The Emerging Role of NaF-PET/CT in Detecting Vascular Microcalcification in the Pathogenesis of Neurological Dysfunction
Abstract
1. Introduction
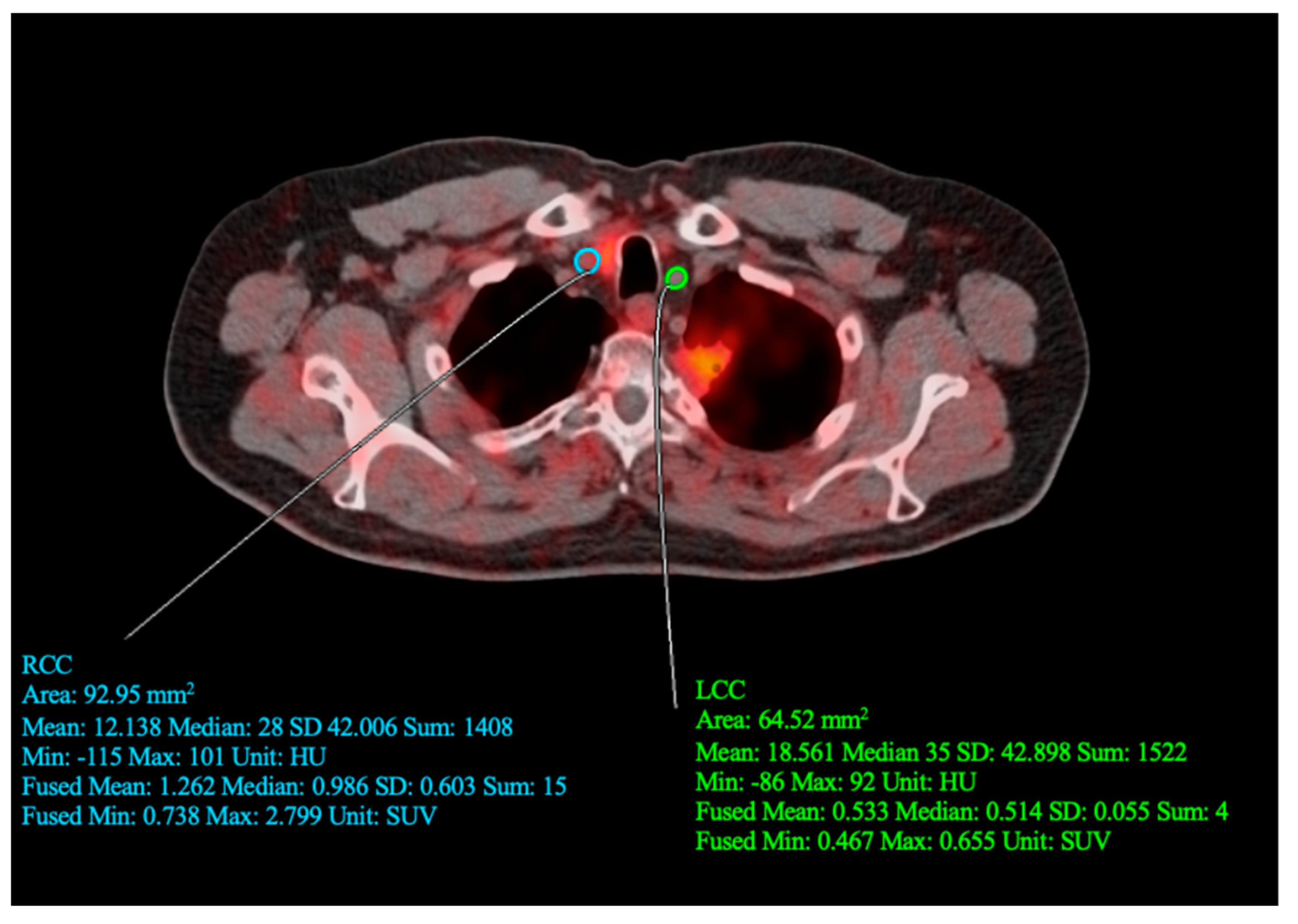
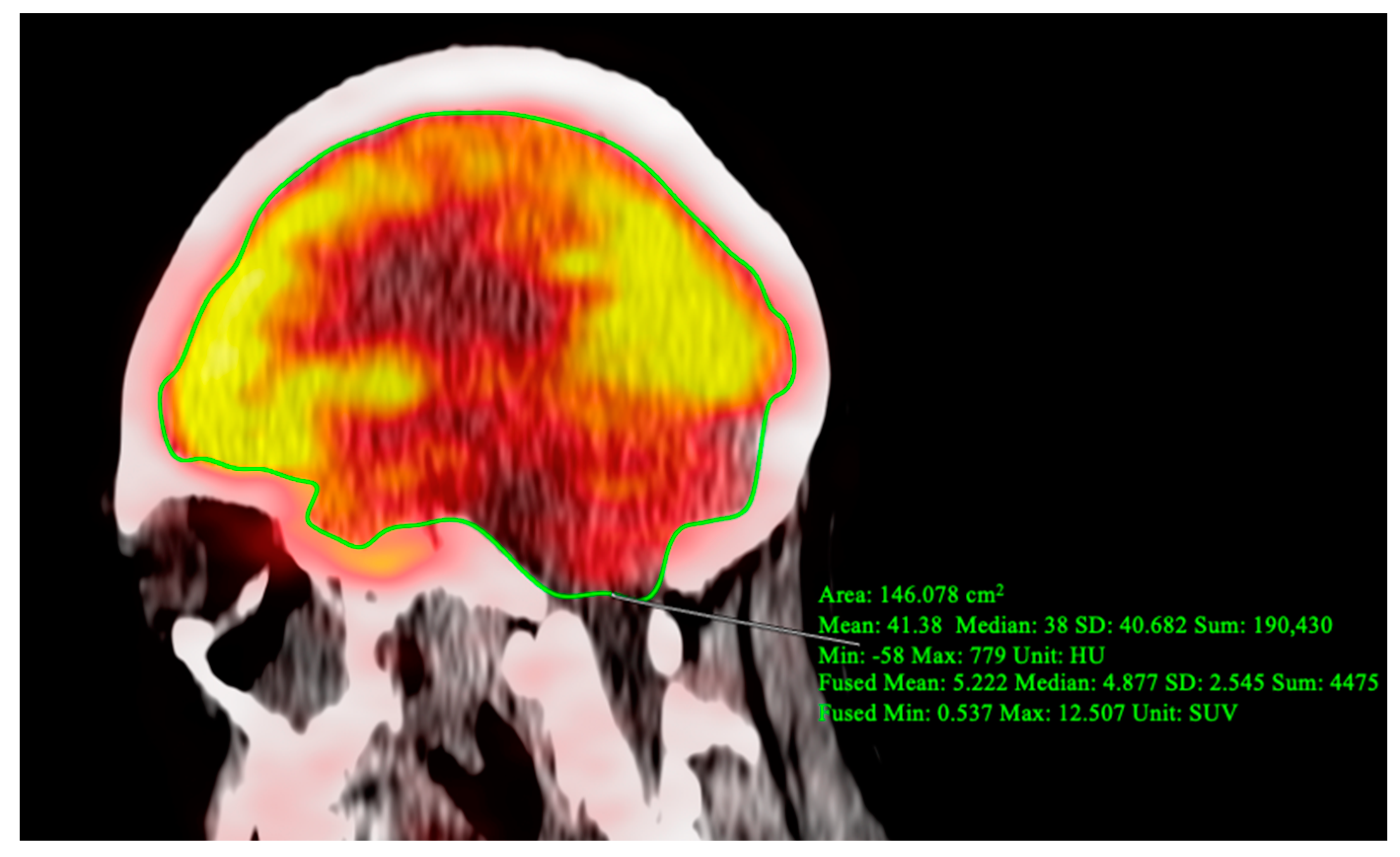
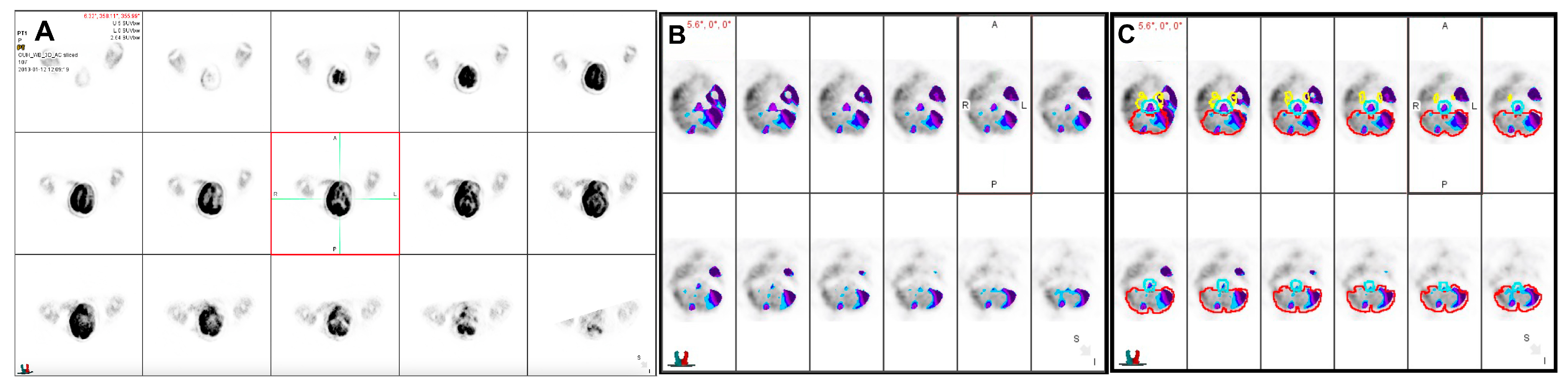
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J. Heart disease and stroke statistics: 2016 update—A report from the American Heart Association. Circulation 2016, 133, e38–360. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Ng, S.J.; Lau, H.C.; Khanal, K.; Bhattarai, S.; Paudyal, P.; Shrestha, B.B.; Naseer, R.; Sandhu, S.; Gokhale, S.; et al. Emerging PET Tracers in Cardiac Molecular Imaging. Cardiol. Ther. 2023, 12, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The Use of (18)F-FDG-PET/CT for Diagnosis and Treatment Monitoring of Inflammatory and Infectious Diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.L.; Subramanian, S.S.; Cury, R.C.; Truong, Q.A.; Gardecki, J.A.; Tearney, G.J.; Hoffmann, U.; Brady, T.J.; Tawakol, A. Distribution of Inflammation within Carotid Atherosclerotic Plaques with High-Risk Morphological Features: A Comparison between Positron Emission Tomography Activity, Plaque Morphology, and Histopathology. Circ. Cardiovasc. Imaging 2012, 5, 69–77. [Google Scholar] [CrossRef]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of Coronary Atherosclerotic Plaques From Computed Tomography Imaging: A Review of Recent Methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef] [PubMed]
- Tzolos, E.; Dweck, M.R. 18F-Sodium Fluoride (18F-NaF) for Imaging Microcalcification Activity in the Cardiovascular System. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.A.; Thomassen, A.; de Jong, P.A.; Simonsen, J.A.; Lam, M.G.E.H.; Nielsen, A.L.; Mickley, H.; Mali, W.P.T.M.; Alavi, A.; Høilund-Carlsen, P.F. Impact of Personal Characteristics and Technical Factors on Quantification of Sodium 18F-Fluoride Uptake in Human Arteries: Prospective Evaluation of Healthy Subjects. J. Nucl. Med. 2015, 56, 1534–1540. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.V.; Calvert, P.A.; Craighead, F.H.M.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-Fluoride Positron Emission Tomography for Identification of Ruptured and High-Risk Coronary Atherosclerotic Plaques: A Prospective Clinical Trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Piri, R.; Gerke, O.; Sturek, M.; Werner, T.J.; Revheim, M.-E.; Alavi, A. NaF-PET Imaging of Atherosclerosis Burden. J. Imaging 2023, 9, 31. [Google Scholar] [CrossRef]
- Paydary, K.; Revheim, M.-E.; Emamzadehfard, S.; Gholami, S.; Pourhassan, S.; Werner, T.J.; Høilund-Carlsen, P.F.; Alavi, A. Quantitative Thoracic Aorta Calcification Assessment by 18F-NaF PET/CT and Its Correlation with Atherosclerotic Cardiovascular Disorders and Increasing Age. Eur. Radiol. 2021, 31, 785–794. [Google Scholar] [CrossRef]
- Teichner, E.M.; You, J.C.; Hriso, C.; Wintering, N.A.; Zabrecky, G.P.; Alavi, A.; Bazzan, A.J.; Monti, D.A.; Newberg, A.B. Alterations in Cerebral Glucose Metabolism as Measured by 18F-Fluorodeoxyglucose-PET in Patients with Persistent Postconcussion Syndrome. Nucl. Med. Commun. 2021, 42, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Partovi, S.; Yuh, R.; Pirozzi, S.; Lu, Z.; Couturier, S.; Grosse, U.; Schluchter, M.D.; Nelson, A.; Jones, R.; O’Donnell, J.K.; et al. Diagnostic Performance of an Automated Analysis Software for the Diagnosis of Alzheimer’s Dementia with 18F FDG PET. Am J. Nucl. Med. Mol. Imaging 2017, 7, 12–23. [Google Scholar] [PubMed]
- Jadvar, H.; Alavi, A.; Gambhir, S.S. 18F-FDG Uptake in Lung, Breast, and Colon Cancers: Molecular Biology Correlates and Disease Characterization. J. Nucl. Med. 2009, 50, 1820–1827. [Google Scholar] [CrossRef]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.E.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying Active Vascular Microcalcification by 18F-Sodium Fluoride Positron Emission Tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.A.; Thomassen, A.; Takx, R.A.P.; Vilstrup, M.H.; Hess, S.; Nielsen, A.L.; Diederichsen, A.C.P.; Mickley, H.; Alavi, A.; Høilund-Carlsen, P.F. Delayed Sodium 18F-Fluoride PET/CT Imaging Does Not Improve Quantification of Vascular Calcification Metabolism: Results from the CAMONA Study. J. Nucl. Cardiol. 2014, 21, 293–304. [Google Scholar] [CrossRef]
- Leng, X.; Lan, L.; Ip, V.H.L.; Liu, H.; Abrigo, J.; Liebeskind, D.S.; Wong, L.K.S.; Leung, T.W. Noninvasive Fractional Flow in Intracranial Atherosclerotic Stenosis: Reproducibility, Limitations, and Perspectives. J. Neurol. Sci. 2017, 381, 150–152. [Google Scholar] [CrossRef]
- Leng, X.; Lan, L.; Ip, H.L.; Abrigo, J.; Scalzo, F.; Liu, H.; Feng, X.; Chan, K.L.; Fan, F.S.Y.; Ma, S.H.; et al. Hemodynamics and Stroke Risk in Intracranial Atherosclerotic Disease. Ann. Neurol. 2019, 85, 752–764. [Google Scholar] [CrossRef]
- Beheshti, M.; Saboury, B.; Mehta, N.N.; Torigian, D.A.; Werner, T.; Mohler, E.; Wilensky, R.; Newberg, A.B.; Basu, S.; Langsteger, W.; et al. Detection and Global Quantification of Cardiovascular Molecular Calcification by Fluoro18-Fluoride Positron Emission Tomography/Computed Tomography--A Novel Concept. Hell J. Nucl. Med. 2011, 14, 114–120. [Google Scholar]
- Dweck, M.R.; Chow, M.W.L.; Joshi, N.V.; Williams, M.C.; Jones, C.; Fletcher, A.M.; Richardson, H.; White, A.; McKillop, G.; van Beek, E.J.R.; et al. Coronary Arterial 18F-Sodium Fluoride Uptake: A Novel Marker of Plaque Biology. J. Am. Coll. Cardiol. 2012, 59, 1539–1548. [Google Scholar] [CrossRef]
- Tawakol, A.; Fayad, Z.A.; Mogg, R.; Alon, A.; Klimas, M.T.; Dansky, H.; Subramanian, S.S.; Abdelbaky, A.; Rudd, J.H.F.; Farkouh, M.E.; et al. Intensification of Statin Therapy Results in a Rapid Reduction in Atherosclerotic Inflammation: Results of a Multicenter Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Feasibility Study. J. Am. Coll. Cardiol. 2013, 62, 909–917. [Google Scholar] [CrossRef]
- Cistaro, A.; Pagani, M.; Montuschi, A.; Calvo, A.; Moglia, C.; Canosa, A.; Restagno, G.; Brunetti, M.; Traynor, B.J.; Nobili, F.; et al. The Metabolic Signature of C9ORF72-Related ALS: FDG PET Comparison with Nonmutated Patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Normandin, M.D.; Vanderburg, C.R.; Costantino, I.M.; Bien, E.A.; Rycyna, L.G.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.L.; et al. Validating Novel Tau Positron Emission Tomography Tracer [F-18]-AV-1451 (T807) on Postmortem Brain Tissue. Ann Neurol 2015, 78, 787–800. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teichner, E.M.; Subtirelu, R.C.; Ashok, A.B.; Su, Y.; Anderson, V.A.; Writer, M.; Al-Daoud, O.; Ismoilov, M.; Raynor, W.Y.; Werner, T.J.; et al. The Emerging Role of NaF-PET/CT in Detecting Vascular Microcalcification in the Pathogenesis of Neurological Dysfunction. J. Vasc. Dis. 2023, 2, 310-316. https://doi.org/10.3390/jvd2030023
Teichner EM, Subtirelu RC, Ashok AB, Su Y, Anderson VA, Writer M, Al-Daoud O, Ismoilov M, Raynor WY, Werner TJ, et al. The Emerging Role of NaF-PET/CT in Detecting Vascular Microcalcification in the Pathogenesis of Neurological Dysfunction. Journal of Vascular Diseases. 2023; 2(3):310-316. https://doi.org/10.3390/jvd2030023
Chicago/Turabian StyleTeichner, Eric M., Robert C. Subtirelu, Arjun B. Ashok, Yvonne Su, Victoria A. Anderson, Milo Writer, Omar Al-Daoud, Miraziz Ismoilov, William Y. Raynor, Thomas J. Werner, and et al. 2023. "The Emerging Role of NaF-PET/CT in Detecting Vascular Microcalcification in the Pathogenesis of Neurological Dysfunction" Journal of Vascular Diseases 2, no. 3: 310-316. https://doi.org/10.3390/jvd2030023
APA StyleTeichner, E. M., Subtirelu, R. C., Ashok, A. B., Su, Y., Anderson, V. A., Writer, M., Al-Daoud, O., Ismoilov, M., Raynor, W. Y., Werner, T. J., Høilund-Carlsen, P. F., Alavi, A., & Revheim, M.-E. (2023). The Emerging Role of NaF-PET/CT in Detecting Vascular Microcalcification in the Pathogenesis of Neurological Dysfunction. Journal of Vascular Diseases, 2(3), 310-316. https://doi.org/10.3390/jvd2030023








