Survey on Cardiogenic Shock and the Use of ECMO and Impella in Spanish Cardiac Critical Care Units
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Population
2.3. Sample Size and Statistical Analysis
3. Results
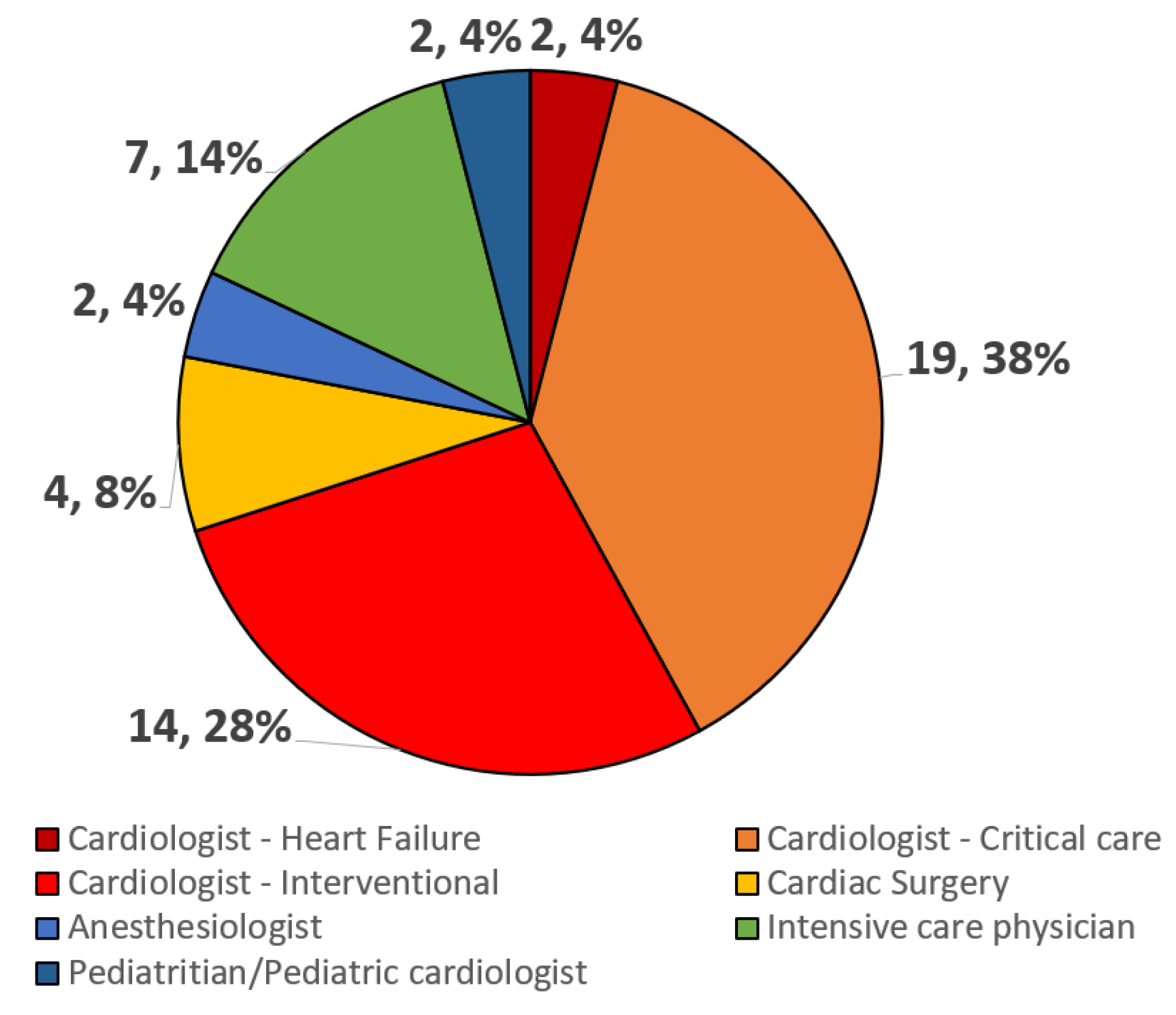
3.1. Participants and Centers
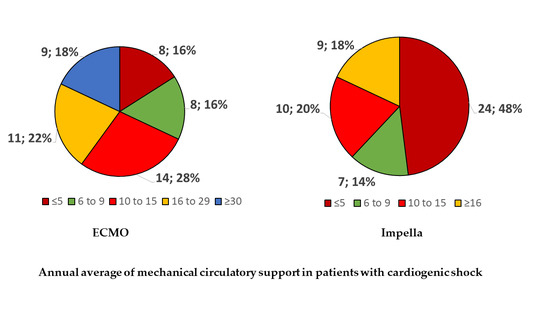
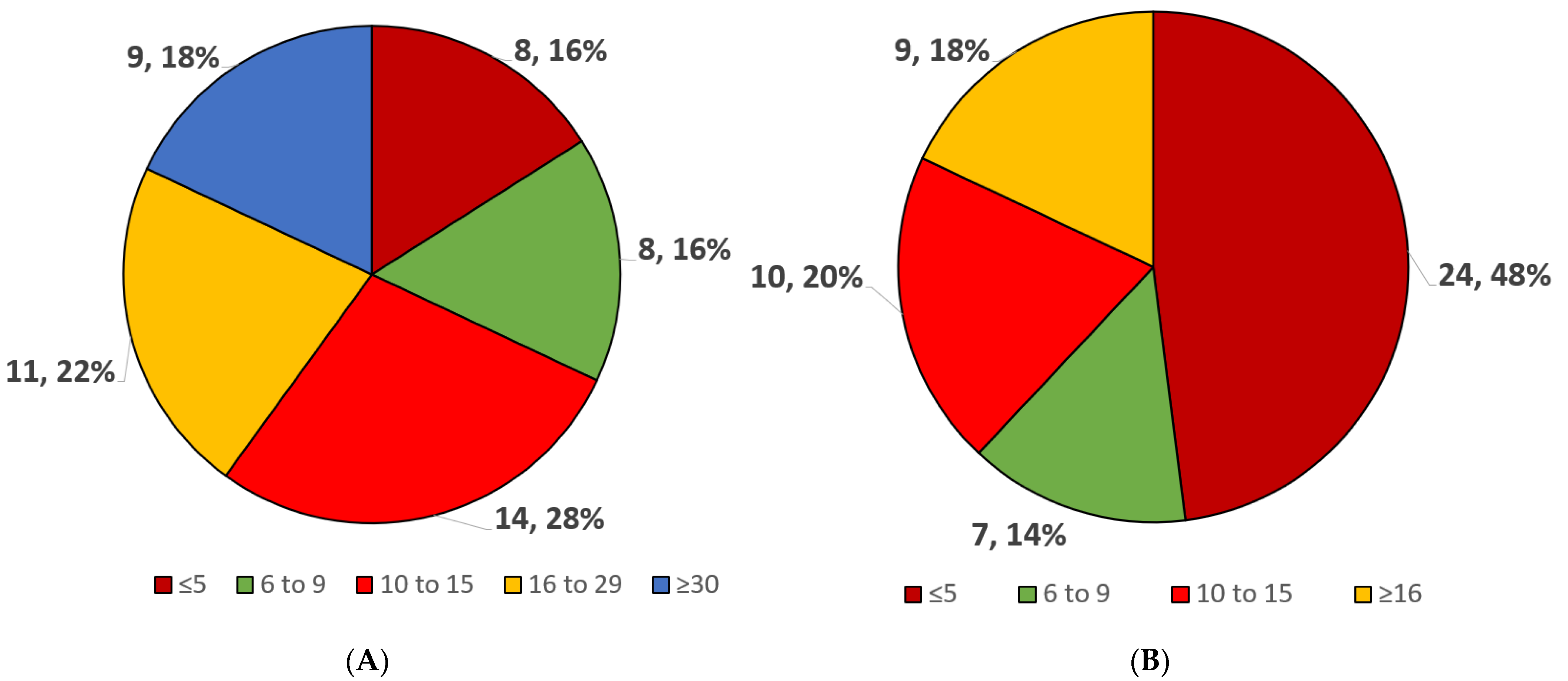
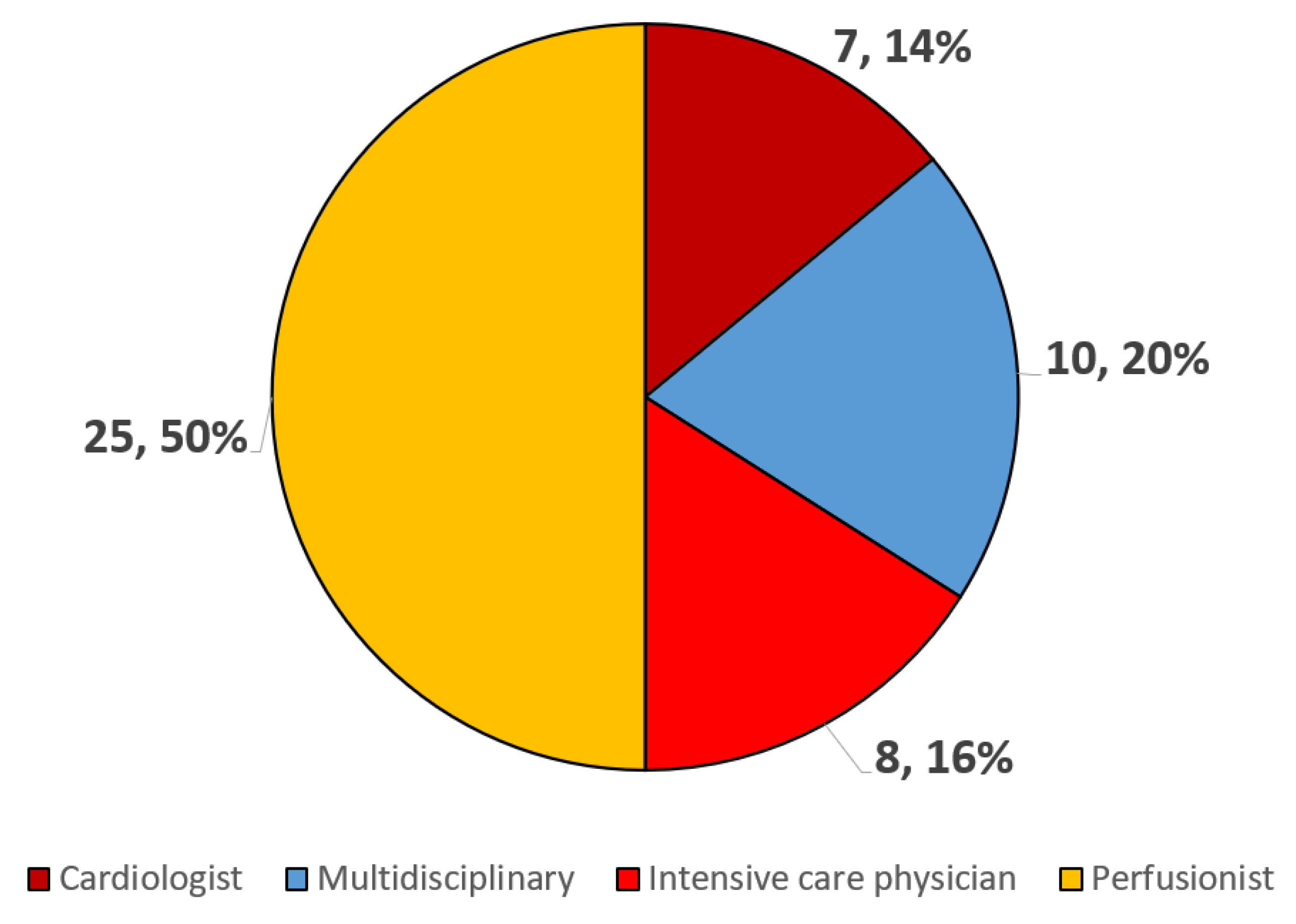
3.2. MCS Volume, Type, and Logistics
3.3. Protocols and Training
3.4. Patient Management and Drugs
3.5. Pulmonary Thromboembolism
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Martínez-Solano, J.; Sousa-Casasnovas, I.; Bellón-Cano, J.M.; García-Carreño, J.; Juárez-Fernández, M.; Díez-Delhoyo, F.; Sanz-Ruiz, R.; Devesa-Cordero, C.; Elízaga-Corrales, J.; Fernández-Avilés, F.; et al. Lactate levels as a prognostic predict in cardiogenic shock under venoarterial extracorporeal membrane oxygenation support. Rev. Esp. Cardiol. 2022, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Parlow, S.; Di Santo, P.; Sterling, L.H.; Goodliffe, L.; Motazedian, P.; Prosperi-Porta, G.; Morgan, B.; Koopman, Z.; Jung, R.G.; Lepage-Ratte, M.F.; et al. Inotrope versus placebo therapy in cardiogenic shock: Rationale and study design of the CAPITAL DOREMI2 trial. Am. Heart J. 2023, 262, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Sundermeyer, J.; Beer, B.N.; Bertoldi, L.; Bernhardt, A.; Blankenberg, S.; Dauw, J.; Dindane, Z.; Eckner, D.; Eitel, I.; et al. Use of mechanical circulatory support in patients with non-ischaemic cardiogenic shock. Eur. J. Heart Fail. 2023, 25, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Schmack, L.; Schmack, B.; Papathanasiou, M.; Al-Rashid, F.; Weymann, A.; Pizanis, N.; Kamler, M.; Ruhparwar, A.; Rassaf, T.; Luedike, P. Central extracorporeal circulatory life support (cECLS) in selected patients with critical cardiogenic shock. Front. Cardiovasc. Med. 2023, 10, 1142953. [Google Scholar] [CrossRef] [PubMed]
- Ardito, V.; Sarucanian, L.; Rognoni, C.; Pieri, M.; Scandroglio, A.M.; Tarricone, R. Impella Versus VA-ECMO for Patients with Cardiogenic Shock: Comprehensive Systematic Literature Review and Meta-Analyses. J. Cardiovasc. Dev. Dis. 2023, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Belford, P.M.; Truesdell, A.G.; Sinha, S.S.; Zhao, D.X.; Vallabhajosyula, S. Meta-Analysis on Left Ventricular Unloading with Impella in Patients with Cardiogenic Shock Receiving Venoarterial Extracorporeal Membrane Oxygenation. Am. J. Cardiol. 2023, 193, 52–54. [Google Scholar] [CrossRef]
- Pinna, S.M.; Casasnovas, I.S.; Olmedo, M.; Machado, M.; Fernández, M.J.; Devesa-Cordero, C.; Galar, A.; Alvarez-Uria, A.; Fernández-Avilés, F.; Carreño, J.G.; et al. Nosocomial Infections in Adult Patients Supported by Extracorporeal Membrane Oxygenation in a Cardiac Intensive Care Unit. Microorganisms 2023, 11, 1079. [Google Scholar] [CrossRef]
- Sklar, M.C.; Sy, E.; Lequier, L.; Fan, E.; Kanji, H.D. Anticoagulation Practices during Venovenous Extracorporeal Membrane Oxygenation for Respiratory Failure. A Systematic Review. Ann. Am. Thorac. Soc. 2016, 13, 2242–2250. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Currò, J.M.; La Via, L.; Dezio, V.; Martucci, G.; Brancati, S.; Murabito, P.; Pappalardo, F.; Astuto, M. Use of nafamostat mesilate for anticoagulation during extracorporeal membrane oxygenation: A systematic review. Artif. Organs 2022, 46, 2371–2381. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Murabito, P.; Pappalardo, F.; Astuto, M. More evidence available for the use of Bivalirudin in patients supported by extracorporeal membrane oxygenation. Thromb. Res. 2022, 211, 148–149. [Google Scholar] [CrossRef]
- Beavers, C.J.; DiDomenico, R.J.; Dunn, S.P.; Cox, J.; To, L.; Weeks, P.; Trujillo, T.C.; Jennings, D.L. Optimizing anticoagulation for patients receiving Impella support. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Vandenbriele, C.; Arachchillage, D.J.; Frederiks, P.; Giustino, G.; Gorog, D.A.; Gramegna, M.; Janssens, S.; Meyns, B.; Polzin, A.; Scandroglio, M.; et al. Anticoagulation for Percutaneous Ventricular Assist Device-Supported Cardiogenic Shock: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Succar, L.; Sulaica, E.M.; Donahue, K.R.; Wanat, M.A. Management of Anticoagulation with Impella® Percutaneous Ventricular Assist Devices and Review of New Literature. J. Thromb. Thrombolysis 2019, 48, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Martin-Villen, L.; Martínez-Sellés, M.; Diaz-Fernandez, J.F.; Zapata-Fenor, L. Cardiogenic shock code 2023, towards a quality multidisciplinary organization. Med. Intensiv. 2023, 47, 406–410. [Google Scholar] [CrossRef]
- Araki, T.; Kondo, T.; Imaizumi, T.; Sumita, Y.; Nakai, M.; Tanaka, A.; Okumura, T.; Yang, M.; Butt, J.H.; Petrie, M.C.; et al. Relationship between the volume of cases and in-hospital mortality in patients with cardiogenic shock receiving short-term mechanical circulatory support. Am. Heart J. 2023, 261, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Linke, N.J.; Fulcher, B.J.; Engeler, D.M.; Anderson, S.; Bailey, M.J.; Bernard, S.; Board, J.V.; Brodie, D.; Buhr, H.; Burrell, A.J.C.; et al. A survey of extracorporeal membrane oxygenation practice in 23 Australian adult intensive care units. Crit. Care Resusc. 2020, 22, 166–170. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Hernández-Pérez, F.J.; Uribarri, A.; Villén, L.M.; Zapata, L.; Alonso, J.J.; Amat-Santos, I.J.; Ariza-Solé, A.; Barrabés, J.A.; Barrio, J.M.; et al. Cardiogenic shock code 2023. Expert document for a multidisciplinary organization that allows quality care. Rev. Esp. Cardiol. (Engl. Ed.) 2023, 76, 261–269. [Google Scholar] [CrossRef]
- Sharma, A.; Minh Duc, N.T.; Luu Lam Thang, T.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef]
- Gómez-Sánchez, R.; García-Carreño, J.; Martínez-Solano, J.; Sousa-Casasnovas, I.; Juárez-Fernández, M.; Devesa-Cordero, C.; Sanz-Ruiz, R.; Gutiérrez-Ibañes, E.; Elízaga, J.; Fernández-Avilés, F.; et al. Off-Hours versus Regular-Hours Implantation of Peripheral Venoarterial Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock. J. Clin. Med. 2023, 12, 1875. [Google Scholar] [CrossRef]
- Xiao, X.; Bloom, J.E.; Andrew, E.; Dawson, L.P.; Nehme, Z.; Stephenson, M.; Anderson, D.; Fernando, H.; Noaman, S.; Cox, S.; et al. Age as a predictor of clinical outcomes and determinant of therapeutic measures for emergency medical services treated cardiogenic shock. J. Geriatr. Cardiol. 2023, 20, 1–10. [Google Scholar] [CrossRef]
- Scupakova, N.; Urbonas, K.; Jankuviene, A.; Puodziukaite, L.; Andrijauskas, P.; Janusauskas, V.; Zorinas, A.; Laurusonis, K.; Serpytis, P.; Samalavicius, R. Extracorporeal Life Support for Cardiogenic Shock in Octogenarians: Single Center Experience. J. Clin. Med. 2023, 12, 585. [Google Scholar] [CrossRef]
- Delmas, C.; Pernot, M.; Le Guyader, A.; Joret, R.; Roze, S.; Lebreton, G. Budget Impact Analysis of Impella CP® Utilization in the Management of Cardiogenic Shock in France: A Health Economic Analysis. Adv. Ther. 2022, 39, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.N.J.; Dammassa, V.; Klersy, C.; Camporotondo, R.; Pellegrini, C.; Mojoli, F.; Tavazzi, G. Heart rate control and haemodynamic improvement with ivabradine in cardiogenic shock patient on mechanical circulatory support. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Yao, Y.; Li, Y.; Qin, W.; Li, Y.; Xue, W.; Li, P.; Chen, Y.; Chen, X.; et al. Effects of levosimendan on the outcome of veno-arterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. Clin. Res. Cardiol. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Horowitz, J.M.; Beam, D.; Jaber, W.A.; Khandhar, S.; Toma, C.; Weinberg, M.D.; Mina, B. Prevalence and Predictors of Cardiogenic Shock in Intermediate-Risk Pulmonary Embolism: Insights From the FLASH Registry. JACC Cardiovasc. Interv. 2023, 16, 958–972. [Google Scholar] [CrossRef]
| Specific CS Training in Other Center | Receptors of Professionals to Be Trained on CS |
|---|---|
| No (3–6%) | No (24–48%) |
| Only physicians (17–34%) | Only physicians (13–26%) |
| Only nurses (1–2%) | Only nurses (1–2%) |
| Both (29–58%) | Both (12–24%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sellés, M.; García Carreño, J.; Martínez-Solano, J.; Sousa, I.; Juárez-Fernández, M. Survey on Cardiogenic Shock and the Use of ECMO and Impella in Spanish Cardiac Critical Care Units. J. Vasc. Dis. 2023, 2, 299-309. https://doi.org/10.3390/jvd2030022
Martínez-Sellés M, García Carreño J, Martínez-Solano J, Sousa I, Juárez-Fernández M. Survey on Cardiogenic Shock and the Use of ECMO and Impella in Spanish Cardiac Critical Care Units. Journal of Vascular Diseases. 2023; 2(3):299-309. https://doi.org/10.3390/jvd2030022
Chicago/Turabian StyleMartínez-Sellés, Manuel, Jorge García Carreño, Jorge Martínez-Solano, Iago Sousa, and Miriam Juárez-Fernández. 2023. "Survey on Cardiogenic Shock and the Use of ECMO and Impella in Spanish Cardiac Critical Care Units" Journal of Vascular Diseases 2, no. 3: 299-309. https://doi.org/10.3390/jvd2030022
APA StyleMartínez-Sellés, M., García Carreño, J., Martínez-Solano, J., Sousa, I., & Juárez-Fernández, M. (2023). Survey on Cardiogenic Shock and the Use of ECMO and Impella in Spanish Cardiac Critical Care Units. Journal of Vascular Diseases, 2(3), 299-309. https://doi.org/10.3390/jvd2030022