Abstract
Sickle cell disease (SCD) imposes a significant health burden, particularly in low- and middle-income countries where healthcare professionals and resources are scarce. This opinion paper delves into the management strategies employed for vaso-occlusive crises (VOCs) in pediatric patients with SCD, advocating for the adoption of a transformative strategy. We explore the integration of functional assessment approaches into existing procedures, highlighting the potential of technology-assisted rehabilitation, including wearable sensors and digital biomarkers, to enhance the effectiveness of managing and preventing VOCs. Rehabilomics, as a comprehensive framework, merges rehabilitation-related data with biomarkers, providing a basis for personalized therapeutic interventions. Despite the promising advantages of these approaches, persistent obstacles such as the limited availability of rehabilitation programs, especially in resource-limited settings, pose challenges. This paper underscores the importance of a collaborative strategy to effectively address the unique obstacles faced by patients with SCD. This collaborative approach involves improving accessibility to rehabilitation services, incorporating technology-supported therapy, and fostering focused research endeavors. The primary objective of this comprehensive approach is to enhance the overall care of SCD patients, with a specific focus on preventing VOCs, as well as providing tailored (neuro)rehabilitation services in resource-limited settings. By examining the current state of SCD management and proposing transformative strategies, this opinion paper seeks to inspire collective action and collaboration to improve outcomes for pediatric SCD patients globally.
1. Introduction
Sickle cell disease (SCD) is a hereditary disorder characterized by the deformation of erythrocytes into a sickle-shaped morphology [1]. This genetic condition manifests as chronic hemolytic anemia, intermittent vaso-occlusive events, and extreme susceptibility to infections. Vaso-occlusive events lead to tissue ischemia, resulting in acute and chronic pain, as well as organ damage that can affect various organ systems, including the bones, spleen, liver, brain, lungs, kidneys, and joints [2]. The Democratic Republic of Congo (DRC) is positioned as the third-most impacted nation on a global scale, with an estimated annual occurrence of 39,700 newborns affected by SCD, trailing after Nigeria and India [3,4]. The variability in the clinical manifestation of SCD can be attributed to a complex interplay of environmental and genetic factors. These factors include the presence of both α-triplication and α-homozygous deletion, which contribute to milder symptoms and manifestations of the disease [5].
At the cerebral level, SCD increases the vulnerability of affected children to cerebrovascular accidents, with an estimated stroke incidence rate 220 to 300 times greater compared to their healthy peers of the same age. According to existing research, the prevalence of strokes in SCD ranges from 3.7% to 7% [6]. Moreover, approximately two-thirds of patients experience recurrent cerebral infarctions within 2–3 years following their initial stroke [7]. The subsequent occurrence of microvascular injury and limited vasoreactivity plays a role in the development of silent infarcts, which have been associated with delayed cognitive development. A stroke in children or adolescents with SCD is characterized by the enduring presence of localized neurological impairment lasting for a period exceeding 24 h [8]. In some cases, a stroke may be the clinical manifestation suggestive of the diagnosis of SCD, as brain lesions appear early, associated with cognitive deficits.
At the pulmonary level, hyperalgesic vaso-occlusive crises can be complicated by acute chest syndrome, a major complication of SCD associated with significant morbidity and mortality. Recurrence of intraerythrocyte polymerization of hemoglobin S promotes ischemia–reperfusion injury and hemolysis, causing permanent sterile inflammation. This results in the progressive establishment of pulmonary vaso-occlusive lesions following the aggregation of neutrophils and platelets. These aggregates arise from the intravascular migration of neutrophil extracellular traps from the liver to the lungs [9]. This inflammatory pulmonary environment predisposes the patient to an alteration of pulmonary function, often associated with hypoxemia and restrictive lung disease, even though there is no formally established link between this alteration and functional capacities, as well as low oxygen saturation in sickle cell hemoglobin at a steady state [10,11,12].
The ultimate progression of this vaso-occlusion in the lung leads to the occurrence of pulmonary hypertension, increasing the mortality associated with SCD [13]. Vaso-occlusive events in SCD also lead to bone complications, the most common of which are avascular necrosis of the femoral head, bone infarction, and spinal cord necrosis. These bone complications worsen the morbidity of the disease and expose patients to the occurrence of disabling complications. In the context of Lubumbashi, where access to imaging and biomarker testing is extremely limited, the early identification of patients at risk of these complications is a major challenge.
The management of SCD continues to provide significant challenges, particularly in regions such as the DRC and other low- and middle-income countries (LMICs), as SCD management is impeded by socioeconomic vulnerability, a limited availability of essential therapies such as hydroxyurea [14], and an inadequate supply of appropriate blood products [15]. This study argues that in order to tackle these issues, it is necessary to reassess the assessment and management of these patients, with a specific emphasis on vaso-occlusive crises (VOCs). This can be achieved by integrating functional evaluation methodologies into current practices. Such evaluations are integral to the rehabilitation process, and they have demonstrated their beneficial effects in managing the complications of SCD even in the context of personalized endurance exercise [16,17].
This paper argues for the adoption of a transformative approach to optimize patient outcomes and improve the quality of life for patients with SCD by exploring the possibility of utilizing functional evaluation for alleviating the impact of motor and cognitive deficits particularly in the context of cerebrovascular problems [18].
2. Epidemiology of Vaso-Occlusive Crises in SCD Patients
Due to genetic and environmental factors, the region of Katanga in the DR of Congo is the most severely affected by SCD. It is estimated that in the region of Katanga (Lubumbashi), between 30 and 40% of the population has at least one drepanocyte trait, and 1–2% of the population will develop a severe form of the disease [19]. The management of these patients—particularly when they suffer from frequent VOCs—poses significant challenges in the DRC.
The most common VOC-associated complications are presented in Figure 1.
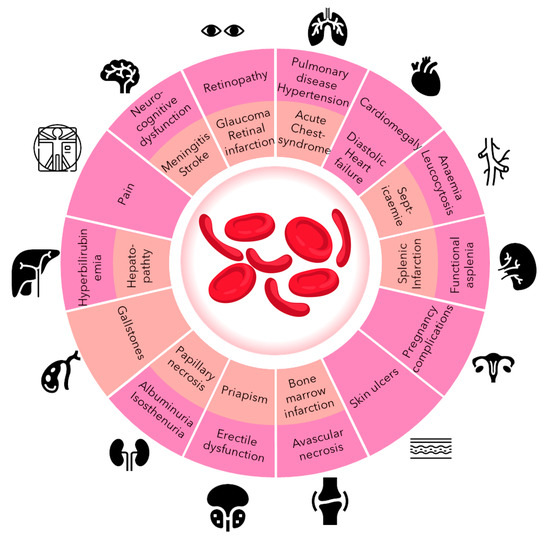
Figure 1.
Most common clinical manifestations of SCD: in pink are those in the chronic phase; in orange are those in the acute phase [20].
Many studies classify VOCs based on various criteria, with location and intensity being the most commonly used for typing since large multicenter studies have demonstrated that qualifying VOCs based on the combination of their location and intensity constitutes an essential foundation that assists clinicians in planning efficient patient management [21,22].
To evaluate the magnitude of this problem and to determine which type of crisis was most prevalent, we conducted a retrospective analysis, examining pediatric and adolescent patients at the Sickle Cell Reference Center (CRDL) in Lubumbashi, part of the Institute of Research in Health Sciences.
At the CRDL, VOCs are categorized into three locations: osteoarticular (when ischemic pain is localized to the bone), abdominal (if ischemic pain is situated in the abdomen), and mixed (if ischemic pain affects both previously mentioned compartments) [23]. Painful crises were classified according to symptom severity into three categories: mild, moderate, and severe.
Pain was assessed using two scales: the Faces Pain Scale (FPS) and the Numerical Pain Scale (NPS) [24]. The FPS was used as a hetero-assessment in children under 6 years of age, or in those who did not have sufficient schooling for self-assessment. For children over 6, self-evaluation was performed with the FPS or the NPS. Pain is defined as mild when the score obtained from the assessments is less than 4, moderate when the score is 4 or 5, and severe when the pain score is equal to or greater than 6.
Our focus is on understanding the incidence and dynamics of VOCs within this patient cohort. In total, 838 patients were included in this study (433 female and 405 male), with a median age of 10 years old [p25 = 5–p75 = 17].
During the follow-up period, 2910 VOCs were monitored. Amongst these, the majority were classified in relation to pain as mild (46.7%, n = 1360) and moderate (45.2%, n = 1316); severe crises were found in 8.0% of the cases (n = 234). These results align with earlier findings in the adult population, which showed that 40% of SCD patients experience neuropathic pain [25]. Another study conducted in the USA reported an average pain intensity of 4.5 on a scale of 10 using the Visual Analog Scale (VAS); however, it is important to note that this study specifically targeted an adult population [26].
Concerning the localization, the vast majority of the crises were osteoarticular (59.5%, n = 1730), followed by mixed (27.2%, n = 793); the abdominal type was found in only 13.2% of the crises (n = 387). These results align with findings from other studies, indicating that osteoarticular crises are the most prevalent. Specifically, 42.6% were knee/shin crises, 42% were low back crises, and 33.8% were hip crises. Abdominal crises were found in 16.2% of the reported cases [26].
Regrettably, none of these patients underwent rehabilitation services.
3. Functional Evaluation as a More-Multidimensional Diagnostic Tool
As previously highlighted, we observed that the majority of the VOCs were osteoarticular. In this context, rehabilitation strategies play a pivotal role in ameliorating functionality and reducing impairment in individuals grappling with chronic diseases such as SCD [27]. Rehabilitation is defined by the World Health Organization as “a set of interventions designed to optimize functioning and reduce disability in individuals with health conditions in interaction with their environment” [28]. Person-centered rehabilitation underscores the importance of tailoring therapies based on individual goals and preferences. Rehabilitation interventions are administered in diverse settings, ranging from inpatient or outpatient hospital environments to private clinics and community spaces like a patient’s home [29]. A cadre of rehabilitation professionals, including physiotherapists, occupational therapists, speech and language therapists, audiologists, orthotists and prosthetists, clinical psychologists, physical medicine and rehabilitation physicians, and rehabilitation nurses, collaboratively work to optimize patient outcomes.
In recent decades, the profound impact of the advancement of technology and computer science on numerous facets of human existence has become increasingly evident. The use of advanced technologies to enhance and optimize physiotherapy and rehabilitation procedures is commonly known as technology-supported rehabilitation [30]. This novel methodology not only utilizes pre-existing technology to provide advantages for both patients and clinicians, but also exhibits significant potential in the specific domain of preventing and handling VOCs in individuals diagnosed with SCD.
An important benefit of incorporating technology into the rehabilitation framework is its capacity to measure a wide range of functional parameters such as balance, gait, ranges of motion, strengths, spasticity, coordination, etc. throughout interventions targeted at managing VOCs. Continuous monitoring plays a crucial role in dynamically monitoring the progress of patients who are undergoing targeted interventions such as high-intensity training, stretching, and balance training [31]. This monitoring allows for the adaptation of rehabilitation plans based on the real-time needs of each patient and the specific characteristics associated with various forms of SCD: i.e., precision rehabilitation [32].
4. The Role of Functional Evaluation in Improving Vaso-Occlusive Crisis Management
The increasing prevalence of affordable and portable technologies integrated with wearable sensors in rehabilitation holds significant relevance in the context of VOC management. These systems have the capability to be smoothly incorporated into routine clinical practices for the purpose of quantifying essential parameters like upper limb motion, hand function, and gait. This integration provides significant insights into the physiological reactions that occur during the implementation of techniques for managing VOCs. Significantly, these devices facilitate the continuous monitoring and functional follow-up of patients during the rehabilitation process [33]. This capability allows for a comprehensive and nuanced evaluation of the effectiveness of therapies. In the specific case of acute thoracic syndrome, incentive spirometry plays a key role in the therapeutic arsenal, emphasizing the importance of rehabilitation. More generally, pulmonary function assessments and functional capacity testing in routine clinical practice would enable the early identification of patients at risk of hypoxemia-related complications [10]. This identification will pave the way for the implementation of a treatment plan to prevent the consequences of hypoxemia, such as improving arterial oxygen levels [34]. Gait and balance assessments are also crucial, especially in cases of aseptic necrosis. They can facilitate the collection of clinical parameters likely to identify patients in the pre-clinical phase of necrosis, or at least at the very beginning of the clinical phase. This early identification will streamline the initiation of prompt management. In patients who have already been affected, these assessments remain equally important, as they allow us to evaluate post-treatment functional results in relation to the initial abnormalities [35,36].
As demonstrated earlier, effectively managing VOCs, and preventing strokes represents a significant therapeutic challenge in the realm of SCD care. Hence, the significance of technology-assisted rehabilitation becomes more pronounced. The concept of “biomarkers”, which comprises a wide range of medical indicators that represent different biological and pathologic processes, can be utilized to assess the real-time efficacy of therapies [37,38]. The application of biomarkers in the context of SCD rehabilitation presents a focused and individualized method for therapy. This allows for prompt modifications to the specific kind, dosage, and intensity of interventions.
Wagner’s concept of rehabilomics presents a comprehensive framework that combines the methodical gathering of data on rehabilitation-related characteristics with a transdisciplinary examination of biomarkers [39,40]. By using the physiologically based conceptual framework, rehabilitation procedures can be tailored to individual needs, considering the biomolecular perspective. This approach aims to enhance the recovery of individuals, particularly during crucial periods characterized by VOCs, and more importantly, tries to sooner identify risk factors and patients at increased risk.
The use of mobile health technology, such as wearable sensors and home-integrated devices, is increasingly being recognized as highly valuable for independent assessments of mobility in everyday activities [41]. This aspect holds significant importance in the effective management of SCD patients. The use of these technologies allows for ongoing collection of data, which offers a thorough understanding of the patient’s condition. This enables the detection of tiny variations that may indicate either progress or decline, a crucial element in customizing therapies for the management of crises. The incorporation of non-invasive and wearable sensors into remote health monitoring serves to augment patient safety by providing ongoing insights into patients’ actions during the intervals between clinical appointments.
In summary, the implementation of technology-assisted rehabilitation, guided by the principles of rehabilomics and digital biomarkers, presents a transformative approach to a better understanding of the factors driving VOCs and the treatment of SCD, as presented in Figure 2.
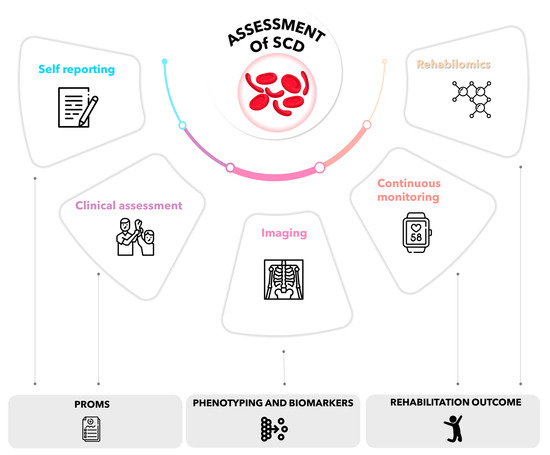
Figure 2.
Multidimensional and functional data collection.
5. Challenges and Opportunities
Enhancing the management of VOC of children with SCD encounters significant obstacles.
As highlighted by the WHO, the lack of access to essential rehabilitation services, encompassing healthcare professionals, infrastructures, and financial resources, constitutes a substantial barrier in some countries [42]. In Lubumbashi, where healthcare resources are often constrained, the scarcity of rehabilitation services equipped with new technologies exacerbates the burden of children with SCD. This shortage underscores the imperative need for concerted efforts to enhance the availability of skilled professionals, establish robust infrastructures, and secure funding for comprehensive rehabilitation services.
In the context of VOC management, a multifaceted approach is imperative. Indeed, it has been established that a medical approach alone does not facilitate optimal management, and that non-medicinal adjuvants make it possible to reduce the time to remission after a crisis and the quantity of opiates used [43,44]. Against this backdrop, establishing (neuro)rehabilitation programs geared towards children becomes not only a necessity but a pivotal aspect of preventing and improving therapeutic efficacy during acute crises [1,27]. By addressing these challenges head-on, we would not only alleviate the burden on the affected children and their families, but also pave the way for a comprehensive strategy that integrates stroke prevention and tailored neurorehabilitation programs.
The primary goal in the field of pediatric stroke rehabilitation is to improve the quality of life and functional capacities of children affected by this condition. Although post-stroke rehabilitation is the main focus, we argue that the importance of prevention should not be disregarded. Preventive measures implemented by proficient rehabilitation experts are crucial in reducing the probability of recurring strokes [45].
After a first stroke, rehabilitation programs have proven effective in reducing the risk of recurrence, notably by encouraging increased levels of physical activity. In children with sickle cell disease, early rehabilitation combined with appropriate management, including transfusion exchange programs and hydroxyurea, has been shown to be crucial in improving outcomes and reducing morbidity and mortality [46].
Currently, there are biomarkers such as the Brain-Derived Neurotrophic Factor (BDNF) whose elevated levels are associated with the risk of stroke and silent cerebral infarction, even in children with SCD [47]. However, their measurement is not feasible in countries with limited resources. In this context, functional assessments remain a better option. Indeed, the early detection of children with elevated velocimetry on transcranial echo Doppler scans [48], coupled with disturbances in cognitive function assessments, would make it possible to identify patients with silent cerebral infarction and, consequently, facilitate the introduction of appropriate management likely to prevent the onset of clinical stroke.
Tablets equipped with cognitive assessment software therefore appear to be an ideal solution [49], especially given the scarcity of specialists capable of carrying out cognitive assessments using conventional clinical examinations.
We strongly support the adoption of a proactive strategy, where we argue for the deployment of systematic functional assessments specifically designed for juvenile patients. This methodology would allow us to pinpoint individuals who are at the greatest risk of experiencing VOCs. This, in turn, would enable us to implement focused interventions that are customized to mitigate the specific risk factors linked with recurring incidents in this susceptible population.
Efforts to enhance access to rehabilitation services in Lubumbashi must be approached comprehensively, involving strategic measures for the placement of healthcare professionals, the establishment of dedicated rehabilitation infrastructures, and the mobilization of essential financial resources [50]. This initiative necessitates collaborative efforts, engaging governmental bodies, non-governmental organizations, and the international community to build a sustainable framework [51]. Such concerted endeavors are imperative to ensure ongoing and comprehensive rehabilitation support, particularly for pediatric SCD patients on the path to recovery from strokes [52].
In the future, it is imperative to conduct economic evaluations to assess the cost-effectiveness of both rehabilitation interventions and functional assessments to further support this change of paradigm.
In resource-limited countries, the implementation of rehabilitation strategies for SCD presents intricate challenges, as succinctly outlined in the aforementioned concerns. The hurdles associated with the expense and accessibility of suitable sensors, along with the imperative need for comprehensive patient education within home environments, underscore the formidable barriers faced when introducing rehabilitation in regions like the DRC. The acknowledgment of limited skilled personnel in these areas emphasizes the urgent requirement for a robust argument on achieving cost-effectiveness. It becomes imperative to incorporate strategies that optimize resource utilization and ensure effective patient outreach, especially in regions where fundamental necessities like hydroxyurea and blood for transfusion are scarce. Addressing these intricacies is not only vital for reinforcing the overarching case for rehabilitation but also for establishing it as a viable and sustainable option in regions grappling with significant resource constraints.
The challenges hindering the management of children with SCD who have suffered a VOC necessitate a comprehensive and concerted effort. Improving access to rehabilitation services and tailoring strategies to the distinctive needs of pediatric populations are fundamental steps. By addressing these challenges, we would not only enhance the rehabilitation journey of affected children but also contribute significantly to stroke prevention efforts, underscoring the vital role of neurorehabilitation programs in comprehensive SCD management. Through such targeted efforts, the feasibility and effectiveness of rehabilitation strategies in resource-limited settings can be maximized, contributing to improved patient outcomes and overall SCD management.
6. Conclusions
In summary, this perspective highlights the significant obstacles and potential advantages associated with evaluating and controlling VOCs in pediatric patients diagnosed with SCD, particularly in relation to the prevention of strokes and the process of neurorehabilitation. The high occurrence of SCD, exacerbated by the intricate interaction of hereditary and environmental elements, presents a significant challenge in areas such as the DRC and other nations with limited economic resources. The obstacles experienced by pediatric patients with SCD are intensified by the lack of rehabilitative programs, as well as socioeconomic vulnerabilities and the restricted availability of important therapies.
We propose an innovative strategy that focuses on integrating functional evaluation approaches to effectively address the distinct requirements of these patients, particularly with regard to VOCs. A significant proportion of VOC incidents were found to primarily affect the osteoarticular system, underscoring the necessity for focused rehabilitative interventions and evaluation.
The utilization of technology-supported rehabilitation has emerged as a potential approach in effectively controlling these crises.
Despite the potential advantages of using technology in rehabilitation, there are notable obstacles that continue to exist, particularly in areas with little resources. The lack of access to rehabilitation services continues to be a significant obstacle, necessitating the need for cooperation among healthcare practitioners, infrastructure improvements, and financial investments. Moreover, addressing the existing knowledge deficit in the field of pediatric stroke rehabilitation necessitates a deliberate redistribution of resources towards the exploration of the distinct intricacies of SCD in the pediatric population. This endeavor will ultimately contribute to the development of evidence-based rehabilitation approaches that are specifically designed to cater to the distinctive requirements of children affected by this condition.
In conclusion, effectively managing VOCs in SCD patients requires a comprehensive and collaborative approach. Enhancing access to rehabilitation services, integrating technology-supported therapy, and expanding targeted research are crucial measures for improving the rehabilitation process for children in need. By implementing such measures, it is possible to not only mitigate the challenges faced by individuals and their families, but also to formulate comprehensive approaches that incorporate stroke prevention and customized neurorehabilitation initiatives. This would greatly enhance the overall management of SCD in resource-limited environments.
Author Contributions
Conceptualization, P.M.B., A.A.K., J.P. and B.B.; writing—original draft preparation, B.B.; writing—review and editing, P.M.B., A.A.K., J.P. and B.B.; visualization, B.B.; supervision, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piel, F.B.; Rees, D.C.; DeBaun, M.R.; Nnodu, O.; Ranque, B.; Thompson, A.A.; Ware, R.E.; Abboud, M.R.; Abraham, A.; Ambrose, E.E.; et al. Defining Global Strategies to Improve Outcomes in Sickle Cell Disease: A Lancet Haematology Commission. Lancet Haematol. 2023, 10, e633–e686. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.A.; Carlberg, K. Sickle Cell Disease. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Piel, F.B.; Hay, S.I.; Gupta, S.; Weatherall, D.J.; Williams, T.N. Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions. PLoS Med. 2013, 10, e1001484. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Sickle Cell Disease Collaborators Global, Regional, and National Prevalence and Mortality Burden of Sickle Cell Disease, 2000-2021: A Systematic Analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [CrossRef]
- Mikobi, T.M.; Lukusa Tshilobo, P.; Aloni, M.N.; Akilimali, P.Z.; Mvumbi-Lelo, G.; Mbuyi-Muamba, J.M. Clinical Phenotypes and the Biological Parameters of Congolese Patients Suffering from Sickle Cell Anemia: A First Report from Central Africa. J. Clin. Lab. Anal. 2017, 31, e22140. [Google Scholar] [CrossRef] [PubMed]
- Ohene-Frempong, K.; Weiner, S.J.; Sleeper, L.A.; Miller, S.T.; Embury, S.; Moohr, J.W.; Wethers, D.L.; Pegelow, C.H.; Gill, F.M. Cerebrovascular Accidents in Sickle Cell Disease: Rates and Risk Factors. Blood 1998, 91, 288–294. [Google Scholar]
- Njamnshi, A.; Mbong, E.; Wonkam, A.; Ongolo-Zogo, P.; Djientcheu, V.-D.; Sunjoh, F.; Wiysonge, C.; Sztajzel, R.; Mbanya, D.; Blackett, K.N.; et al. The Epidemiology of Stroke in Sickle Cell Patients in Yaounde, Cameroon. J. Neurol. Sci. 2006, 250, 79–84. [Google Scholar] [CrossRef]
- Kossorotoff, M.; Grevent, D.; de Montalembert, M. Drépanocytose et atteinte vasculaire cérébrale chez l’enfant. Arch. Pédiatrie 2014, 21, 404–414. [Google Scholar] [CrossRef]
- Vats, R.; Kaminski, T.W.; Brzoska, T.; Leech, J.A.; Tutuncuoglu, E.; Katoch, O.; Jonassaint, J.; Tejero, J.; Novelli, E.M.; Pradhan-Sundd, T.; et al. Liver-to-Lung Microembolic NETs Promote Gasdermin D–Dependent Inflammatory Lung Injury in Sickle Cell Disease. Blood 2022, 140, 1020–1037. [Google Scholar] [CrossRef]
- Vieira, A.K.; Alvim, C.G.; Carneiro, M.C.M.; Ibiapina, C.D.C.; Fundação Hemominas, Brasil; Universidade Federal de Minas Gerais, Brazil. Pulmonary Function in Children and Adolescents with Sickle Cell Disease: Have We Paid Proper Attention to This Problem? J. Bras. Pneumol. 2016, 42, 409–415. [Google Scholar] [CrossRef]
- Campbell, A.; Minniti, C.P.; Nouraie, M.; Arteta, M.; Rana, S.; Onyekwere, O.; Sable, C.; Ensing, G.; Dham, N.; Luchtman-Jones, L.; et al. Prospective Evaluation of Haemoglobin Oxygen Saturation at Rest and after Exercise in Paediatric Sickle Cell Disease Patients. Br. J. Haematol. 2009, 147, 352–359. [Google Scholar] [CrossRef]
- Arigliani, M.; Kitenge, R.; Castriotta, L.; Ndjule, P.; Barbato, V.; Cogo, P.; Tshilolo, L. Lung Function in Children with Sickle Cell Disease from Central Africa. Thorax 2019, 74, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.; El-Baba, F.; Dhillon, K.; Daoud, A.; Soubani, A. Pulmonary Complications of Sickle Cell Disease: A Narrative Clinical Review. Adv. Respir. Med. 2021, 89, 173–187. [Google Scholar] [CrossRef]
- Mukinayi, B.M.; Cibeyibeyi, G.K.; Tumba, G.D.; Gulbis, B. Drépanocytose En République Démocratique Du Congo: Quels Sont Les Obstacles à Un Traitement Par Hydroxyurée? Pan Afr. Med. J. 2021, 38, 41. [Google Scholar] [CrossRef] [PubMed]
- Boma Muteb, P.; Kaluila Mamba, J.F.J.; Muhau Pfutila, P.; Bilo, V.; Panda Mulefu, J.D.; Diallo, D.A. Effectiveness, Safety, and Cost of Partial Exchange Transfusions in Patients with Sickle-Cell Anemia at a Sickle Cell Disease Center in Sub-Saharan Africa. Med. Sante Trop. 2017, 27, 387–391. [Google Scholar] [CrossRef]
- Boma, P.M.; Panda, J.; Ngoy Mande, J.P.; Bonnechère, B. Rehabilitation: A Key Service, yet Highly Underused, in the Management of Young Patients with Sickle Cell Disease after Stroke in DR of Congo. Front. Neurol. 2023, 14, 1104101. [Google Scholar] [CrossRef] [PubMed]
- Messonnier, L.A.; Gellen, B.; Lacroix, R.; Peyrot, S.; Rupp, T.; Mira, J.; Peyrard, A.; Berkenou, J.; Galactéros, F.; Bartolucci, P.; et al. Physiological Evaluation for Endurance Exercise Prescription in Sickle Cell Disease. Med. Sci. Sports Exerc. 2019, 51, 1795–1801. [Google Scholar] [CrossRef]
- King, A.A.; DeBaun, M.R.; White, D.A. Need for Cognitive Rehabilitation for Children with Sickle Cell Disease and Strokes. Expert Rev. Neurother. 2008, 8, 291–296. [Google Scholar] [CrossRef]
- Tshilolo, L.; Mukendi, R.; Girot, R. La drépanocytose dans le sud du Zaïre. Étude de deux séries de 251 et 340 malades suivis entre 1988 et 1992. Archives de Pédiatrie 1996, 3, 104–111. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle Cell Disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef]
- Jacob, E.; Miaskowski, C.; Savedra, M.; Beyer, J.E.; Treadwell, M.; Styles, L. Changes in Intensity, Location, and Quality of Vaso-Occlusive Pain in Children with Sickle Cell Disease. Pain 2003, 102, 187–193. [Google Scholar] [CrossRef]
- Darbari, D.S.; Sheehan, V.A.; Ballas, S.K. The Vaso-Occlusive Pain Crisis in Sickle Cell Disease: Definition, Pathophysiology, and Management. Eur. J. Haematol. 2020, 105, 237–246. [Google Scholar] [CrossRef]
- Smith, W.R.; Scherer, M. Sickle-Cell Pain: Advances in Epidemiology and Etiology. Hematol. Am. Soc. Hematol. Educ. Program. 2010, 2010, 409–415. [Google Scholar] [CrossRef]
- Jensen, M.P.; Miró, J.; Euasobhon, P. Assessing Pain Intensity: Critical Questions for Researchers and Clinicians. Anaesthesia 2023, 79, 114–118. [Google Scholar] [CrossRef]
- Cregan, M.; Puri, L.; Kang, G.; Anghelescu, D. Prevalence of Neuropathic Pain in Adolescents with Sickle Cell Disease: A Single Center Experience. Pediatr. Blood Cancer 2022, 69, e29583. [Google Scholar] [CrossRef]
- McClish, D.K.; Smith, W.R.; Dahman, B.A.; Levenson, J.L.; Roberts, J.D.; Penberthy, L.T.; Aisiku, I.P.; Roseff, S.D.; Bovbjerg, V.E. Pain Site Frequency and Location in Sickle Cell Disease: The PiSCES Project. Pain 2009, 145, 246–251. [Google Scholar] [CrossRef]
- Bonnechère, B. Sickle Cell Disease Strategies and Priorities. Lancet Haematol. 2023, 10, e793–e794. [Google Scholar] [CrossRef]
- WHO Fact Sheets: Rehabilitation. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed on 3 October 2023).
- Wade, D.T. What Is Rehabilitation? An Empirical Investigation Leading to an Evidence-Based Description. Clin. Rehabil. 2020, 34, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Young, M.; Shoman, H.; Punnoose, A.; Norrish, A.R.; Khanduja, V. Advanced Rehabilitation Technology in Orthopaedics-a Narrative Review. Int. Orthop. 2021, 45, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, B. Integrating Rehabilomics into the Multi-Omics Approach in the Management of Multiple Sclerosis: The Way for Precision Medicine? Genes 2022, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Adans-Dester, C.; Hankov, N.; O’Brien, A.; Vergara-Diaz, G.; Black-Schaffer, R.; Zafonte, R.; Dy, J.; Lee, S.I.; Bonato, P. Enabling Precision Rehabilitation Interventions Using Wearable Sensors and Machine Learning to Track Motor Recovery. NPJ Digit. Med. 2020, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Komaris, D.-S.; Tarfali, G.; O’Flynn, B.; Tedesco, S. Unsupervised IMU-Based Evaluation of at-Home Exercise Programmes: A Feasibility Study. BMC Sports Sci. Med. Rehabil. 2022, 14, 28. [Google Scholar] [CrossRef]
- Koelbel, M.; Hamdule, S.; Kirkham, F.J.; Stotesbury, H.; Hood, A.M.; Dimitriou, D. Mind the Gap: Trajectory of Cognitive Development in Young Individuals with Sickle Cell Disease: A Cross-Sectional Study. Front. Neurol. 2023, 14, 1087054. [Google Scholar] [CrossRef] [PubMed]
- Severyns, M.; Gayet, L.E. Aseptic Osteonecrosis of the Femoral Head in Patients with Sickle Cell Anemia. Morphologie 2021, 105, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kenanidis, E.; Kapriniotis, K.; Anagnostis, P.; Potoupnis, M.; Christofilopoulos, P.; Tsiridis, E. Total Hip Arthroplasty in Sickle Cell Disease: A Systematic Review. EFORT Open Rev. 2020, 5, 180–188. [Google Scholar] [CrossRef]
- Babrak, L.M.; Menetski, J.; Rebhan, M.; Nisato, G.; Zinggeler, M.; Brasier, N.; Baerenfaller, K.; Brenzikofer, T.; Baltzer, L.; Vogler, C.; et al. Traditional and Digital Biomarkers: Two Worlds Apart? Digit. Biomark. 2019, 3, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Papapetropoulos, S.; Xiong, M.; Kieburtz, K. The First Frontier: Digital Biomarkers for Neurodegenerative Disorders. Digit. Biomark. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Wagner, A.K. A Rehabilomics Framework for Personalized and Translational Rehabilitation Research and Care for Individuals with Disabilities: Perspectives and Considerations for Spinal Cord Injury. J. Spinal Cord. Med. 2014, 37, 493–502. [Google Scholar] [CrossRef]
- Wagner, A.K. TBI Translational Rehabilitation Research in the 21st Century: Exploring a Rehabilomics Research Model. Eur. J. Phys. Rehabil. Med. 2010, 46, 549–556. [Google Scholar]
- Pratap, A.; Grant, D.; Vegesna, A.; Tummalacherla, M.; Cohan, S.; Deshpande, C.; Mangravite, L.; Omberg, L. Evaluating the Utility of Smartphone-Based Sensor Assessments in Persons With Multiple Sclerosis in the Real-World Using an App (elevateMS): Observational, Prospective Pilot Digital Health Study. JMIR Mhealth Uhealth 2020, 8, e22108. [Google Scholar] [CrossRef]
- WHO The World Rehabilitation Alliance. Available online: https://www.who.int/initiatives/world-rehabilitation-alliance (accessed on 10 November 2023).
- Puri, L.; Nottage, K.A.; Hankins, J.S.; Anghelescu, D.L. State of the Art Management of Acute Vaso-Occlusive Pain in Sickle Cell Disease. Pediatr. Drugs 2018, 20, 29–42. [Google Scholar] [CrossRef]
- Muteb, P.B.; Matungulu, C.; Luntadila, S.N.; Mwamba, D.; Kambale, S.K.; Wela, J.-J.I.; Mukengeshayi, A.N.; Mulefu, P.J.; Mulefu, A. Phase 2 Randomized Clinical Trial Evaluating the Use of the Vascular Electrical Stimulation Therapy by Device Diavein in Severe Painful Vaso-Occlusive Crisis of Sickle Cell Disease: Reduction of Crisis Exit Time and Hospital Stay Duration. J. Clin. Med. Ther. 2022, 7. Available online: https://www.imedpub.com/articles/phase-2-randomized-clinical-trial-evaluating-the-use-of-the-vascular-electrical-stimulation-therapy-by-device-diavein-in-severe-pa.php?aid=46788 (accessed on 10 November 2023).
- Gladstone, D.J.; Lindsay, M.P.; Douketis, J.; Smith, E.E.; Dowlatshahi, D.; Wein, T.; Bourgoin, A.; Cox, J.; Falconer, J.B.; Graham, B.R.; et al. Canadian Stroke Best Practice Recommendations: Secondary Prevention of Stroke Update 2020. Can. J. Neurol Sci. 2022, 49, 315–337. [Google Scholar] [CrossRef]
- Hakami, F.; Alhazmi, E.; Busayli, W.M.; Althurwi, S.; Darraj, A.M.; Alamir, M.A.; Hakami, A.; Othman, R.A.; Moafa, A.I.; Mahasi, H.A.; et al. Overview of the Association Between the Pathophysiology, Types, and Management of Sickle Cell Disease and Stroke. Cureus 2023, 15, e50577. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Abd El Naby, S.A.; Abdelgawad, A.S.; Rizq, M.S.; Abd El Hady, N.M.S. Brain-Derived Neurotrophic Factor and Neuroimaging in Pediatric Patients with Sickle Cell Disease. Pediatr. Res. 2023, 93, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Verlhac, S.; Balandra, S.; Cussenot, I.; Kasbi, F.; Vasile, M.; Kheniche, A.; Elmaleh-Bergès, M.; Ithier, G.; Benkerrou, M.; Bernaudin, F.; et al. Extracranial Carotid Arteriopathy in Stroke-Free Children with Sickle Cell Anemia: Detection by Submandibular Doppler Sonography. Pediatr. Radiol. 2014, 44, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, B.; Klass, M. Cognitive Computerized Training for Older Adults and Patients with Neurological Disorders: Do the Amount and Training Modality Count? An Umbrella Meta-Regression Analysis. Games Health J. 2023, 12, 100–117. [Google Scholar] [CrossRef]
- Richter, M.; Dragano, N. Micro, Macro, but What about Meso? The Institutional Context of Health Inequalities. Int. J. Public Health 2018, 63, 163–164. [Google Scholar] [CrossRef]
- O’Brien, P.; Kajja, I.; Potter, J.M.; O’Hara, N.N.; Kironde, E.; Petrisor, B. Role of North-South Partnership in Trauma Management: Uganda Sustainable Trauma Orthopaedic Program. J. Orthop. Trauma 2018, 32 (Suppl. 7), S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Louw, Q.; Dizon, J.; van Niekerk, S.-M.; Ernstzen, D.; Grimmer, K. Contextualised Evidence-Based Rehabilitation Recommendations to Optimise Function in African People with Stroke. In Collaborative Capacity Development to Complement Stroke Rehabilitation in Africa; Louw, Q., Ed.; Human Functioning, Technology and Health; AOSIS: Cape Town, South Africa, 2020; ISBN 978-1-928523-85-7. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).