Small Paracentral Acute Middle Maculopathy Lesions as Biomarker of Vascular Morbidity: Natural Course
Abstract
1. Introduction
2. Material and Methods
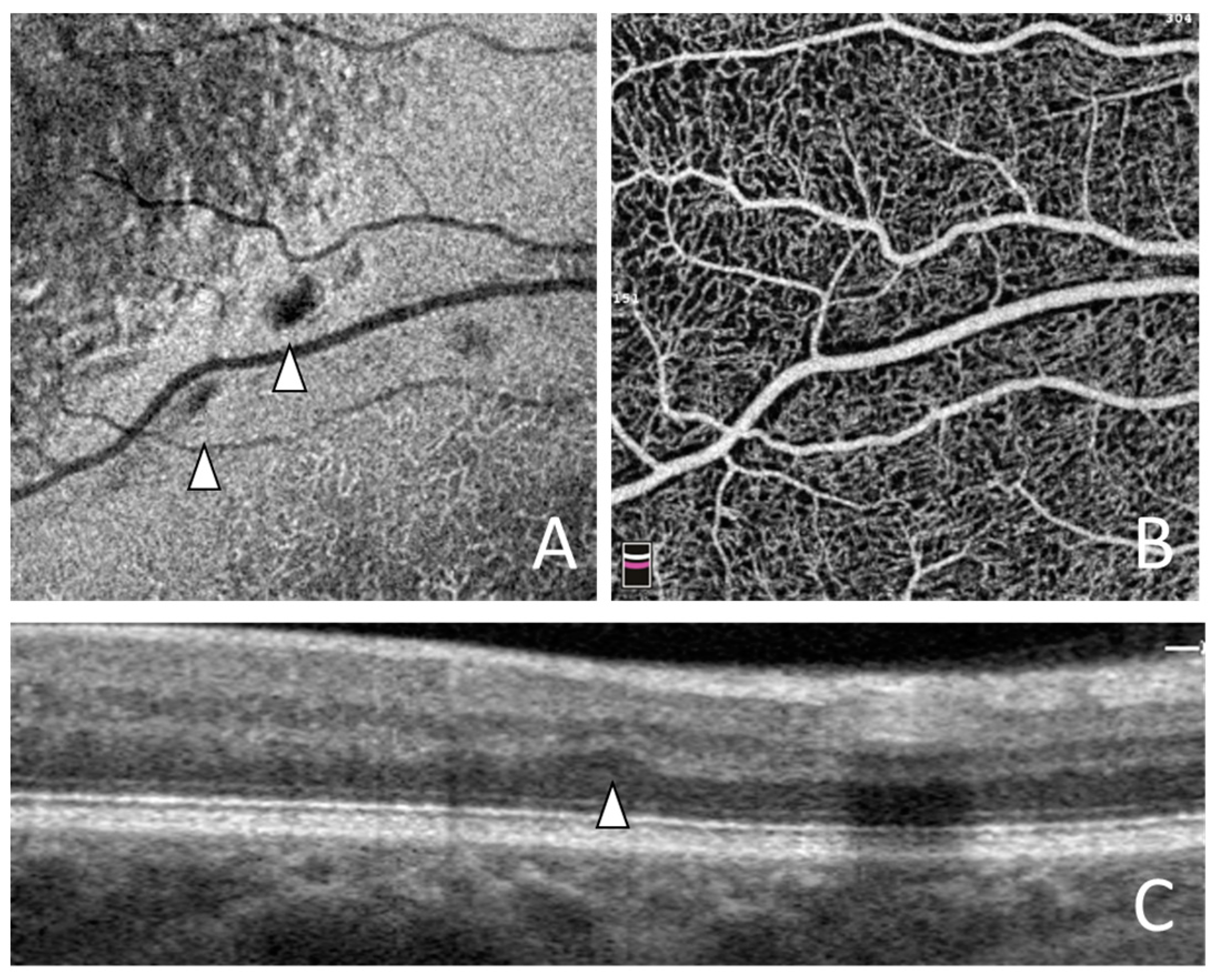
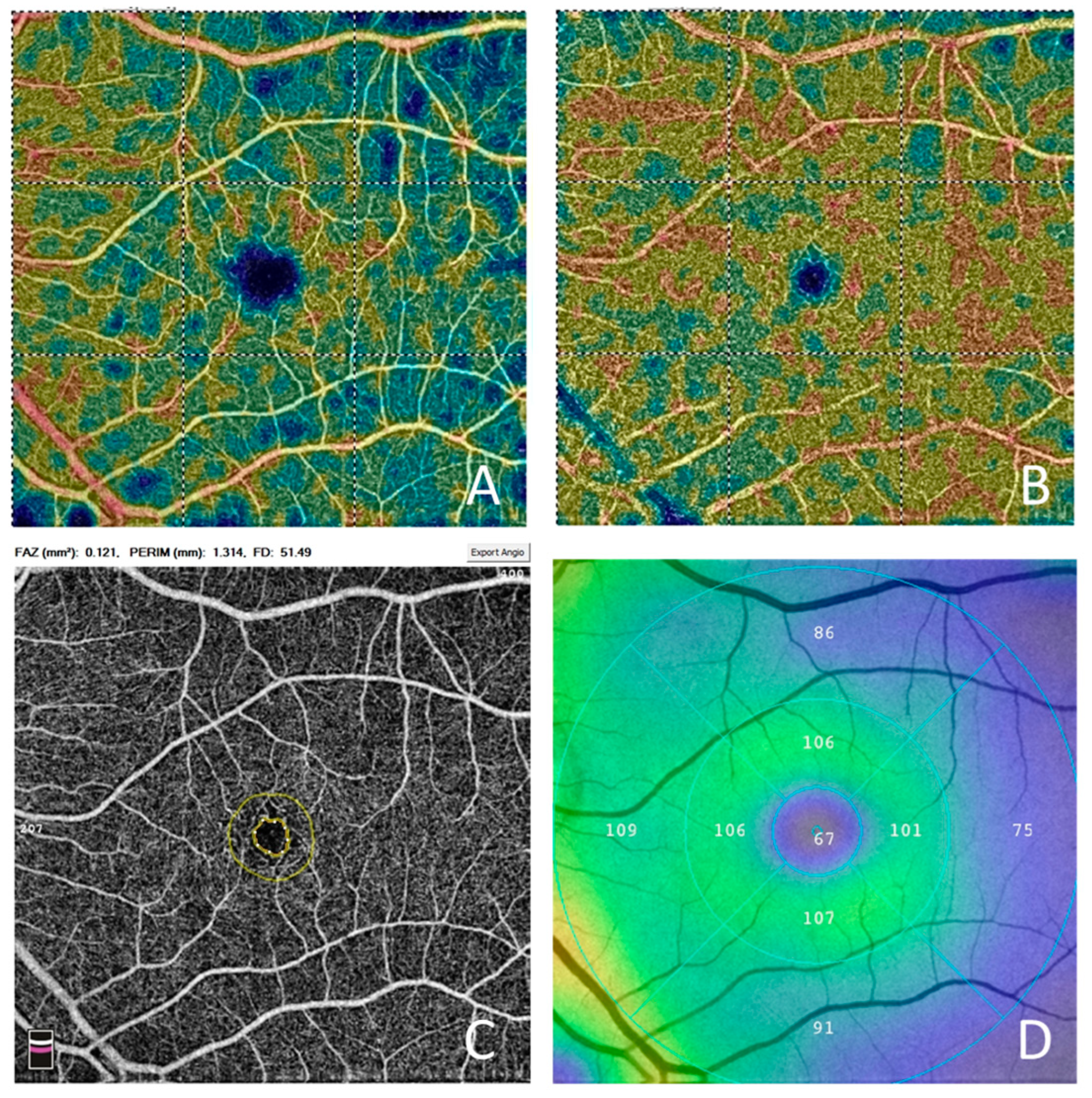
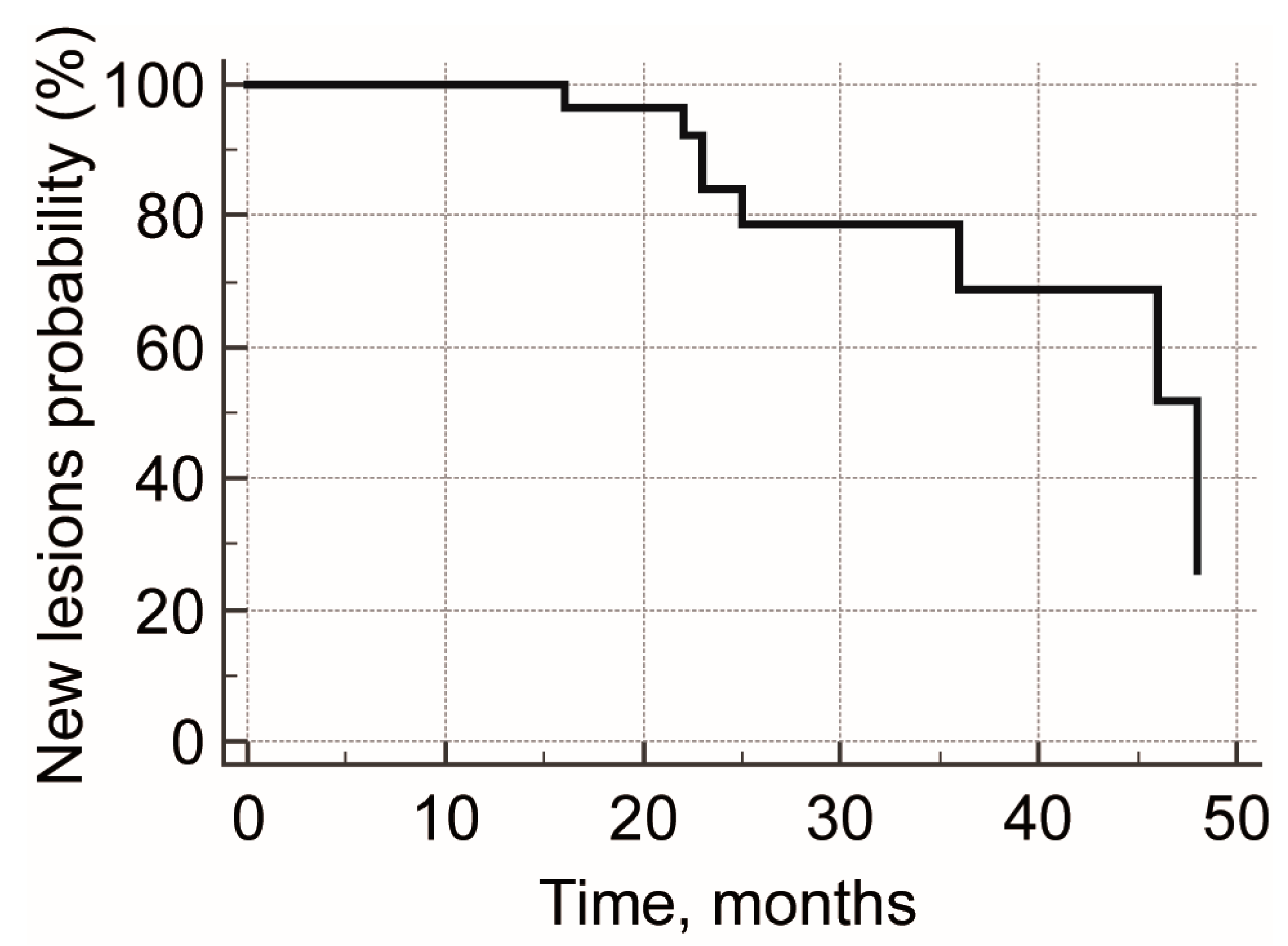
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jia, Y.; Wei, E.; Wang, X.; Zhang, X.; Morrison, J.C.; Parikh, M.; Lombardi, L.H.; Gattey, D.M.; Armour, R.L.; Edmunds, B.; et al. Optical Coherence Tomography Angiography of Optic Disc Perfusion in Glaucoma. Ophthalmology 2014, 121, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [PubMed]
- Nemiroff, J.; Kuehlewein, L.; Rahimy, E.; Tsui, I.; Doshi, R.; Gaudric, A.; Gorin, M.B.; Sadda, S.; Sarraf, D. Assessing Deep Retinal Capillary Ischemia in Paracentral Acute Middle Maculopathy by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 162, 121–132.e1. [Google Scholar] [CrossRef]
- Kulikov, A.N.; Maltsev, D.S.; Leongardt, T.A. Retinal Microvasculature Alteration in Paracentral Acute Middle Maculopathy and Acute Macular Neuroretinopathy. Retin. Cases Brief Rep. 2018; ahead of print. [Google Scholar] [CrossRef]
- Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M.A.; Chhablani, J. Prevalence of resolved paracentral acute middle maculopathy lesions in fellow eyes of patients with unilateral retinal vein occlusion. Acta Ophthalmol. 2019, 98, e22–e28. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M.A.; Freund, K.B. Vascular Microanatomy of Small Resolved Paracentral Acute Middle Maculopathy Lesions. Ophthalmol. Retin. 2020, 5, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Burnasheva, M.A.; Maltsev, D.S.; Kulikov, A.N.; Sherbakova, K.A.; Barsukov, A.V. Association of Chronic Paracentral Acute Middle Maculopathy Lesions with Hypertension. Ophthalmol. Retin. 2020, 4, 504–509. [Google Scholar] [CrossRef]
- Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M. Association of Resolved Paracentral Acute Middle Maculopathy Lesions with Diabetic Retinopathy. J. Curr. Ophthalmol. 2022, 34, 318–322. [Google Scholar] [CrossRef]
- Long, C.P.; Chan, A.X.; Bakhoum, C.Y.; Toomey, C.B.; Madala, S.; Garg, A.K.; Freeman, W.R.; Goldbaum, M.H.; DeMaria, A.N.; Bakhoum, M.F. Prevalence of subclinical retinal ischemia in patients with cardiovascular disease—A hypothesis driven study. EClinicalMedicine 2021, 33, 100775. [Google Scholar] [CrossRef]
- Maltsev, D.S.; Kulikov, A.N.; Kazak, A.A.; Burnasheva, M.A. Status of Choriocapillaris in Fellow Eyes of Patients with Unilateral Retinal Vein Occlusions. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, 23–28. [Google Scholar] [CrossRef]
- Sarraf, D.; Rahimy, E.; Fawzi, A.A.; Sohn, E.; Barbazetto, I.; Zacks, D.N.; Mittra, R.A.; Klancnik, J.M.; Mrejen, S.; Goldberg, N.R.; et al. Paracentral Acute Middle Maculopathy: A New Variant of Acute Macular Neuroretinopathy Associated with Retinal Capillary Ischemia. JAMA Ophthalmol. 2013, 131, 1275–1287. [Google Scholar] [CrossRef]
- Chen, X.; Rahimy, E.; Sergott, R.C.; Nunes, R.P.; Souza, E.C.; Choudhry, N.; Cutler, N.E.; Houston, S.K.; Munk, M.R.; Fawzi, A.A.; et al. Spectrum of Retinal Vascular Diseases Associated with Paracentral Acute Middle Maculopathy. Am. J. Ophthalmol. 2015, 160, 26–34.e1. [Google Scholar] [CrossRef] [PubMed]
- Dansingani, K.K.; Freund, K.B. Paracentral Acute Middle Maculopathy and Acute Macular Neuroretinopathy: Related and Distinct Entities. Am. J. Ophthalmol. 2015, 160, 1–3.e2. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, M.F.; Freund, K.B.; Dolz-Marco, R.; Leong, B.C.; Baumal, C.R.; Duker, J.S.; Sarraf, D. Paracentral Acute Middle Maculopathy and the Ischemic Cascade Associated with Retinal Vascular Occlusion. Arch. Ophthalmol. 2018, 195, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Pichi, F.; Fragiotta, S.; Freund, K.B.; Au, A.; Lembo, A.; Nucci, P.; Sebastiani, S.; Hernandez, J.C.G.; Interlandi, E.; Pellegrini, F.; et al. Cilioretinal artery hypoperfusion and its association with paracentral acute middle maculopathy. Br. J. Ophthalmol. 2019, 103, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M.A. Choriocapillaris alteration in patients with paracentral acute middle maculopathy. Eur. J. Ophthalmol. 2022, 32, 3622–3628. [Google Scholar] [CrossRef]
- Sridhar, J.; Shahlaee, A.; Rahimy, E.; Hong, B.K.; Khan, M.A.; Maguire, J.I.; Dunn, J.P.; Mehta, S.; Ho, A.C. Optical Coherence Tomography Angiography and En Face Optical Coherence Tomography Features of Paracentral Acute Middle Maculopathy. Arch. Ophthalmol. 2015, 160, 1259–1268.e2. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Phasukkijwatana, N.; Freund, K.B.; Cunningham, E.T.; Kalevar, A.; McDonald, H.R.; Dolz-Marco, R.; Roberts, P.K.; Tsui, I.; Rosen, R.; et al. En Face Optical Coherence Tomography Analysis to Assess the Spectrum of Perivenular Ischemia and Paracentral Acute Middle Maculopathy in Retinal Vein Occlusion. Am. J. Ophthalmol. 2017, 177, 131–138. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Huang, X.; Wu, Z.; Ke, B. Clinical Characteristics of Paracentral Acute Middle Maculopathy in Eyes with Retinal Vascular Occlusion Diseases in Chinese Patients. J. Ophthalmol. 2021, 2021, 8867570. [Google Scholar] [CrossRef]
- Hussnain, S.A.; Coady, P.A.; Stoessel, K.M. Paracentral acute middle maculopathy: Precursor to macular thinning in sickle cell retinopathy. BMJ Case Rep. 2017, 2017, bcr2016216124. [Google Scholar] [CrossRef]
- Ong, S.S.; Ahmed, I.; Scott, A.W. Association of Acute Macular Neuroretinopathy or Paracentral Acute Middle Maculopathy with Sickle Cell Disease. Ophthalmol. Retin. 2021, 5, 1146–1155. [Google Scholar] [CrossRef]
- Ahuja, A.S.; El-Dairi, M.A.; Hadziahmetovic, M.; Gospe, S.M. Paracentral Acute Middle Maculopathy as a Manifestation of Giant Cell Arteritis. J. Neuro-Ophthalmol. 2021, 41, e153–e156. [Google Scholar] [CrossRef] [PubMed]
- Broyles, H.; Chacko, J.; Chancellor, J.; LoRusso, F.; Phillips, P.H.; Mashayekhi, A.; Uwaydat, S. Paracentral Acute Middle Maculopathy as the Initial Presentation of Giant Cell Arteritis. J. Neuro-Ophthalmol. 2021, 41, e157–e159. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, J.J.; Echegaray, P.M.; Townsend-Pico, W. Penile Filler Injection Leading to Silicone Embolism Syndrome and Paracentral Acute Middle Maculopathy. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 635–638. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, B.M.R.; de Souza, L.M.; Andrade, G.C.; Lobos, C.Z.M.; de Souza, E.C.; de Moraes, N.S.B. Purtscher-like retinopathy and paracentral acute middle maculopathy caused by industrial silicone embolism. Retin. Cases Brief Rep. 2022, 16, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Iwama, Y.; Tanioka, K.; Emi, K. Paracentral Acute Middle Maculopathy following Vitrectomy for Proliferative Diabetic Retinopathy. Ophthalmology 2018, 125, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Dasari, V.R.; Selliyan, A.; Gratton, S.M. Paracentral acute middle maculopathy in a patient with frequent migraine with aura: A case report. Retin. Cases Brief Rep. 2022, 16, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Shahlaee, A.; Sridhar, J.; Rahimy, E.; Shieh, W.S.; Ho, A.C. Paracentral acute middle maculopathy associated with postviral purtscher-like retinopathy. Retin. Cases Brief Rep. 2019, 13, 50–53. [Google Scholar] [CrossRef]
- Çebi, A.Y.; Kılıçarslan, O.; Uçar, D. Coincident Acute Macular Neuroretinopathy and Paracentral Acute Middle Maculopathy in COVID-19. Turk. J. Ophthalmol. 2023, 53, 120–123. [Google Scholar] [CrossRef]
- Madala, S.; Adabifirouzjaei, F.; Lando, L.; Yarmohammadi, A.; Long, C.P.; Bakhoum, C.Y.; Goldbaum, M.H.; Sarraf, D.; DeMaria, A.N.; Bakhoum, M.F. Retinal Ischemic Perivascular Lesions, a Biomarker of Cardiovascular Disease. Ophthalmol. Retin. 2022, 6, 865–867. [Google Scholar] [CrossRef]
- Kido, A.; Uji, A.; Morooka, S.; Kuroda, Y.; Arichika, S.; Akagi, T.; Tsujikawa, A. Outer Plexiform Layer Elevations as a Marker for Prior Ocular Attacks in Patients with Behcet’s Disease. Investig. Opthalmol. Vis. Sci. 2018, 59, 2828–2832. [Google Scholar] [CrossRef]
- Spaide, R.F. Diminutive paracentral acute middle maculopathy lesion. Retin. Cases Brief Rep. 2021, 15, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, L.; Nguyen, M.; Chang, M.; Park, S.S. Repeatability of Vascular Density Measurement of the Three Retinal Plexus Layers Using OCT Angiography in Pathologic Eyes (OCTA Vascular Density Repeatability of Three Plexus Layers). Clin. Ophthalmol. 2021, 15, 93–103. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Freeman, W.R.; Weinreb, R.N.; Zangwill, L.; Manalastas, P.I.C.; Saunders, L.J.; Nudleman, E. Reproducibility of vessel density measurement with optical coherence tomography angiography in eyes with and without retinopathy. Retina 2017, 37, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, J.; Zhang, B.; Lu, P. Association of glaucoma with risk of retinal vein occlusion: A meta-analysis. Acta Ophthalmol. 2019, 97, 652–659. [Google Scholar] [CrossRef]

| RVO (n = 41) | BRVO (n = 25) | CRVO (n = 16) | |
|---|---|---|---|
| Age (y) | 63.46 ± 10.13 | 62.44 ± 10.95 | 65.06 ± 8.77 |
| Males/Females | 29/12 | 17/8 | 12/4 |
| PAMM lesions at baseline | 6.5 ± 5.7 | 5.4 ± 4.7 | 8.3 ± 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M.A.; Kalinicheva, Y.A.; Vasiliev, A.S. Small Paracentral Acute Middle Maculopathy Lesions as Biomarker of Vascular Morbidity: Natural Course. J. Vasc. Dis. 2024, 3, 67-76. https://doi.org/10.3390/jvd3010006
Maltsev DS, Kulikov AN, Burnasheva MA, Kalinicheva YA, Vasiliev AS. Small Paracentral Acute Middle Maculopathy Lesions as Biomarker of Vascular Morbidity: Natural Course. Journal of Vascular Diseases. 2024; 3(1):67-76. https://doi.org/10.3390/jvd3010006
Chicago/Turabian StyleMaltsev, Dmitrii S., Alexei N. Kulikov, Maria A. Burnasheva, Yana A. Kalinicheva, and Alexander S. Vasiliev. 2024. "Small Paracentral Acute Middle Maculopathy Lesions as Biomarker of Vascular Morbidity: Natural Course" Journal of Vascular Diseases 3, no. 1: 67-76. https://doi.org/10.3390/jvd3010006
APA StyleMaltsev, D. S., Kulikov, A. N., Burnasheva, M. A., Kalinicheva, Y. A., & Vasiliev, A. S. (2024). Small Paracentral Acute Middle Maculopathy Lesions as Biomarker of Vascular Morbidity: Natural Course. Journal of Vascular Diseases, 3(1), 67-76. https://doi.org/10.3390/jvd3010006






