultrasonography. N. Engl. J. Med. 1990, 323, 1566–1567. [Google Scholar]
- Alfonso, F.; Goicolea, J.; Aragoncillo, P.; Hernandez, R.; Macaya, C. Diagnosis of aortic intramural hematoma by intravascular ultrasound imaging. Am. J. Cardiol. 1995, 76, 735–738. [Google Scholar] [CrossRef]
- Mileva, N.; Vassilev, D.; Gil, R.; Rigatelli, G. Misdiagnosed aortic intramural hematoma and the role of intravascular ultrasound imaging in detection of acute aortic syndrome: A Case Report. Cardiovasc. Innov. Appl. 2018, 2, 447–449. [Google Scholar] [CrossRef]
- Wei, H.; Schiele, F.; Meneveau, N.; Seronde, M.-F.; Legalery, P.; Caulfield, F.; Bonneville, J.-F.; Chocron, S.; Bassand, J.-P. The value of intravascular ultrasound imaging in diagnosis of aortic penetrating atherosclerotic ulcer. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2006, 1, 432–437. [Google Scholar]
- Hughes, D.J.; Fearnot, N.E.; Babbs, C.F.; Bourland, J.D.; Geddes, L.A.; Eggelton, R. Continuous measurement of aortic radius change in vivo with an intra-aortic ultrasonic catheter. Med. Biol. Eng. Comput. 1985, 23, 197–202. [Google Scholar] [CrossRef]
- Hansen, M.E.; Yucel, E.K.; Megerman, J.; L’Italien, G.J.; Abbott, W.M.; Waltman, A.C. In vivo determination of human arterial compliance: Preliminary investigation of a new technique. Cardiovasc. Interv. Radiol. 1994, 17, 22–26. [Google Scholar] [CrossRef]
- Mileva, N.B.; Vassilev, D.I. Intravascular Ultrasound Imaging for Evaluation of Aortic Elastic Properties: Review of the Literature and a Single-Center Experience. Cardiol. Cardiovasc. Med. 2020, 4, 396–399. [Google Scholar]
- Mitchell, G.F. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J. Appl. Physiol. 2018, 125, 1871–1880. [Google Scholar] [CrossRef]
- Martínez-Ayala, P.; Alanis-Sánchez, G.A.; González-Hernández, L.A.; Álvarez-Zavala, M.; Cabrera-Silva, R.I.; Andrade-Villanueva, J.F.; Sánchez-Reyes, K.; Ramos-Solano, M.; Castañeda-Zaragoza, D.A.; Cardona-Müller, D.; et al. Aortic stiffness and central hemodynamics in treatment-naïve HIV infection: A cross-sectional study. BMC Cardiovasc. Disord. 2020, 20, 440. [Google Scholar] [CrossRef]
- Bell, V.; McCabe, E.L.; Larson, M.G.; Rong, J.; Merz, A.A.; Osypiuk, E.; Lehman, B.T.; Stantchev, P.; Aragam, J.; Benjamin, E.J.; et al. Relations between aortic stiffness and left ventricular mechanical function in the community. J. Am. Heart Assoc. 2017, 6, e004903. [Google Scholar] [CrossRef]
- Cuomo, F.; Roccabianca, S.; Dillon-Murphy, D.; Xiao, N.; Humphrey, J.D.; Figueroa, C.A. Effects of age-associated regional changes in aortic stiffness on human hemodynamics revealed by computational modeling. PLoS ONE 2017, 12, e0173177. [Google Scholar] [CrossRef] [PubMed]
- Kuwajima, I.; Suzuki, Y.; Shimosawa, T.; Kanemaru, A.; Hoshino, S.; Kuramoto, K. Diminished nocturnal decline in blood pressure in elderly hypertensive patients with left ventricular hypertrophy. Am. Heart J. 1992, 123, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Adamopoulos, C.; Bureau, J.-M.; Temmar, M.; Labat, C.; Bean, K.; Thomas, F.; Pannier, B.; Asmar, R.; Zureik, M.; et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circ. 2002, 105, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Tropeano, A.I.; Asmar, R.; Gautier, I.; Benetos, A.; Lacolley, P.; Laurent, S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 2002, 39, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Toprak, A.; Reddy, J.; Chen, W.; Srinivasan, S.; Berenson, G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the bogalusa heart Study). Am. J. Cardiol. 2009, 103, 978–984. [Google Scholar] [CrossRef]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. Jama 2012, 308, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef]
- Choi, C.U.; Park, E.B.; Suh, S.Y.; Kim, J.W.; Kim, E.J.; Rha, S.-W.; Seo, H.S.; Oh, D.J.; Park, C.G. Impact of aortic stiffness on cardiovascular disease in patients with chest pain*assessment with direct intra-arterial measurement. Am. J. Hypertens. 2007, 20, 1163–1169. [Google Scholar]
- Warnholtz, A.; Wild, P.; Ostad, M.A.; Elsner, V.; Stieber, F.; Schinzel, R.; Walter, U.; Peetz, D.; Lackner, K.; Blankenberg, S.; et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: Results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis 2009, 204, 216–221. [Google Scholar] [CrossRef]
- Van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.; van der Kuip, D.A.; Hofman, A.; Witteman, J.C. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef]
- Eren, M.; Gorgulu, S.; Uslu, N.; Celik, S.; Dagdeviren, B.; Tezel, T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart 2004, 90, 37–43. [Google Scholar] [CrossRef]
- Dart, A.M.; Lacombe, F.; Yeoh, J.K.; Cameron, J.D.; Jennings, G.L.; Laufer, E.; Esmore, D.S. Aortic distensibility in patients with isolated hypercholesterolaemia, coronary artery disease, or cardiac transplant. Lancet 1991, 338, 270–273. [Google Scholar] [CrossRef]
- Oliver, J.J.; Webb, D.J. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 554–566. [Google Scholar] [CrossRef]
- Devlin, W.H.; Petrusha, J.; Briesmiester, K.; Montgomery, D.; Starling, M.R. Impact of vascular adaptation to chronic aortic regurgitation on left ventricular performance. Circulation 1999, 99, 1027–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nemes, A.; Forster, T.; Csanády, M. Decreased aortic distensibility and coronary flow velocity reserve in patients with significant aortic valve stenosis with normal epicardial coronary arteries. J. Heart Valve Dis. 2004, 13, 567–573. [Google Scholar] [PubMed]
- Leung, M.C.H.; Meredith, I.T.; Cameron, J.D. Aortic stiffness affects the coronary blood flow response to percutaneous coronary intervention. Am. J. Physiol. Circ. Physiol. 2006, 290, H624–H630. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Cheng, H.M.; Sung, S.H.; Chuang, S.Y.; Li, C.H.; Spurgeon, H.A.; Ting, C.T.; Najjar, S.S.; Lakatta, E.G.; Yin, F.C.; et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: A community-based study. Hypertension 2010, 55, 799–805. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef]
- Terai, M.; Ohishi, M.; Ito, N.; Takagi, T.; Tatara, Y.; Kaibe, M.; Komai, N.; Rakugi, H.; Ogihara, T. Comparison of arterial functional evaluations as a predictor of cardiovascular events in hypertensive patients: The non-invasive atherosclerotic evaluation in hypertension (noah) study. Hypertens. Res. 2008, 31, 1135–1145. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Havlik, R.; Lakatta, E.G.; Spurgeon, H.; Kritchevsky, S.; et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005, 111, 3384–3390. [Google Scholar] [CrossRef]
- Laurent, S.; Katsahian, S.; Fassot, C.; Tropeano, A.-I.; Gautier, I.; Laloux, B.; Boutouyrie, P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003, 34, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Willum Hansen, T.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006, 113, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Haluska, B.A.; Jeffries, L.; Carlier, S.; Marwick, T.H. Measurement of arterial distensibility and compliance to assess prognosis. Atherosclerosis 2010, 209, 474–480. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Staessen, J.A.; Vlachopoulos, C.; Duprez, D.; Plante, G.E. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 2002, 15, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arter. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lekakis, J.; Papadopoulos, C.; Triantafyllidi, H.; Paraskevaidis, I.; Georgoula, G.; Tzortzis, S.; Revela, I.; Kremastinos, D.T. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am. J. Hypertens. 2008, 21, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ohtsuka, S.; Kakihana, M.; Sugishita, Y. Coronary circulation in dogs with an experimental decrease in aortic compliance. J. Am. Coll. Cardiol. 1993, 21, 1497–1506. [Google Scholar] [CrossRef]
- Schnabel, R.; Larson, M.G.; Dupuis, J.; Lunetta, K.L.; Lipinska, I.; Meigs, J.B.; Yin, X.; Rong, J.; Vita, J.A.; Newton-Cheh, C.; et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 2008, 51, 1651–1657. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).