Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health
Abstract
1. Introduction
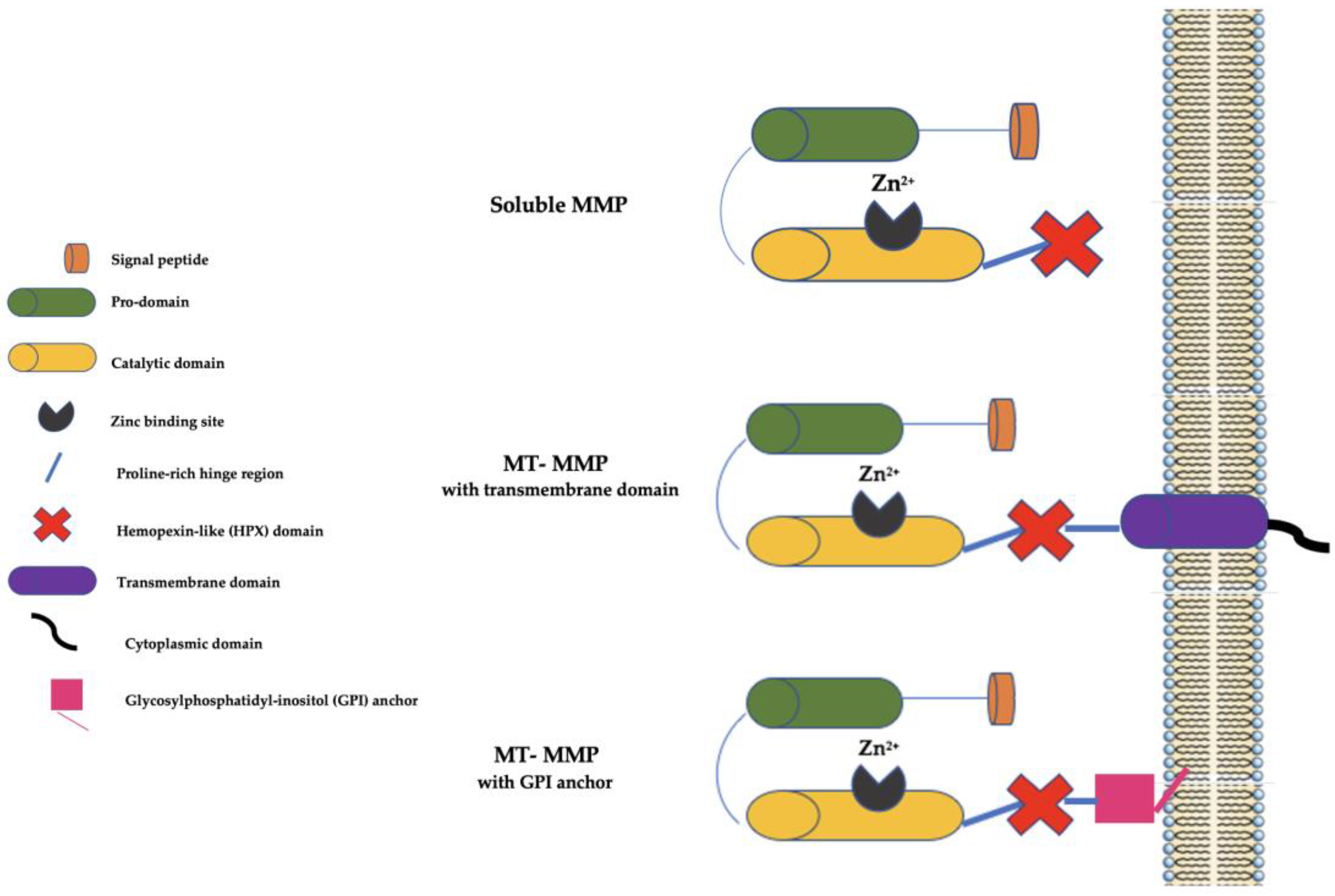
2. The Matrix Metalloproteinase (MMP) Family
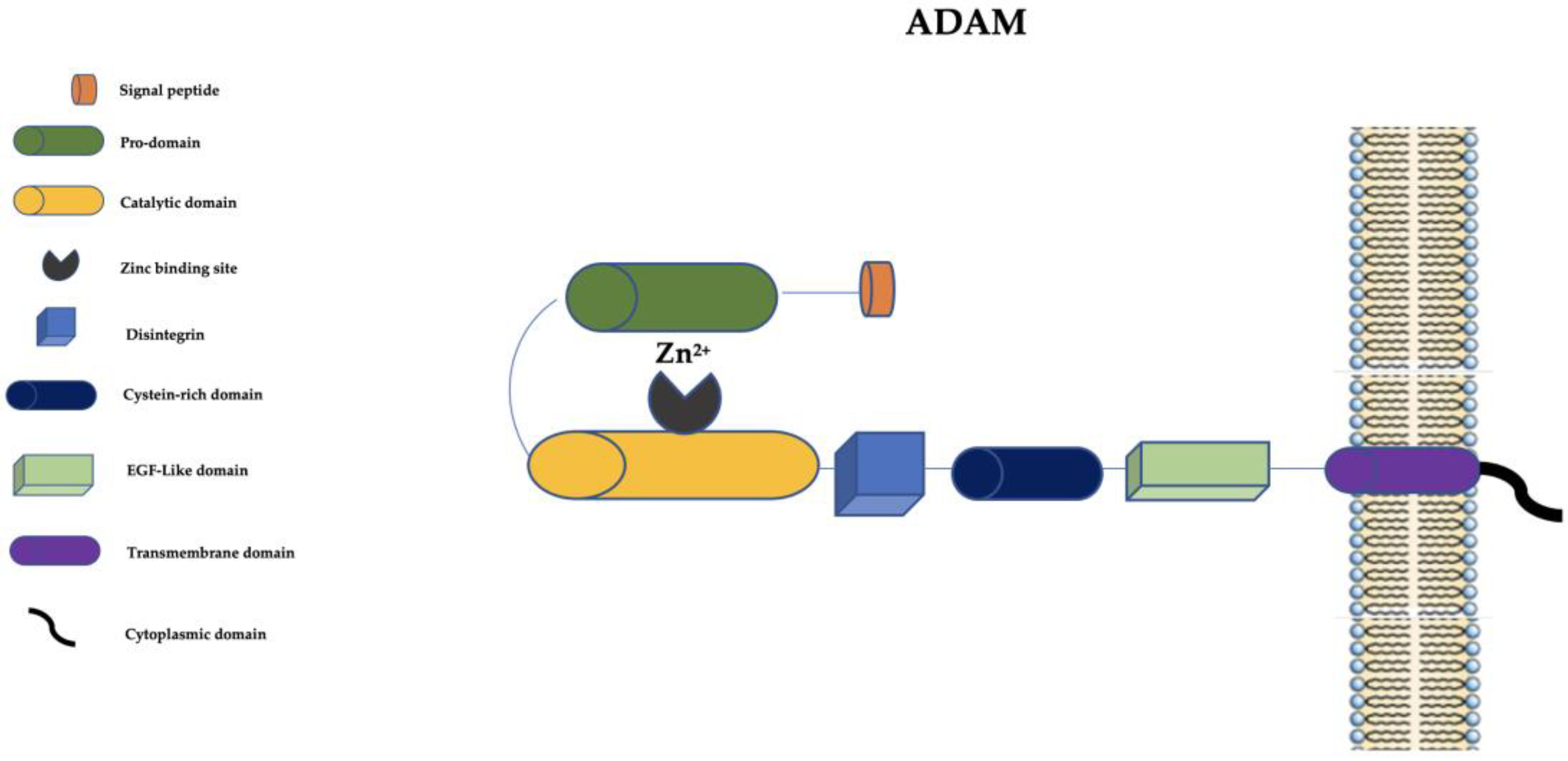
3. The “A Disintegrin and Metalloprotease” (ADAM) Family
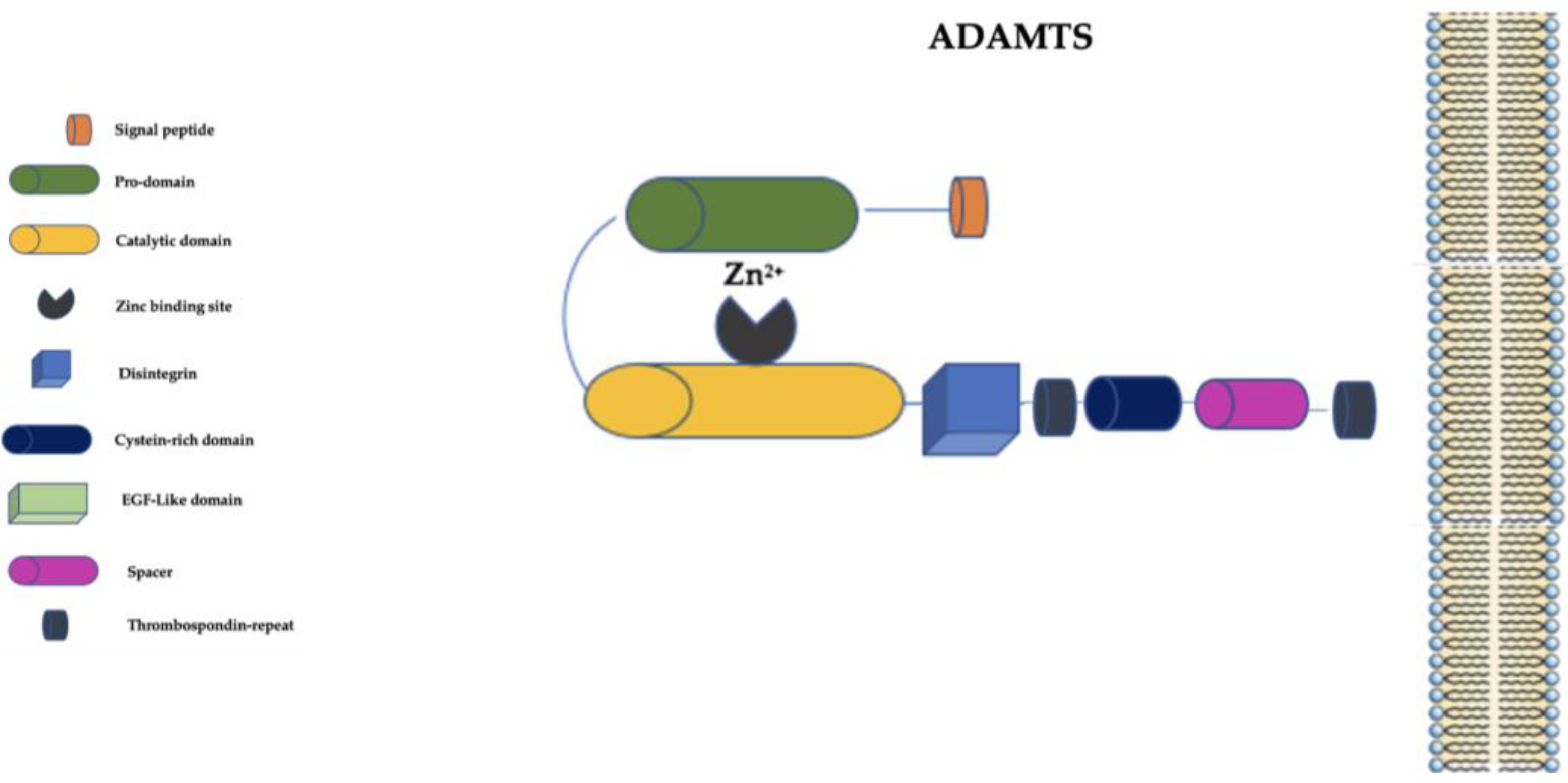
4. The “A Disintegrin and Metalloprotease with Thrombospondin Motifs” (ADAMTS) Family
5. Inhibition of Metalloproteinases
6. Metalloproteinases as Biomarkers
7. Metalloproteinases and the Paradigm of Complexity
8. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ielapi, N.; Andreucci, M.; Licastro, N.; Faga, T.; Grande, R.; Buffone, G.; Mellace, S.; Sapienza, P.; Serra, R. Precision Medicine and Precision Nursing: The Era of Biomarkers and Precision Health. Int. J. Gen. Med. 2020, 13, 1705–1711. [Google Scholar] [CrossRef]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 1928. [Google Scholar] [CrossRef]
- Serra, R.; Gallelli, L.; Butrico, L.; Buffone, G.; Caliò, F.G.; De Caridi, G.; Massara, M.; Barbetta, A.; Amato, B.; Labonia, M.; et al. From varices to venous ulceration: The story of chronic venous disease described by metalloproteinases. Int. Wound J. 2017, 14, 233–240. [Google Scholar] [CrossRef]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Vascular Biology of arterial aneurysms. Ann. Vasc. Surg. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Stöcker, W.; Bode, W. Structural features of a superfamily of zinc-endopeptidases: The metzincins. Curr. Opin. Struct. Biol. 1995, 5, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wächter, J.; Shannon, M.J.; Beristain, A.G. Transcriptomic mapping of the metzincin landscape in human trophoblasts. Gene Expr. Patterns GEP 2022, 46, 119283. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem. J. 1962, 83, 304–314. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Nagai, Y.; Lapiere, C.M.; Gross, J. Tadpole collagenase. Preparation and purification. Biochemistry 1966, 5, 3123–3130. [Google Scholar] [CrossRef]
- Iyer, R.P.; Patterson, N.L.; Fields, G.B.; Lindsey, M.L. The history of matrix metalloproteinases: Milestones, myths, and misperceptions. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H919–H930. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H. From tadpole collagenase to a family of matrix metalloproteinases. J. Oral Pathol. 1988, 17, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Khalil, R.A. A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem. Pharmacol. 2019, 164, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.E.; Zhu, X.; Prater, C.E.; Oh, E.; Evans, J.P. Analysis of fertilin alpha (ADAM1)-mediated sperm-egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J. Biol. Chem. 2001, 276, 24937–24945. [Google Scholar] [CrossRef]
- Giebeler, N.; Zigrino, P. A Disintegrin and Metalloprotease (ADAM): Historical Overview of Their Functions. Toxins 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Kuno, K.; Kanada, N.; Nakashima, E.; Fujiki, F.; Ichimura, F.; Matsushima, K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J. Biol. Chem. 1997, 272, 556–562. [Google Scholar] [CrossRef]
- Bond, J.S. Proteases: History, discovery, and roles in health and disease. J. Biol. Chem. 2019, 294, 1643–1651. [Google Scholar] [CrossRef]
- Noël, A.; Gutiérrez-Fernández, A.; Sounni, N.E.; Behrendt, N.; Maquoi, E.; Lund, I.K.; Cal, S.; Hoyer-Hansen, G.; López-Otín, C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front. Pharmacol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef]
- Alford, V.M.; Kamath, A.; Ren, X.; Kumar, K.; Gan, Q.; Awwa, M.; Tong, M.; Seeliger, M.A.; Cao, J.; Ojima, I.; et al. Targeting the Hemopexin-like Domain of Latent Matrix Metalloproteinase-9 (proMMP-9) with a Small Molecule Inhibitor Prevents the Formation of Focal Adhesion Junctions. ACS Chem. Biol. 2017, 12, 2788–2803. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, X.; Fang, G.; Tian, X.; Ge, C. Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: Recent progress and current challenges. NanoImpact 2021, 21, 100293. [Google Scholar] [CrossRef]
- Jones, L.; Ghaneh, P.; Humphreys, M.; Neoptolemos, J.P. The matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann. N. Y. Acad. Sci. 1999, 880, 288–307. [Google Scholar] [CrossRef] [PubMed]
- Leber, T.M.; Balkwill, F.R. Zymography: A single-step staining method for quantitation of proteolytic activity on substrate gels. Anal. Biochem. 1997, 249, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Jian, M.; Li, X.; Wei, J.; Meng, X.; Wang, Z. Biosensors and bioassays for determination of matrix metalloproteinases: State of the art and recent advances. J. Mater. Chem. B 2020, 8, 3261–3291. [Google Scholar] [CrossRef] [PubMed]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, C.; Wu, D.; Chen, G.; Tan, R.; Ran, J. AGEs-induced MMP-9 activation mediated by Notch1 signaling is involved in impaired wound healing in diabetic rats. Diabetes Res. Clin. Pract. 2022, 186, 109831. [Google Scholar] [CrossRef]
- Cook, L.; Sengelmann, M.; Winkler, B.; Nagl, C.; Koch, S.; Schlomann, U.; Slater, E.P.; Miller, M.A.; von Strandmann, E.P.; Dörsam, B.; et al. ADAM8-Dependent Extracellular Signaling in the Tumor Microenvironment Involves Regulated Release of Lipocalin 2 and MMP-9. Int. J. Mol. Sci. 2022, 23, 1976. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. S2), 91–114. [Google Scholar] [CrossRef] [PubMed]
- Braundmeier, A.G.; Fazleabas, A.T.; Nowak, R.A. Extracellular matrix metalloproteinase inducer expression in the baboon endometrium: Menstrual cycle and endometriosis. Reproduction 2010, 140, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Korach, K.S.; Burns, K.A. Unique Sensitivity of Uterine Tissue and the Immune System for Endometriotic Lesion Formation. Front. Physiol. 2021, 12, 805784. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Kaczmarek, L.; Lapinska-Dzwonek, J.; Szymczak, S. Matrix metalloproteinases in the adult brain physiology: A link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO J. 2002, 21, 6643–6648. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Buffone, G.; Falcone, D.; Molinari, V.; Scaramuzzino, M.; Gallelli, L.; de Franciscis, S. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2013, 21, 395–401. [Google Scholar] [CrossRef]
- Horecka, A.; Hordyjewska, A.; Biernacka, J.; Dąbrowski, W.; Zubilewicz, T.; Malec, A.; Musik, I.; Kurzepa, J. Intense remodeling of extracellular matrix within the varicose vein: The role of gelatinases and vascular endothelial growth factor. Ir. J. Med. Sci. 2021, 190, 255–259. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, W.; Raffetto, J.D.; Khalil, R.A. Matrix Metalloproteinases in Remodeling of Lower Extremity Veins and Chronic Venous Disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 267–299. [Google Scholar]
- Busceti, M.T.; Grande, R.; Amato, B.; Gasbarro, V.; Buffone, G.; Amato, M.; Gallelli, L.; Serra, R.; de Franciscis, S. Pulmonary embolism, metalloproteinsases and neutrophil gelatinase associated lipocalin. Acta Phlebol. 2013, 14, 115–121. [Google Scholar]
- Raffetto, J.D.; Ross, R.L.; Khalil, R.A. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: Relevance to varicose vein formation. J. Vasc. Surg. 2007, 45, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Serraino, G.F.; Jiritano, F.; Costa, D.; Ielapi, N.; Battaglia, D.; Bracale, U.M.; Mastroroberto, P.; Andreucci, M.; Serra, R. Metalloproteinases in Cardiac Surgery: A Systematic Review. Biomolecules 2023, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Provenzano, M.; Faga, T.; Michael, A.; Patella, G.; Mastroroberto, P.; Serraino, G.F.; Bracale, U.M.; Ielapi, N.; Serra, R. Aortic Aneurysms, Chronic Kidney Disease and Metalloproteinases. Biomolecules 2021, 11, 194. [Google Scholar] [CrossRef]
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Faga, T.; Michael, A.; Ielapi, N.; Grande, R.; Sapienza, P.; Franciscis, S.; Mastroroberto, P.; et al. The Association of Matrix Metalloproteinases with Chronic Kidney Disease and Peripheral Vascular Disease: A Light at the End of the Tunnel? Biomolecules 2020, 10, 154. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Montemurro, R.; Butrico, L.; Caliò, F.G.; Mastrangelo, D.; Scarcello, E.; Gallelli, L.; Buffone, G.; de Franciscis, S. The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015, 157, 155–162. [Google Scholar] [CrossRef]
- de Franciscis, S.; Mastroroberto, P.; Gallelli, L.; Buffone, G.; Montemurro, R.; Serra, R. Increased plasma levels of metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in a rare case of multiple artery aneurysm. Ann. Vasc. Surg. 2013, 27, 1185.e5–1185.e7. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Chen, Z.; Wang, Q.; Luo, Y.; Huang, Y.; Wang, B.; Gu, R. The role of intestinal immune cells and matrix metalloproteinases in inflammatory bowel disease. Front. Immunol. 2023, 13, 1067950. [Google Scholar] [CrossRef]
- Chowkwale, M.; Lindsey, M.L.; Saucerman, J.J. Intercellular model predicts mechanisms of inflammation-fibrosis coupling after myocardial infarction. J. Physiol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Wagner, J.; Kumar, Y.; Lautenbach, A.; von Kroge, P.; Wolter, S.; Mann, O.; Izbicki, J.; Gagliani, N.; Duprée, A. Fatty acid-binding protein-4 (FABP4) and matrix metalloproteinase-9 (MMP9) as predictive values for nonalcoholic steatohepatitis (NASH). Lipids Health Dis. 2023, 22, 1. [Google Scholar] [CrossRef]
- Seegar, T.C.; Blacklow, S.C. Domain integration of ADAM family proteins: Emerging themes from structural studies. Exp. Biol. Med. 2019, 244, 1510–1519. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C.; Seipold, L.; Saftig, P. Ectodomain shedding by ADAM proteases as a central regulator in kidney physiology and disease. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119165. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 2018, 37, e99456. [Google Scholar] [CrossRef] [PubMed]
- Clark, P. Protease-mediated ectodomain shedding. Thorax 2014, 69, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Kishi, T.; Ishida, S.; Kawana, H.; Uwamizu, A.; Ono, Y.; Kawakami, K.; Aoki, J.; Inoue, A. Ectodomain shedding of EGFR ligands serves as an activation readout for TRP channels. PLoS ONE 2023, 18, e0280448. [Google Scholar] [CrossRef]
- Ma, X.; Takahashi, Y.; Wu, W.; Liang, W.; Chen, J.; Chakraborty, D.; Li, Y.; Du, Y.; Benyajati, S.; Ma, J.X. ADAM17 mediates ectodomain shedding of the soluble VLDL receptor fragment in the retinal epithelium. J. Biol. Chem. 2021, 297, 101185. [Google Scholar] [CrossRef]
- Brown, M.S.; Ye, J.; Rawson, R.B.; Goldstein, J.L. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 2000, 100, 391–398. [Google Scholar] [CrossRef]
- Butrico, L.; Barbetta, A.; Ciranni, S.; Mastroroberto, P.; Andreucci, M.; De Franciscis, S.; Serra, R. Role of metalloproteinases and their inhibitors in the development of abdominal aortic aneurysm: Current insights and systematic review of the literature. Chirurgia 2017, 30, 151–159. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Porter, S.; Clark, I.M.; Kevorkian, L.; Edwards, D.R. The ADAMTS metalloproteinases. Biochem. J. 2005, 386, 15–27. [Google Scholar] [CrossRef]
- Santamaria, S.; de Groot, R. ADAMTS proteases in cardiovascular physiology and disease. Open Biol. 2020, 10, 200333. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, V.; Singh, K.; Kretz, C.A. Mechanisms of ADAMTS13 regulation. J. Thromb. Haemost. 2022, 20, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.M.; Leotescu, A.; Sieweke, J.T.; Schneppenheim, S.; Budde, U.; Ziegler, N.L.; Biber, S.; Gabriel, M.M.; Ernst, J.; Schuppner, R.; et al. ADAMTS-13 activity in stroke of known and unknown cause: Relation to vascular risk factor burden. Front. Neurol. 2023, 13, 1045478. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Bauer, E.A.; Stricklin, G.P.; Jeffrey, J.J.; Eisen, A.Z. Collagenase production by human skin fibroblasts. Biochem. Biophys. Res. Commun. 1975, 64, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, J.; Von den Hoff, J.W. Tissue inhibitors of metalloproteinases (TIMPs): Their biological functions and involvement in oral disease. J. Dent. Res. 2006, 85, 1074–1084. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Rose, K.W.J.; Taye, N.; Karoulias, S.Z.; Hubmacher, D. Regulation of ADAMTS Proteases. Front. Mol. Biosci. 2021, 8, 701959. [Google Scholar] [CrossRef]
- Khanafer, K.; Ghosh, A.; Vafai, K. Correlation between MMP and TIMP levels and elastic moduli of ascending thoracic aortic aneurysms. Cardiovasc. Revascularization Med. Incl. Mol. Interv. 2019, 20, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Gallelli, L.; Buffone, G.; Molinari, V.; Stillitano, D.M.; Palmieri, C.; de Franciscis, S. Doxycycline speeds up healing of chronic venous ulcers. Int. Wound J. 2015, 12, 179–184. [Google Scholar] [CrossRef]
- Serra, R.; Gallelli, L.; Conti, A.; De Caridi, G.; Massara, M.; Spinelli, F.; Buffone, G.; Caliò, F.G.; Amato, B.; Ceglia, S.; et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des. Dev. Ther. 2014, 8, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Buffone, G.; Gallelli, L.; De Franciscis, S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Acta Phlebol. 2013, 14, 99–107. [Google Scholar]
- de Franciscis, S.; Gallelli, L.; Battaglia, L.; Molinari, V.; Montemurro, R.; Stillitano, D.M.; Buffone, G.; Serra, R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int. Wound J. 2015, 12, 250–253. [Google Scholar] [CrossRef]
- Cione, E.; Piegari, E.; Gallelli, G.; Caroleo, M.C.; Lamirata, E.; Curcio, F.; Colosimo, F.; Cannataro, R.; Ielapi, N.; Colosimo, M.; et al. Expression of MMP-2, MMP-9, and NGAL in Tissue and Serum of Patients with Vascular Aneurysms and Their Modulation by Statin Treatment: A Pilot Study. Biomolecules 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tay, F.R.; Yiu, C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465. [Google Scholar] [CrossRef]
- Serra, R.; Ielapi, N.; Barbetta, A.; Andreucci, M.; de Franciscis, S. Novel biomarkers for cardiovascular risk. Biomark. Med. 2018, 12, 1015–1024. [Google Scholar] [CrossRef]
- Galliera, E.; Tacchini, L.; Corsi Romanelli, M.M. Matrix metalloproteinases as biomarkers of disease: Updates and new insights. Clin. Chem. Lab. Med. 2015, 53, 349–355. [Google Scholar] [CrossRef]
- Fonseca, F.L.; da Costa Aguiar Alves, B.; Azzalis, L.A.; Belardo, T.M. Matrix Metalloproteases as Biomarkers of Disease. Methods Mol. Biol. 2017, 1579, 299–311. [Google Scholar]
- Liu, C.H.; Di, Y.P. Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes. Int. J. Mol. Sci. 2023, 24, 2382. [Google Scholar] [CrossRef]
- Sun, W.P.; Du, X.; Chen, J.J. Biomarkers for Predicting the Occurrence and Progression of Atrial Fibrillation: Soluble Suppression of Tumorigenicity 2 Protein and Tissue Inhibitor of Matrix Metalloproteinase-1. Int. J. Clin. Pract. 2022, 2022, 6926510. [Google Scholar] [CrossRef]
- Stojanovic, S.K.; Stamenkovic, B.N.; Cvetkovic, J.M.; Zivkovic, V.G.; Apostolovic, M.R.A. Matrix Metalloproteinase-9 Level in Synovial Fluid-Association with Joint Destruction in Early Rheumatoid Arthritis. Medicina 2023, 59, 167. [Google Scholar] [CrossRef] [PubMed]
- Brusa, S.; Terracciano, D.; Bruzzese, D.; Fiorenza, M.; Stanziola, L.; Pinchera, B.; Valente, V.; Gentile, I.; Cittadini, A.; Mormile, I.; et al. Circulating tissue inhibitor of metalloproteinases 1 (TIMP-1) at COVID-19 onset predicts severity status. Front. Med. 2022, 9, 1034288. [Google Scholar] [CrossRef]
- Kicman, A.; Niczyporuk, M.; Kulesza, M.; Motyka, J.; Ławicki, S. Utility of Matrix Metalloproteinases in the Diagnosis, Monitoring and Prognosis of Ovarian Cancer Patients. Cancer Manag. Res. 2022, 14, 3359–3382. [Google Scholar] [CrossRef]
- Noh, J.W.; Jang, J.H.; Yoon, H.S.; Kim, K.B.; Heo, M.H.; Jang, H.E.; Kim, Y.J.; Lee, Y. Evaluation of Salivary Biomarkers of Periodontal Disease Based on Smoking Status: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14619. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Gallelli, L.; Grande, R.; Amato, B.; De Caridi, G.; Sammarco, G.; Ferrari, F.; Butrico, L.; Gallo, G.; Rizzuto, A.; et al. Hemorrhoids and matrix metalloproteinases: A multicenter study on the predictive role of biomarkers. Surgery 2016, 159, 487–494. [Google Scholar] [CrossRef]
- Tsiknia, A.A.; Sundermann, E.E.; Reas, E.T.; Edland, S.D.; Brewer, J.B.; Galasko, D.; Banks, S.J.; Alzheimer’s Disease Neuroimaging Initiative. Sex differences in Alzheimer’s disease: Plasma MMP-9 and markers of disease severity. Alzheimer’s Res. Ther. 2022, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Marks, J. Dossier: Le groupe des Dix, des précurseurs de l’interdisciplinarité–Biology and complexity: Edgar Morin and Henri Atlan. Nat. Sci. Sociétés 2019, 27, 159–168. [Google Scholar] [CrossRef]
- Waldrop, M.M. Complexity: The Emerging Science at the Edge of Order and Chaos; Simon and Schuster: New York, NY, USA, 1993. [Google Scholar]
- Coveney, P.V. Self-Organization and Complexity: A New Age for Theory, Computation and Experiment. Philos. Trans. Math. Phys. Eng. Sci. 2003, 361, 1057–1079. [Google Scholar] [CrossRef]
- Morin, E. Introduction à la Pensée Complexe; Editors du Seuil: Paris, France, 2005. [Google Scholar]
- Serra, R. Matrix Metalloproteinases in Health and Disease. Biomolecules 2020, 10, 1138. [Google Scholar] [CrossRef]
- Morin, E. Restricted complexity, general complexity. In Worldviews, Science and Us: Philosophy and Complexity; Gershenson, C., Aerts, D., Edmonds, B., Eds.; World Scientific: Singapore, 2007; pp. 5–29. [Google Scholar]
- Heath-Carpentier, A. The Challenge of Complexity: Essays by Edgar Morin; Liverpool University Press: Liverpool, UK, 2022. [Google Scholar]
- Costa, D. Diversity and Health: Two Sides of the Same Coin. Ital. Sociol. Rev. 2023, 13, 69–90. [Google Scholar]
- Costa, D.; Ielapi, N.; Caprino, F.; Giannotta, N.; Sisinni, A.; Abramo, A.; Ssempijja, L.; Andreucci, M.; Bracale, U.M.; Serra, R. Social Aspects of Diabetic Foot: A Scoping Review. Soc. Sci. 2022, 11, 149. [Google Scholar] [CrossRef]
- Kalra, S.; Baruah, M.P.; Sahay, R. Salutogenesis in Type 2 Diabetes Care: A Biopsychosocial Perspective. Indian J. Endocrinol. Metab. 2018, 22, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Soon, K.; Acton, C. Pain-induced stress: A barrier to wound healing. Wounds UK 2006, 2, 92–101. [Google Scholar]
- Alexander, S.J. Time to get serious about assessing- and managing-psychosocial issues associated with chronic wounds. Curr. Opin. Support. Palliat. Care 2013, 7, 95–100. [Google Scholar] [CrossRef]
- Fu, K.; Zheng, X.; Chen, Y.; Wu, L.; Yang, Z.; Chen, X.; Song, W. Role of matrix metalloproteinases in diabetic foot ulcers: Potential therapeutic targets. Front. Pharmacol. 2022, 13, 1050630. [Google Scholar] [CrossRef] [PubMed]
- Hariono, M.; Yuliani, S.H.; Istyastono, E.P.; Riswanto, F.D.O.; Adhipandito, C.F. Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and structure-based drug design. Vasc. Med. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Jones, J.I.; Nguyen, T.T.; Peng, Z.; Chang, M. Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals 2019, 12, 79. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Yang, S.H.; Chen, T.Y.; Pang, J.H. Cilostazol inhibits matrix invasion and modulates the gene expressions of MMP-9 and TIMP-1 in PMA-differentiated THP-1 cells. Eur. J. Pharmacol. 2011, 670, 419–426. [Google Scholar] [CrossRef]
- Li, H.; Sheng, Z.; Khan, S.; Zhang, R.; Liu, Y.; Zhang, Y.; Yong, V.W.; Xue, M. Matrix Metalloproteinase-9 as an Important Contributor to the Pathophysiology of Depression. Front. Neurol. 2022, 13, 861843. [Google Scholar] [CrossRef]
- Cukor, D. Introduction: Psychosocial Issues in Kidney Disease. Semin. Nephrol. 2021, 41, 485–486. [Google Scholar] [CrossRef]
- Cukor, D.; Cohen, S.D.; Kimmel, P.L. Psychosocial Aspects of Chronic Kidney Disease: Exploring the Impact of CKD, Dialysis, and Transplantation on Patients; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Bayne, D.F.; Shune, S.E. A Biopsychosocial Model of Mealtime Management in Persons with Dementia, an Asset-Based Approach to Patient-Centered Care. Geriatrics 2022, 7, 112. [Google Scholar] [CrossRef]
- Ismail, Z.; Smith, E.E.; Geda, Y.; Sultzer, D.; Brodaty, H.; Smith, G.; Agüera-Ortiz, L.; Sweet, R.; Miller, D.; Lyketsos, C.G.; et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s Dement. 2015, 12, 195–202. [Google Scholar] [CrossRef]
- Edmondson, D.; Newman, J.; Whang, W.; Davidson, K. Emotional triggers in myocardial infarction: Do they matter? Eur. Heart J. 2013, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.; Mostofsky, E. Physical, psychological and chemical triggers of acute cardiovascular events. Circulation 2011, 124, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Cilli, E.; Ranieri, J.; Guerra, F.; Ferri, C.; Di Giacomo, D. Cardiovascular disease, self-care and emotional regulation processes in adult patients: Balancing unmet needs and quality of life. BioPsychoSocial Med. 2022, 16, 20. [Google Scholar] [CrossRef]
- Monami, M.; Marchionni, N. Psychological disorders and cardiovascular diseases. G. Ital. Cardiol. 2007, 8, 335–348. [Google Scholar]
- WHO. Adherence to Long-Term Therapies: Evidence for Action; WHO: Geneva, Switzerland, 2003.
- Thomas, H.N.; Thurston, R.C. A biopsychosocial approach to women’s sexual function and dysfunction at midlife: A narrative review. Maturitas 2016, 87, 49–60. [Google Scholar] [CrossRef]
- Ventegodt, S. Sex and the quality of life in Denmark. Arch. Sex Behav. 1998, 27, 295–307. [Google Scholar] [CrossRef]
- Mercer, C.H.; Fenton, K.A.; Johnson, A.M.; Wellings, K.; Macdowall, W.; McManus, S.; Nanchahal, K.; Erens, B. Sexual function problems and help seeking behaviour in Britain: National probability sample survey. BMJ Clin. Res. Ed. 2003, 327, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Jin, L.; Zhang, R.; Wang, T.; Wang, Q.; Chen, J.; Yang, F.; Siebert, H.C.; Zheng, X. Ketogenic Diet Elicits Antitumor Properties through Inducing Oxidative Stress, Inhibiting MMP-9 Expression, and Rebalancing M1/M2 Tumor-Associated Macrophage Phenotype in a Mouse Model of Colon Cancer. J. Agric. Food Chem. 2020, 68, 11182–11196. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, P.M.; Leal, E.C.; Moura, J.; Gonçalves, P.; Gonçalves, J.P.; Carvalho, E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020, 254, 117813. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hong, H.; Sun, Y.; Chen, C.; Wu, C.; Xu, G.; Bao, G.; Cui, Z. Role of macrophage polarization in osteoarthritis (Review). Exp. Ther. Med. 2022, 24, 757. [Google Scholar] [CrossRef]
- Serra, R.; Jiritano, F.; Bracale, U.M.; Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Rizzuto, A.; Mastroroberto, P.; Serraino, G.F. Novel biomarkers in cardiovascular surgery. Biomark. Med. 2021, 15, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Serraino, G.F.; Jiritano, F.; Costa, D.; Ielapi, N.; Napolitano, D.; Mastroroberto, P.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases and Hypertrophic Cardiomyopathy: A Systematic Review. Biomolecules 2023, 13, 665. [Google Scholar] [CrossRef]
- Hunt, M.A.; Birmingham, T.B.; Skarakis-Doyle, E.; Vandervoort, A.A. Towards a biopsychosocial framework of osteoarthritis of the knee. Disabil. Rehabil. 2008, 30, 54–61. [Google Scholar] [CrossRef]
- Ali, S.A.; Lee, K.; MacDermid, J.C. Applying the International Classification of Functioning, Disability and Health to understand osteoarthritis management in urban and rural community-dwelling seniors. Osteoarthr. Cartil. Open 2021, 3, 100132. [Google Scholar] [CrossRef]
- Chen, Y.W.; Camp, P.G.; Coxson, H.O.; Road, J.D.; Guenette, J.A.; Hunt, M.A.; Reid, W.D. A Comparison of Pain, Fatigue, Dyspnea and their Impact on Quality of Life in Pulmonary Rehabilitation Participants with Chronic Obstructive Pulmonary Disease. COPD 2018, 15, 65–72. [Google Scholar] [CrossRef]
- Novy, D.M.; Aigner, C.J. The biopsychosocial model in cancer pain. Curr. Opin. Support. Palliat. Care 2014, 8, 117–123. [Google Scholar] [CrossRef]
- Syrjala, K.L.; Chapko, M.E. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain 1995, 61, 69–79. [Google Scholar] [CrossRef]
- Badura, A.S.; Grohmann, J.M. Psychological issues in pain perception and treatment in the elderly. Ann. Long-Term Care 2002, 10, 29–34. [Google Scholar]
- Masselin-Dubois, A.; Attal, N.; Fletcher, D.; Jayr, C.; Albi, A.; Fermanian, J.; Bouhassira, D.; Baudic, S. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J. Pain 2013, 14, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ielapi, N.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Serra, R. Social Determinants of Health and Vascular Diseases: A Systematic Review and Call for Action. Soc. Sci. 2023, 12, 214. [Google Scholar] [CrossRef]
- Shafi, B.H.; Bøttcher, M.; Ejupi, A.; Jensen, G.; Osler, M.; Lange, T.; Prescott, E. Socioeconomic disparity in cardiovascular disease: Possible biological pathways based on a proteomic approach. Atherosclerosis 2022, 352, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Carey, G.; Malbon, E.; Carey, N.; Joyce, A.; Crammond, B.; Carey, A. Systems science and systems thinking for public health: A systematic review of the field. BMJ Open 2015, 5, e009002. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.D.F.C.T.; Viana, A.L.; Gontijo, D.T. Use of the complexity paradigm in the field of health: Scope review. Esc. Anna Nery 2020, 24, e20190235. [Google Scholar] [CrossRef]
- de Franciscis, S.; Fregola, S.; Gallo, A.; Argirò, G.; Barbetta, A.; Buffone, G.; Caliò, F.G.; De Caridi, G.; Amato, B.; Serra, R. PredyCLU: A prediction system for chronic leg ulcers based on fuzzy logic; part I—Exploring the venous side. Int. Wound J. 2016, 13, 1349–1353. [Google Scholar] [CrossRef]
- Serra, R.; Bracale, U.M.; Barbetta, A.; Ielapi, N.; Licastro, N.; Gallo, A.; Fregola, S.; Turchino, D.; Gasbarro, V.; Mastroroberto, P.; et al. PredyCLU: A prediction system for chronic leg ulcers based on fuzzy logic; part II-Exploring the arterial side. Int. Wound J. 2020, 17, 987–991. [Google Scholar] [CrossRef]
- Garvin, P.; Jonasson, L.; Nilsson, L.; Falk, M.; Kristenson, M. Plasma Matrix Metalloproteinase-9 Levels Predict First-Time Coronary Heart Disease: An 8-Year Follow-Up of a Community-Based Middle Aged Population. PLoS ONE 2015, 10, e0138290. [Google Scholar] [CrossRef]
- Lundberg, A.K.; Jönsson, S.; Stenmark, J.; Kristenson, M.; Jonasson, L. Stress-induced release of matrix metalloproteinase-9 in patients with coronary artery disease: The possible influence of cortisol. Psychoneuroendocrinology 2016, 73, 117–124. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Baumer, Y.; Baah, F.O.; Baez, A.S.; Farmer, N.; Mahlobo, C.T.; Pita, M.A.; Potharaju, K.A.; Tamura, K.; Wallen, G.R. Social determinants of cardiovascular disease. Circ. Res. 2022, 130, 782–799. [Google Scholar] [CrossRef] [PubMed]
- Kasthurirathne, S.N.; Vest, J.R.; Menachemi, N.; Halverson, P.K.; Grannis, S.J. Assessing the capacity of social determinants of health data to augment predictive models identifying patients in need of wraparound social services. J. Am. Med. Inform. Assoc. 2018, 25, 47–53. [Google Scholar] [CrossRef] [PubMed]
| Subgroup | Members | Enzymatic Name |
|---|---|---|
| Collagenases | MMP-1 MMP-8 MMP-13 MMP-18 | Collagenase-1 Collagenase-2/neutrophil collagenase Collagenase-3 Collagenase-4 |
| Gelatinases | MMP-2 MMP-9 | Gelatinase A Gelatinase B |
| Stromelysins | MMP-3 MMP-10 MMP-11 MMP-19 MMP-27 | Stromelysin-1/transin-1 Stromelysin-2/transin-2 Stromelysin-3 Stromelysin-4/RASI-1 n/d |
| Matrilysins | MMP-7 MMP-26 | Matrilysin-1/putative MMP (PUMP) Matrilysin-2 |
| MT-MMPs | MMP-14 MMP-15 MMP-16 MMP-4/MMP-17 MMP-5/MMP-24 MMP-6/MMP-25 | MT1-MMP MT2-MMP MT3-MMP MT4-MMP * MT5-MMP * MT6-MMP |
| Ungrouped MMPs | MMP-12 MMP-20 MMP-21 MMP-22 MMP-23 MMP-28 | Macrophage metalloelastase Enamelysin Xenopous MMP (XMPP) Chicken MMP (CMMP) Cysteine array MMP (CA-MMP) Epilysin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.; Ielapi, N.; Minici, R.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Di Taranto, M.D.; Bracale, U.M.; Andreucci, M.; et al. Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health. J. Vasc. Dis. 2023, 2, 282-298. https://doi.org/10.3390/jvd2030021
Costa D, Ielapi N, Minici R, Bevacqua E, Ciranni S, Cristodoro L, Torcia G, Di Taranto MD, Bracale UM, Andreucci M, et al. Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health. Journal of Vascular Diseases. 2023; 2(3):282-298. https://doi.org/10.3390/jvd2030021
Chicago/Turabian StyleCosta, Davide, Nicola Ielapi, Roberto Minici, Egidio Bevacqua, Salvatore Ciranni, Lucia Cristodoro, Giuseppina Torcia, Maria Donata Di Taranto, Umberto Marcello Bracale, Michele Andreucci, and et al. 2023. "Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health" Journal of Vascular Diseases 2, no. 3: 282-298. https://doi.org/10.3390/jvd2030021
APA StyleCosta, D., Ielapi, N., Minici, R., Bevacqua, E., Ciranni, S., Cristodoro, L., Torcia, G., Di Taranto, M. D., Bracale, U. M., Andreucci, M., & Serra, R. (2023). Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health. Journal of Vascular Diseases, 2(3), 282-298. https://doi.org/10.3390/jvd2030021









