Zoledronate-Induced Large Vessel Vasculitis Diagnosed by PET/CT
Abstract
1. Introduction
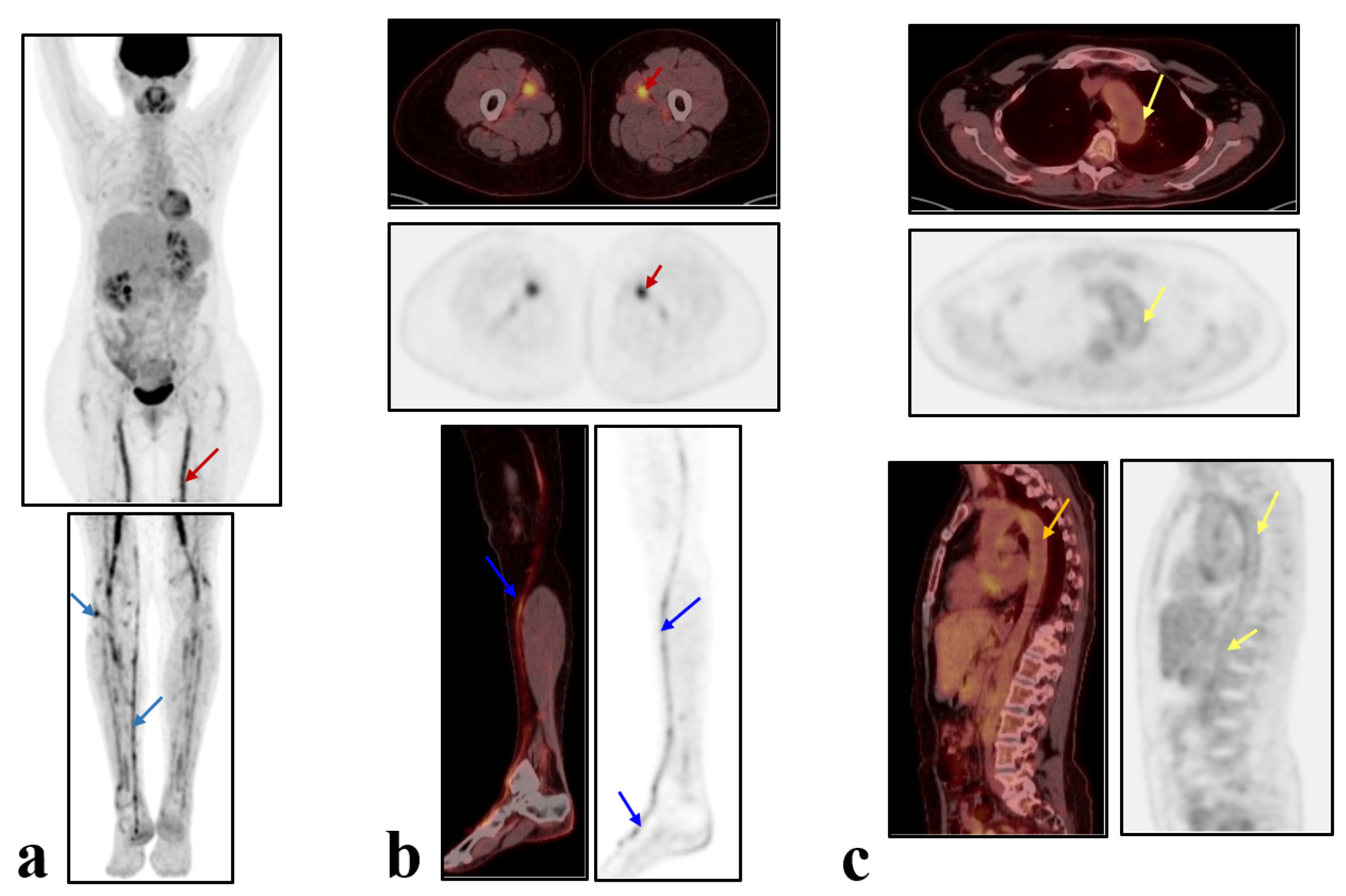
2. Case Presentation
3. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef] [PubMed]
- Metyas, S.; Ibrahim, M.; Solyman, J.; Yeter, K.C.; Arkfeld, D.G. Giant cell arteritis with visual loss following zoledronic acid infusion. Int. J. Rheum. Dis. 2014, 17, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N. Rare orbital manifestations and vasculitis, giant cell arteritis or side effects of zoledronic acid infusion? Arch. Gen. Intern. Med. 2019, 3, 6–7. [Google Scholar]
- Adler, R.A. Update on Rare Adverse Events from Osteoporosis Therapy and Bisphosphonate Drug Holidays. Endocrinol. Metab. Clin. N. Am. 2021, 50, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, S.; Santini, D.; Vincenzi, B.; Caccamo, N.; Meraviglia, F.; Salerno, A.; Dieli, F.; Tonini, G. Immunomodulating role of bisphosphonates on human gamma delta T cells: An intriguing and promising aspect of their antitumour activity. Expert Opin. Ther. Targets 2007, 11, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Dicuonzo, G.; Vincenzi, B.; Santini, D.; Avvisati, G.; Rocci, L.; Battistoni, F.; Gavasci, M.; Borzomati, D.; Coppola, R.; Tonini, G. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J. Interferon. Cytokine Res. 2003, 23, 649–654. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012, 8, 1227–1236. [Google Scholar] [CrossRef]
- Kabelitz, D.; Fazio, J.; Adam-Klages, S.; Marget, M.; Oberg, H.H.; Wesch, D.; Lamprecht, P. Gammadelta T-cells: Basic features and potential role in vasculitis. Clin. Exp. Rheumatol. 2010, 28 (Suppl. 57), 104–109. [Google Scholar]
- Mansoor, T.; Lynch, N.P.; Rifai, H.; Hamlin, S.; Moneley, D. Utilization of the Revised American College of Rheumatology (rACR) Scoring to Avoid Unnecessary Temporal Artery Biopsies—A Case Series. Med. Sci. 2022, 10, 11. [Google Scholar] [CrossRef]
- Bardi, M.; Diamantopoulos, A.P. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice summary. Radiol. Med. 2019, 124, 965–997. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis: A meta-analysis. Z. Rheumatol. 2016, 75, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kim, J.; Molchanova-Cook, O.P.; Dilsizian, V. The Potential of FDG PET/CT for Early Diagnosis of Cardiac Device and Prosthetic Valve Infection Before Morphologic Damages Ensue. Curr. Cardiol. Rep. 2014, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A. Writing Group; Reviewer Group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees; SNMMI Cardiovascular; Members of Council; PET Interest Group; Members of ASNC & EANM Committee Coordinator. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [PubMed]
- Aslanidi, I.P.; Manukova, V.A.; Mukhortova, O.V.; Katunina, T.A.; Rudas, M.S.; Pozharov, I.V.; Novikov, P.I.; Meshkov, A.D. 18F-fluorodeoxyglucose positron emission tomography in monitoring of therapy effectiveness in large vessel vasculitides. Byulleten’ Nauchnogo Tsentra Serdechno-Sosud. Khirurgii Im. A.N. Bakuleva RAMN 2017, 18, 380–390. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Treglia, G.; Glaudemans, A.W.J.M.; Brouwer, E.; Sandovici, M.; Jamar, F.; Gheysens, O.; Slart, R.H.J.A. Diagnostic value of [18F]FDG-PET/CT for treatment monitoring in large vessel vasculitis: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3886–3902. [Google Scholar] [CrossRef]
- Lefebvre, D.R.; Mandeville, J.T.; Yonekawa, Y.; Arroyo, J.G.; Torun, N.; Freitag, S.K. A case series and review of bisphosphonate-associated orbital inflammation. Ocul. Immunol. Inflamm. 2016, 24, 134–139. [Google Scholar] [CrossRef]
- Swarnkar, B.; Biswal, S.; Agarwal, S.; Gupta, S. Zoledronate induced urticarial vasculitis. Dermatol. Ther. 2021, 34, 15164. [Google Scholar] [CrossRef]
- Mahmood, S.D.; Ji, Y.; Peng, Y.; Abou Zahr, Z. Incidence of giant cell arteritis after bisphosphonate exposure: A retrospective cohort study. Int. J. Rheum. Dis. 2021, 24, 63–68. [Google Scholar] [CrossRef]
- Xiangying, H.; Lili, H.; Yifu, S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric 2006, 9, 283–289. [Google Scholar] [CrossRef]
- Trabuco, E.C.; Moorman, P.G.; Algeciras-Schimnich, A.; Weaver, A.L.; Cliby, W.A. Association of Ovary-Sparing Hysterectomy with Ovarian Reserve. Obstet. Gynecol. 2016, 127, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Durães Simões, R.; Chada Baracat, E.; Szjenfeld, V.L.; de Lima, G.R.; José Gonçalves, W.; de Carvalho Ramos Bortoletto, C. Effects of simple hysterectomy on bone loss. Sao Paulo Med. J. 1995, 113, 1012–1015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larcos, G. Hysterectomy with ovarian conservation: Effect on bone mineral density. Aust. N. Z. J. Obstet. Gynaecol. 1998, 38, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Achenbach, S.J.; Gebhart, J.B.; Babalola, E.O.; Atkinson, E.J.; Bharucha, A.E. Influence of hysterectomy on long-term fracture risk. Fertil. Steril. 2007, 88, 156–162. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchekochikhin, D.; Vinogradskaya, O.; Bublik, E.; Shmyreva, M.; Koroba, G.; Farmanov, A.; Aslanidis, I.; Pursanova, D.; Manukova, V.; Zilov, A.; et al. Zoledronate-Induced Large Vessel Vasculitis Diagnosed by PET/CT. J. Vasc. Dis. 2023, 2, 317-323. https://doi.org/10.3390/jvd2030024
Shchekochikhin D, Vinogradskaya O, Bublik E, Shmyreva M, Koroba G, Farmanov A, Aslanidis I, Pursanova D, Manukova V, Zilov A, et al. Zoledronate-Induced Large Vessel Vasculitis Diagnosed by PET/CT. Journal of Vascular Diseases. 2023; 2(3):317-323. https://doi.org/10.3390/jvd2030024
Chicago/Turabian StyleShchekochikhin, Dmitry, Olga Vinogradskaya, Evgeniia Bublik, Maria Shmyreva, Gregory Koroba, Alexander Farmanov, Irakliy Aslanidis, Diana Pursanova, Veronica Manukova, Alexey Zilov, and et al. 2023. "Zoledronate-Induced Large Vessel Vasculitis Diagnosed by PET/CT" Journal of Vascular Diseases 2, no. 3: 317-323. https://doi.org/10.3390/jvd2030024
APA StyleShchekochikhin, D., Vinogradskaya, O., Bublik, E., Shmyreva, M., Koroba, G., Farmanov, A., Aslanidis, I., Pursanova, D., Manukova, V., Zilov, A., & Zhivov, A. (2023). Zoledronate-Induced Large Vessel Vasculitis Diagnosed by PET/CT. Journal of Vascular Diseases, 2(3), 317-323. https://doi.org/10.3390/jvd2030024




