Abstract
Numerous studies have investigated in-stent restenosis (ISR) predictors in first-generation drug-eluting stents (DESs), but only a few have investigated second-generation DESs. We aimed to investigate the ISR predictors following a successful DES implantation in coronary artery disease (CAD) patients. A systematic review and meta-analysis study was conducted. Diabetes mellitus (DM) (OR 1.47; 95% CI 1.19 to 1.83; p < 0.01), family history of CAD (OR 1.26; 95% CI 1.03 to 1.55; p 0.03), and smoking (OR 1.23; 95% CI 1.02 to 1.48; p 0.03) were the strong predictors for the DES-ISR. The DES-ISR was more common in DESs with smaller stent diameter (MD −0.12; 95% CI −0.16 to −0.08; p < 0.01) and longer stent length (MD 2.24; 95% CI 1.36 to 3.13; p < 0.01). Angiography characteristics, including multi-vessel disease (MVD) (OR 1.45; 95% CI 1.07 to 1.97; p 0.02), type B2/C lesions (OR 1.56; 95% CI 1.06 to 2.30; p 0.02), and type C lesion (OR 1.33; 95% CI 1.09 to 1.62; p < 0.01), were also associated with DES-ISR. We confirmed that DM, family history of CAD, smoking, MVD, smaller stent diameter, longer stent length, and type B2 or C lesions were proven to be ISR predictors following DES implantation.
1. Introduction
Restenosis is described as a narrowing of the lumen diameter after a successful percutaneous coronary intervention (PCI) procedure with or without stent insertion [1]. In-stent restenosis (ISR) is still defined as stenosis of more than 50% of the artery lumen diameter as assessed by coronary angiography within the stented segment or its edge (5 mm segments next to the stent) [2]. Prior to current guideline, BMSs (bare metal stents) were used in PCI, and the rate of not only ISR but also of early thrombosis was high after BMS implantation [2]. DESs are distinguished from BMSs by the presence of drug attached to the stent that is capable of inhibiting proliferation of cells which promote restenosis of the stented vessel [3]. However, the use of a drug-eluting stent (DES) for any PCI is now recommended by the latest European Society of Cardiology/European Association of Cardiothoracic Society guideline [4]. Despite the fact that the prevalence of restenosis in DESs was significantly reduced compared to the BMS era, ISR remained a challenge for post-PCI management. According to a large cohort study, ISR following DES implantation is still relatively high and affects approximately 10% of the population [5].
Factors that play the essential role in the development of ISR can be classified into four main groups: (1) biological, (2) arterial, (3) stent, and (4) implantation factors. The last three factors can be modified [6]. Currently, numerous studies have investigated predictors of restenosis in first-generation DESs, but only a few have investigated second-generation DESs. However, the predictors are inconsistent and conflicting [7]. Diabetes mellitus (DM), for example, has been demonstrated to be unrelated to restenosis-related DESs [8]. On the other hand, DM was found to be one of the strongest predictors in other studies [7,9]. Another issue is a history of coronary artery disease (CAD) in the family. Even though it is commonly recognized as a predictor of CAD, there is no original research or meta-analysis that has specifically addressed the link between family history of CAD and ISR in DES-implanted patients. To confirm or to reject such inconsistencies of predictors in second-generation DESs, a study of pooled analysis of available studies is required. Therefore, we conducted a systematic review and meta-analysis study regarding the ISR predictors following a successful DES implantation procedure. In this study, we reported 17 predictors which are related to ISR following DES implantation.
2. Materials and Methods
This meta-analysis followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for reporting systematic review and meta-analysis study [10]. This protocol of meta-analysis study has been registered in INPLASY (protocol number 202350092) [11].
2.1. Literature Search
As the first DES clinical trial was started in 2002 [3], we conducted an article search from electronic scientific databases from 2002 to 2021 (Embase, ProQuest, PubMed, ClinicalTrials.gov, and CENTRAL) regarding coronary ISR following a successful DES implantation procedure using the following keywords: “coronary artery disease” OR “CAD”, AND “in-stent restenosis” OR “stent restenosis” OR “ISR”, AND “drug-eluting stent” OR “DES”, AND “risk factor” OR “predicting factor” OR “predictor”. The complete list of search terms used in this study is presented in Supplementary Table S1. Duplicate results were also detected and eliminated using reference manager (Zotero) following incorporation of all results. Three investigators conducted the literature search, which was completed on 15 February 2021.
2.2. Eligibility Criteria
The inclusion criteria of included studies were as follows: (1) randomized controlled trial (RCT) or cohort study and (2) included CAD patients undergoing PCI with DES implantation followed by assessment of restenosis for a minimum of 6 months. We excluded the articles with the following criteria: (1) duplications; (2) non-original research article; (3) written in non-English language; (4) non-coronary ISR; (5) non-DES implantation; (6) unclear ISR definition; (7) unreported follow-up duration; and (8) low-quality study. The study selection process was completed by three investigators (SSB, LWS, BBP) led by ANM.
2.3. Study Quality Assessment
We evaluated the quality of studies included using the Modified Jadad Score for RCTs and Newcastle–Ottawa Scales (NOS) for cohort studies [12]. Good-quality studies with Modified Jadad Scores more than 4 or NOS at least 7 were included in the analysis. Three investigators conducted the study quality assessment process. Any disagreements were discussed and, if necessary, resolved with fifth (YW) and sixth reviewer (MSR).
2.4. Data Extraction
We extracted data regarding (1) authors’ name; (2) year of article publication; (3) study design; (4) age; (5) gender; (6) body mass index (BMI); (7) the presence of the cardiovascular disease (CVD) risk factors such as diabetes mellitus, dyslipidemia, hypertension, family history of CVD, smoking status; and (8) the important information about the procedural aspect including DES type, stent diameter, stent length, target vessel, lesion complexity, presence of multi-vessel disease (MVD). Four investigators performed the data extraction (ANM, SSB, LWS, BBP).
2.5. Statistical Analysis
We performed statistical analysis using Comprehensive Meta-Analysis version 3.0 and Review Manager version 5.4. The p-value of heterogeneity (pHet) of <0.1 indicated the presence of heterogeneity. The fixed-effects model was used in the absence of heterogeneity. On the other hand, the random-effect model was used in the absence of heterogeneity. The Egger’s test was performed to assess the small-study effect and publication bias [12,13]. The pooled odds ratio (OR) and 95% confidence interval (CI) for categorical data were calculated using the Mantel–Haenszel statistical method. The pooled mean difference (MD) and 95% CI for continuous data were determined using the inverse variance statistical method. The statistical analysis process was completed by three investigators.
3. Results
3.1. Study Selection Process
We identified 1379 potential articles from the electronic scientific databases in the beginning (Supplementary Table S1). Following duplication removal, we had 672 articles left. In the next stage, a total of 590 non-original research articles were removed and 82 articles underwent a further selection process. In the further selection, we excluded 60 articles because of the following: (1) written in non-English language (n = 4); (2) non-DES implantation (n = 50); (3) unclear ISR definition (n = 3); (4) unreported follow-up duration (n = 2); and (5) low-quality study (n = 1). The final studies included in this systematic review and meta-analysis comprised twenty-two studies, including five RCTs and sixteen cohort studies [8,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Only studies of good quality were involved in the current systematic review and meta-analysis (Supplementary Tables S2 and S3) [8,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. A total of 22,582 patients were included in the data analysis. Figure 1 is showing the study selection process. The summary of baseline characteristics of the included studies is summarized in Table 1.
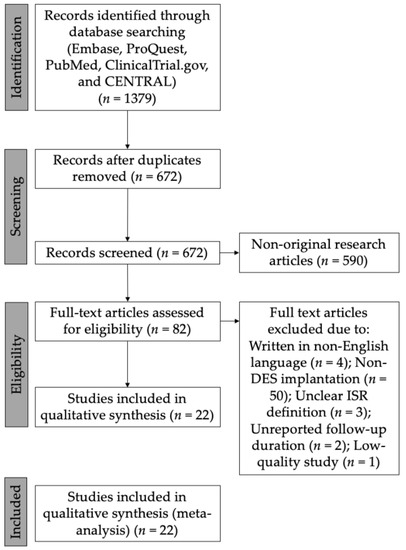
Figure 1.
Flow diagram showing study selection process. DES = drug-eluting stent; ISR = in-stent restenosis.

Table 1.
Baseline characteristic of the included studies.
3.2. In-Stent Restenosis Predictors
We extracted demographic and clinical characteristics data. These covariates were age, gender, BMI, family history of CAD, alcohol consumption, smoking, diabetes mellitus, hypertension, hyperlipidemia, stent diameter, stent length, stent type (limus-eluting stent vs. paclitaxel-eluting stent), target vessel LAD, complex type B2/C lesions, type C lesions, and MVD. The total number of studies included the following: (1) seven studies concerning family history and restenosis [15,20,27,29,30,31,33]; (2) seventeen studies concerning DM, smoking, age, gender, and restenosis [8,15,16,17,18,19,20,21,22,24,26,27,28,30,31,32,33]; (3) eight studies concerning lesion complexity and restenosis [8,19,20,21,22,25,28,30]; (4) fourteen studies concerning stent diameter, stent length, and restenosis [8,14,15,16,18,19,20,21,24,25,28,29,30,33]; (5) eight studies concerning the type of eluted drug and restenosis [8,14,18,21,24,25,26,30]; (6) eleven studies concerning multivessel disease and restenosis [8,17,19,20,21,22,25,28,29,31,34]; and (7) fourteen studies concerning dyslipidemia, hypertension, and restenosis [8,15,16,17,18,19,20,21,22,24,26,28,30,31].
From the clinical characteristics, we found that DM (OR 1.47; 95% CI 1.19 to 1.83; pHet < 0.01; p < 0.01), family history of CAD (OR 1.26; 95% CI 1.03 to 1.55; pHet 0.79; p 0.03), and smoking (OR 1.23; 95% CI 1.02 to 1.48; pHet < 0.01; p 0.03) were the strong predictors for the ISR following the successful DES implantation procedure (Figure 2). Stent characteristics also the important factor for the DES-ISR. Our findings revealed that DES-ISR was more common in DESs with smaller stent diameter (MD −0.12; 95% CI −0.16 to −0.08; pHet < 0.01; p < 0.01) and longer stent length (MD 2.24; 95% CI 1.36 to 3.13; pHet < 0.01; p < 0.01). Our meta-analysis also demonstrated that angiography characteristics, including MVD (OR 1.45; 95% CI 1.07 to 1.97; pHet < 0.01; p 0.02), type B2/C lesions (OR 1.56; 95% CI 1.06 to 2.30; pHet < 0.01; p 0.02), (Figure 3) and type C lesions (OR 1.33; 95% CI 1.09 to 1.62; pHet 0.85; p < 0.01) (Supplementary Figure S1) were associated with restenosis post DES implantation. Other covariates including (1) alcohol consumption (OR 1.00; 95% CI 0.82 to 1.23; pHet 0.10; p 0.97); (2) bifurcation lesion (OR 1.31; 95% CI 0.84 to 2.03; pHet < 0.01; p 0.24); (3) dyslipidemia (OR 1.04; 95% CI 0.88 to 1.23; pHet 0.03; p 0.62); (4) hypertension (OR 1.10; 95% CI 0.81 to 1.48; pHet < 0.01; p 0.55); (5) male gender (OR 0.99; 95% CI 0.75 to 1.30; pHet < 0.01; p 0.94); (6) stent type (limus-eluting stent) (OR 0.83; 95% CI 0.59 to 1.17; pHet < 0.01; p 0.29); (7) target vessel (left anterior descending artery) (OR 1.02; 95% CI 0.91 to 1.13; pHet 0.24; p 0.79); (8) age (MD 0.52; 95% CI −0.90 to 1.95; pHet < 0.01; p 0.47); and BMI (MD 0.54; 95% CI −0.15 to 1.23; pHet < 0.01; p 0.12) showed insignificant results (Supplementary Figures S2–S10).
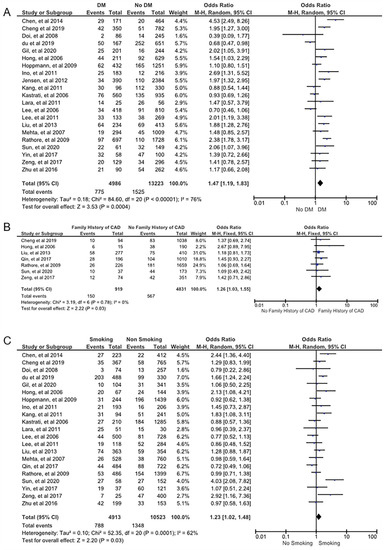
Figure 2.
Forest plot showing the ISR predictors. (A) Diabetes mellitus, (B) family history, and (C) smoking. The studies included in the pooled analysis are from references [8,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
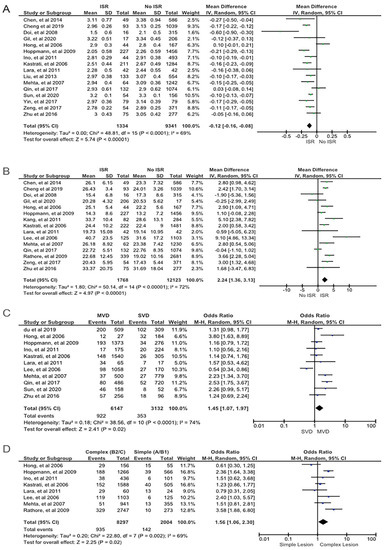
Figure 3.
Forest plot showing the ISR predictors. (A) Smaller stent diameter, (B) longer stent length, (C) multivessel disease, and (D) a complex lesion (type B2 or C lesions). The studies included in the pooled analysis are from references [8,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
3.3. Subgroup Analysis
A subgroup analysis of significant predictors (DM, family history of CAD, smoking, stent diameter, stent length, lesion complexity, and multivessel diseases) based on the generation of DESs was performed to evaluate the association of predictors with the occurrence of ISR in first- and second-generation DESs (Supplementary Figure S11). Diabetes mellitus was associated with the risk of developing ISR in the first-generation DESs (OR 1.62; 95% CI 1.09 to 2.41; pHet < 0.01; p 0.02) but not in the second-generation DESs (OR 1.29; 95% CI 0.84 to 1.97; pHet < 0.01; p 0.24). Smaller stent diameter was associated with the risk of developing ISR both in first-generation (MD −0.12; 95% CI −0.22 to −0.02; pHet < 0.01; p 0.02) and second-generation DESs (MD −0.15; 95% CI −0.24 to −0.06; pHet 0.94; p < 0.01). Likewise, longer stent length was associated with the higher risk of ISR both in the first-generation (MD 2.59; 95% CI 1.3 to 3.88; pHet < 0.01; p < 0.01) and second-generation (MD 3.43; 95% CI 1.37 to 5.49; pHet < 0.01; p < 0.01) DESs. Surprisingly, lesion complexity was associated with higher risk of ISR in the first-generation (OR 1.7; 95% CI 1.06 to 2.71; pHet < 0.01; p 0.03) but not in the second-generation (OR 1.21; 95% CI 0.67 to 2.2; pHet 0.27; p 0.53) DESs.
3.4. Heterogeneity among Studies and Publication Bias
We found heterogeneity between studies in some covariates: DM (p < 0.01), dyslipidemia (p 0.03), hypertension (p < 0.01), smoking (p < 0.01), stent diameter (p < 0.01), age (p < 0.01), male (p < 0.01), BMI (p < 0.01), stent length (p < 0.01), stent type (limus-eluting stent) (p < 0.01), and MVD (p < 0.01). Therefore, we analyze these covariates using a random effect model. The heterogeneity among studies is summarized in Table 2 and Table 3. We performed the Egger’s test to identify the presence of publication bias. As a result, we found no publication bias in all variables (indicated by the p-value of Egger’s test >0.05 in all covariates). The summary of the Egger’s test is described in Table 2 and Table 3. The funnel plots of each predictor are presented in Supplementary Figure S12.

Table 2.
The summary of the meta-analysis results for categorical variable.

Table 3.
The summary of the meta-analysis results for numerical variable.
4. Discussion
Several CVD risk factors had the essential contribution to DES-ISR. In this study, we discovered that DM patients with DES implants are more susceptible to ISR. Similar findings were reported in other investigations [5,20,35]. In the DES subgroup, an earlier meta-analysis study reported comparable findings [36]. Both meta-analyses, however, were conducted in the early 21st century using first-generation DESs. We conducted a meta-analysis using second-generation DESs, as well as a much larger sample size. The effect of DM on atherosclerosis pathogenesis is widely known among traditional CVD risk factors [37,38]. In both the bare metal stent (BMS) and DES categories, DM remains a strong predictor of ISR after coronary stent placement. Family history of CAD was found to be a significant predictor of DES-ISR in our meta-analysis. In this population, we found a 1.26-fold increase in the risk of DES-ISR. There have been several genetic polymorphisms discovered to enhance the risk of ISR. Zhou et al. discovered that polymorphisms in eNOS G298A, AGT M235T, AT1R A1166C, and MMP3 5A/6A genes enhanced the incidence of restenosis in a meta-analysis study [39]. Two previous studies have found a genetic variation linked to ISR, specifically in the DES subgroup [33,34]. To generalize those findings, a study with larger sample size is required. Despite the fact that family history is commonly recognized as a predictor of CAD [40,41], no meta-analysis specifically addressed the link between family history of CAD and ISR in patients undergoing DES implantation. As a result, our meta-analysis is the first meta-analysis to explain the association between family history and DES-ISR. We found that in comparison to non-smokers, smoking increased the risk of DES-ISR by 1.23 times. Tobacco use has long been recognized as a risk factor for CAD [42]. Surprisingly, research on ISR predictors in the BMS demonstrated conflicting results. Smoking has been linked to a decreased risk of ISR following a successful BMS implantation [43,44]. This behavior was named “smoker’s paradox” by one study. This phenomenon occurred in an immediate situation with younger smokers and has more favorable risk factors [43]. In a previous meta-analysis, Hu et al. failed to find a significant link between smoking and ISR in BMS and DES subgroups [45]. Smoking, on the other hand, increased the incidence of ISR in the DM subgroup receiving DESs. Surprisingly, we found the unaligned result with a large study by Moussa et al. [46] showing that dyslipidemia was significantly higher in the participants with restenosis. However, in a study with more specific population, dyslipidemia was not associated with ISR after DES implantation [47] and perfectly fit the result of our study. We found that our meta-analysis study provided a new insight into this topic with a larger sample size in the DES subgroup. In addition, the subgroup analysis in our study gives more understanding that the difference in the generation of DESs has difference association of predictors and the risk of ISR.
“Stent factors” also played the important role in the occurrence of DES-ISR. Our findings revealed that patients in the DES-ISR group have smaller stent diameters and longer stent lengths. A previous study found that a larger stent diameter has a positive long-term outcome, including a lower risk of restenosis [48]. The effect of smaller stent diameter on ischemic burden was consistent among generations of DESs. ISR was associated with smaller stents and vessel areas that were, in turn, associated with a greater frequency of stent under expansion [24]. In comparison to BMSs, within the longest stented lengths (>23 mm) and greatest diameters (>3.4 mm), DESs had a significantly reduced risk of target vessel revascularization, non-fatal myocardial infarction (MI), or mortality at 2 years [49]. In other DES, such as sirolimus-eluting stents, the length of the stent and the length of the lesion have been identified as independent predictors of ISR [50,51]. According to another study, the longer stents in narrower or tapering arteries had smaller in-stent regions, not just at the distal edge. Occult localized stent underexpansion might occur more frequently as the length of larger stents increases, resulting in more severe luminal constriction [52]. While the DES efficacy in reducing neointimal hyperplasia and subsequent restenosis following PCI was outstanding, the greater stent length was also linked to an increased risk of negative clinical outcomes [52]. Another study found that having a longer stent was linked to a higher risk of stent thrombosis, MI, and restenosis [53]. The drugs used on the stents may have an impact on the outcome of restenosis. Limus and paclitaxel were two medications that worked in different ways to prevent restenosis. A prior study found that sirolimus-eluting stents were more effective in the short-term follow-up period than paclitaxel-eluting stents [54]. However, our meta-analysis of limus- vs. paclitaxel-eluting stent found no difference in the risk of restenosis. This result is supported by meta-analysis with larger samples comparing limus and paclitaxel in DM patients [54]. However, when stratified by the generation of DESs, our study revealed that fewer predictors were associated with the higher occurrence of ISR in second-generation compared to first-generation DESs. This result is comparable with of subjects with DM that the risk of target lesion revascularization (TLR) was higher in first-generation DESs [55]. Moreover, our study revealed that lesion complexity is associated with the higher risk to develop ISR in the first-generation DESs. However, there is no study that reported lesion complexity in first-generation versus second-generation DESs.
Many aspects regarding the angiography characteristics also had the significant impact on the DES-ISR. MVD is characterized as stenosis of two or more coronary arteries with a diameter of 50% or less. Compared to single-vessel disease (SVD), MVD involvement was associated with a worse prognosis and considerably higher mortality [56]. The stent or surgery (SOS) trial showed that in the MVD patients, the revascularization requirement was higher in the PCI group using DESs (21%) compared to patients in the coronary artery bypass group (6%) [57]. Our meta-analysis revealed that DES-ISR incidence was significantly higher in MVD patients than in SVD patients. ISR is more common in participants with MVD, which is consistent with our meta-analysis [1]. Although DESs lowered the ISR risk compared to BMSs, late restenosis still occurs, particularly in patients with complicated lesions or high-risk variables for sequelae [58]. The angiography lesions were evaluated using morphological criteria from the American College of Cardiology/American Heart Association. The type A and B1 lesions were categorized as simple lesions, whereas the type B2 or C lesions were classified as complex lesions. Kastrati et al. found that restenosis occurred in 33.2% of complex lesions and 24.9% of simple lesions in a study of 2944 patients. Type A, type B1, type B2, and type C lesions had restenosis rate of 21.7%, 26.3%, 33.7%, and 32.6%, respectively [59]. A higher prevalence of restenosis and worse long-term results were associated with type B2 or C lesions [60]. According to our findings, patients with type B2 or C lesions had a higher risk of ISR than those with type A lesions. Our findings are summarized and illustrated in Figure 4.
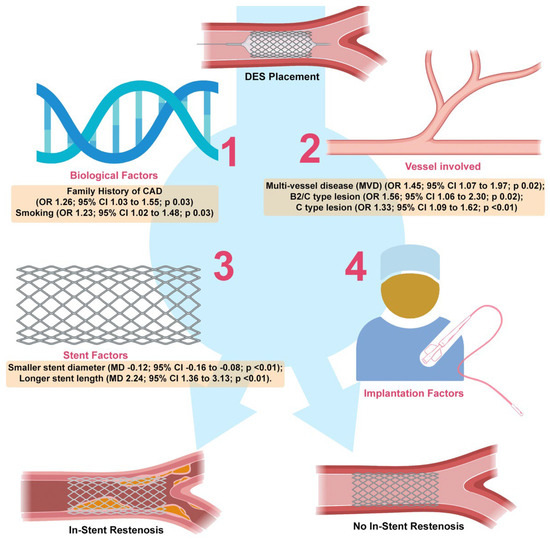
Figure 4.
Summary of major findings. Factors involved in ISR has been found to be classified into biological factors (1), vessel factors (2), stent factors (3), and implantation factors (4). This study revealed new finding that family history is one of predictor following DES implantation. Our study also pointed out that smoking is relevant in-stent restenosis predictor, which is a conflicting issue in the literature so far (1). We also confirmed many previous studies regarding factors of in-stent restenosis (2–4).
This systematic review and meta-analysis had significant limitations, which we identified. First, the majority of the studies in our analysis were cohort studies, which resulted in undesired selection and referral bias. For example, our initial studies included a study by Ino et al. [22] which failed to follow up 13% of their participants. However, we were able to overcome this problem by only including high-quality studies. Second, the likelihood of publishing bias could not be excluded. To mitigate this scenario, we used Egger’s tests to assess publication bias. In this meta-analysis, we found no evidence of publication bias. Third, we were unable to evaluate the true impacts at the patient level due to a lack of data at the individual patient level. Fourth, there could be confounding issues due to the wide variation of follow-up period durations across the studies involved.
5. Conclusions
DM, family history of CAD, smoking, MVD, smaller stent diameter, longer stent length, and type B2 or C lesions were proven to be associated with ISR following DES implantation. Moreover, we demonstrated the association between the family history of CAD and ISR following DES implantation for the first time. In addition, first- and second-generation DESs have different risks of ISR occurrence in each predictor. Our findings contributed to the understanding of the risk of ISR after a successful DES implantation procedure.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jvd2030020/s1. Supplementary Figure S1: Type C lesion was significantly associated with ISR following DES implantation; Supplementary Figure S2: Age was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S3: Alcohol drinking was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S4: Bifurcation lesion was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S5: Body mass index was significantly associated with in stent restenosis following DES implantation. Supplementary Figure S6: Dyslipidemia was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S7: Hypertension was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S8: Left anterior descending lesion was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S9: Male gender was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S10: Stent type (limus-eluting vs. taxel-eluting) was not significantly associated with in stent restenosis following DES implantation. Supplementary Figure S11: Funnel Plot of each predictor. Supplementary Table S1: Detailed search strategy. Supplementary Table S2: Newcastle Ottawa Score of each cohort included in the study. Supplementary Table S3. Modified Jaded Scale of each randomized control trial included in the study.
Author Contributions
M.S.R.: conceptualization, study quality assessment, data interpretation, writing—review and editing, and supervising. Y.W.: conceptualization, study selection process, data interpretation, writing—review and editing, and supervising. A.N.M.: study selection process, study quality assessment, data extraction, data analysis, and writing—original draft. S.S.B.: literature search, investigation, data extraction, data analysis, and writing—original draft. B.B.P.: literature search, data extraction, and writing—original draft. L.W.S.: literature search, data extraction, and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study protocol has been registered in the IPLASY protocol.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the manuscript and Supplementary Materials.
Acknowledgments
Thanks to Jonny Karunia Fajar from Department of Internal Medicine, Faculty of Medicine, Universitas Brawijaya for providing consultation in the early stage of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and Managing In-Stent Restenosis: A Review of Clinical Data, from Pathogenesis to Treatment. J. Thorac. Dis. 2016, 8, E1150–E1162. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-Stent Restenosis in the Drug-Eluting Stent Era. J. Am. Coll. Cardiol. 2010, 56, 1897–1907. [Google Scholar] [CrossRef]
- Tan, L.; Wang, X.; Yuan, K.; Yin, T.; Du, R.; Shen, L.; Zhu, Z.; Yu, S.; Zhang, H.; Wang, G. Structural and Temporal Dynamics Analysis on Drug-Eluting Stents: History, Research Hotspots and Emerging Trends. Bioact. Mater. 2023, 23, 170–186. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.L.; Kastrati, A. Incidence and Predictors of Restenosis after Coronary Stenting in 10,004 Patients with Surveillance Angiography. Heart 2014, 100, 153–159. [Google Scholar] [CrossRef]
- Farooq, V.; Gogas, B.D.; Serruys, P.W. Restenosis Delineating the Numerous Causes of Drug-Eluting Stent Restenosis. Circ. Cardiovasc. Interv. 2011, 4, 195–205. [Google Scholar] [CrossRef]
- Zheng, C.; Kang, J.; Park, K.W.; Han, J.K.; Yang, H.M.; Kang, H.J.; Koo, B.K.; Kim, H.S. The Predictors of Target Lesion Revascularization and Rate of In-Stent Restenosis in the Second-Generation Drug-Eluting Stent Era. J. Interv. Cardiol. 2019, 2019, 3270132. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Dibra, A.; Mehilli, J.; Mayer, S.; Pinieck, S.; Pache, J.; Dirschinger, J.; Schömig, A. Predictive Factors of Restenosis after Coronary Implantation of Sirolimus- or Paclitaxel-Eluting Stents. Circulation 2006, 113, 2293–2300. [Google Scholar] [CrossRef]
- Takasawa, Y.; Iijima, R.; Shiba, M.; Nakamura, M.; Sugi, K. Predictor of Subsequent Target Lesion Revascularization in Patients with Drug-Eluting Stent Restenosis Undergoing Percutaneous Coronary Intervention. J. Cardiol. 2010, 55, 391–396. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Rohman, M.S.; Waranugraha, Y.; Masbuchin, A.N.; Baskoro, S.S.; Sishartami, L.W.; Pratiwi, B.B. Coronary In-Stent Restenosis Predictors Following Drug-Eluting Stent Implantation: A Meta-Analysis Study. Inplasy Protoc. 2023. [Google Scholar] [CrossRef]
- Waranugraha, Y.; Rizal, A.; Firdaus, A.J.; Sihotang, F.A.; Akbar, A.R.; Lestari, D.D.; Firdaus, M.; Nurudinulloh, A.I. The Superiority of High-power Short-duration Radiofrequency Catheter Ablation Strategy for Atrial Fibrillation Treatment: A Systematic Review and Meta-analysis Study. J. Arrhythmia 2021, 37, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S. From Pre-Registration to Publication: A Non-Technical Primer for Conducting a Meta-Analysis to Synthesize Correlational Data. Front. Psychol. 2015, 6, 1549. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Jin, J.; Tian, F.; Han, Y.; Wang, J.; Liu, J.; Chen, Y. Chronic Treatment with Trimetazidine after Discharge Reduces the Incidence of Restenosis in Patients Who Received Coronary Stent Implantation: A 1-Year Prospective Follow-up Study. Int. J. Cardiol. 2014, 174, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Chang, F.J.; Wang, Y.; You, P.H.; Chen, H.C.; Han, W.Q.; Wang, J.W.; Zhong, N.E.; Min, Z.Q. Factors Influencing Stent Restenosis after Percutaneous Coronary Intervention in Patients with Coronary Heart Disease: A Clinical Trial Based on 1-Year Follow-Up. Med. Sci. Monit. 2019, 25, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Maehara, A.; Mintz, G.S.; Weissman, N.J.; Yu, A.; Wang, H.; Mandinov, L.; Popma, J.J.; Ellis, S.G.; Grube, E.; et al. Impact of In-Stent Minimal Lumen Area at 9 Months Poststent Implantation on 3-Year Target Lesion Revascularization-Free Survival: A Serial Intravascular Ultrasound Analysis from the TAXUS IV, V, and VI Trials. Circ. Cardiovasc. Interv. 2008, 1, 111–118. [Google Scholar] [CrossRef]
- Du, J.B.; Zhang, W.; Li, N.; Jiang, H.; Liu, Y.; Gao, J.; Chen, S.T.; Cong, H.L.; Wei, Y.L. Association Study of Matrix Metalloproteinase 3 5A/6A Polymorphism with in-Stent Restenosis after Percutaneous Coronary Interventions in a Han Chinese Population. J. Int. Med. Res. 2020, 48, 0300060519827145. [Google Scholar] [CrossRef]
- Gil, R.J.; Bil, J.; Kern, A.; Iñigo-Garcia, L.A.; Formuszewicz, R.; Dobrzycki, S.; Vassilev, D.; Mehran, R. Angiographic Restenosis in Coronary Bifurcations Treatment with Regular Drug Eluting Stents and Dedicated Bifurcation Drug-Eluting BiOSS Stents: Analysis Based on Randomized POLBOS i and POLBOS II Studies. Cardiovasc. Ther. 2020, 2020, 6760205. [Google Scholar] [CrossRef]
- Gomez-Lara, J.; Heo, J.H.; Brugaletta, S.; Garg, S.; Garcia-Garcia, H.M.; Van Geuns, R.J.; Silber, S.; Windecker, S.; Serruys, P.W. Risk of Target Lesion Failure in Relationship to Vessel Angiographic Geometry and Stent Conformability Using the Second Generation of Drug-Eluting Stents. Am. Heart J. 2011, 162, 1069–1079.e2. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, M.H.; Ahn, T.H.; Ahn, Y.K.; Bae, J.H.; Shim, W.J.; Ro, Y.M.; Lim, D.S. Multiple Predictors of Coronary Restenosis after Drug-Eluting Stent Implantation in Patients with Diabetes. Heart 2006, 92, 1119–1124. [Google Scholar] [CrossRef]
- Hoppmann, P.; Erl, A.; Türk, S.; Tiroch, K.; Mehilli, J.; Schömig, A.; Kastrati, A.; Koch, W. No Association of Chromosome 9p21.3 Variation With Clinical and Angiographic Outcomes After Placement of Drug-Eluting Stents. JACC Cardiovasc. Interv. 2009, 2, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Kubo, T.; Kitabata, H.; Shimamura, K.; Shiono, Y.; Orii, M.; Okochi, K.; Sougawa, H.; Tanimoto, T.; Komukai, K.; et al. Impact of Hinge Motion on In-Stent Restenosis after Sirolimus-Eluting Stent Implantation. Circ. J. 2011, 75, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.O.; Thayssen, P.; Junker, A.; Maeng, M.; Tilsted, H.H.; Kaltoft, A.; Hansen, K.N.; Christiansen, E.H.; Kristensen, S.D.; Ravkilde, J.; et al. Comparison of Outcomes in Patients with versus without Diabetes Mellitus after Revascularization with Everolimus- and Sirolimus-Eluting Stents (from the SORT OUT IV Trial). Am. J. Cardiol. 2012, 110, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Mintz, G.S.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Han, K.H.; Kim, J.J.; Park, S.W.; Park, S.J. Mechanisms of In-Stent Restenosis after Drug-Eluting Stent Implantation Intravascular Ultrasound Analysis. Circ. Cardiovasc. Interv. 2011, 4, 9–14. [Google Scholar] [CrossRef]
- Lee, C.W.; Park, D.W.; Lee, B.K.; Kim, Y.H.; Hong, M.K.; Kim, J.J.; Park, S.W.; Park, S.J. Predictors of Restenosis after Placement of Drug-Eluting Stents in One or More Coronary Arteries. Am. J. Cardiol. 2006, 97, 506–511. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, D.W.; Kim, Y.H.; Yun, S.C.; Kim, W.J.; Kang, S.J.; Lee, S.W.; Lee, C.W.; Park, S.W.; Park, S.J. Incidence, Predictors, Treatment, and Long-Term Prognosis of Patients with Restenosis after Drug-Eluting Stent Implantation for Unprotected Left Main Coronary Artery Disease. J. Am. Coll. Cardiol. 2011, 57, 1349–1358. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Jiang, H.; Ding, X.; Zhu, R.; Li, B.; Zhao, Y. Plasma Levels of Interleukin 18, Interleukin 10, and Matrix Metalloproteinase-9 and -137G/C Polymorphism of Interleukin 18 Are Associated with Incidence of in-Stent Restenosis after Percutaneous Coronary Intervention. Inflammation 2013, 36, 1129–1135. [Google Scholar] [CrossRef]
- Mehta, R.H.; Leon, M.B.; Sketch, M.H. The Relation Between Clinical Features, Angiographic Findings, and the Target Lesion Revascularization Rate in Patients Receiving the Endeavor Zotarolimus-Eluting Stent for Treatment of Native Coronary Artery Disease: An Analysis of ENDEAVOR I, ENDEAVOR II, ENDEAVOR II Continued Access Registry, and ENDEAVOR III. Am. J. Cardiol. 2007, 100, S62–S70. [Google Scholar] [CrossRef]
- Qin, Z.; Zheng, F.W.; Zeng, C.; Zhou, K.; Geng, Y.; Wang, J.L.; Li, Y.P.; Ji, Q.W.; Zhou, Y.J. Elevated Levels of Very Low-Density Lipoprotein Cholesterol Independently Associated with in-Stent Restenosis in Diabetic Patients after Drug-Eluting Stent Implantation. Chin. Med. J. 2017, 130, 2326–2332. [Google Scholar] [CrossRef]
- Rathore, S.; Terashima, M.; Katoh, O.; Matsuo, H.; Tanaka, N.; Kinoshita, Y.; Kimura, M.; Tuschikane, E.; Nasu, K.; Ehara, M.; et al. Predictors of Angiographic Restenosis after Drug Eluting Stents in the Coronary Arteries: Contemporary Practice in Real World Patients. EuroIntervention 2009, 5, 349–354. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H.; Liu, H.; Pu, D.; Gao, J.; Jin, X.; Liu, X.; Yan, A. Correlation of Pre-Operative Circulating Inflammatory Cytokines with Restenosis and Rapid Angiographic Stenotic Progression Risk in Coronary Artery Disease Patients Underwent Percutaneous Coronary Intervention with Drug-Eluting Stents. J. Clin. Lab. Anal. 2020, 34, e23108. [Google Scholar] [CrossRef]
- Yin, J.; Shen, L.; Ji, M.; Wu, Y.; Cai, S.; Chen, J.; Yao, Z.; Ge, J. Inverse Relationship between Serum VEGF Levels and Late In-Stent Restenosis of Drug-Eluting Stents. BioMed Res. Int. 2017, 2017, 8730271. [Google Scholar] [CrossRef]
- Zeng, W.P.; Zhang, R.; Li, R.; Luo, J.F.; Hu, X.F. Association of the Endothelial Nitric Oxide Synthase Gene T786C Polymorphism with In-Stent Restenosis in Chinese Han Patients with Coronary Artery Disease Treated with Drug-Eluting Stent. PLoS ONE 2017, 12, e0170964. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, M.; Lin, J.; Zhu, H.; Lu, Y.; Wang, B.; Xue, Y.; Fang, C.; Tang, L.; Xu, B.; et al. Association of Seven Renin Angiotensin System Gene Polymorphisms with Restenosis in Patients Following Coronary Stenting. J. Renin-Angiotensin-Aldosterone Syst. 2017, 18, 147032031668877. [Google Scholar] [CrossRef]
- Stettler, C.; Allemann, S.; Egger, M.; Windecker, S.; Meier, B.; Diem, P. Efficacy of Drug Eluting Stents in Patients with and without Diabetes Mellitus: Indirect Comparison of Controlled Trials. Heart 2006, 92, 650–657. [Google Scholar] [CrossRef]
- Gilbert, J.; Raboud, J.; Zinman, B. Meta-Analysis of the Effect of Diabetes on Restenosis Rates among Patients Receiving Coronary Angioplasty Stenting. Diabetes Care 2004, 27, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, M.T.; Setodji, C.M.; Pankow, J.S.; Blumenthal, R.S.; Keeler, E. Relation of Familial Patterns of Coronary Heart Disease, Stroke, and Diabetes to Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Genet. Med. 2008, 10, 879–887. [Google Scholar] [CrossRef]
- Smith, S.C.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2006 Update. Endorsed by the National Heart, Lung, and Blood Institute. J. Am. Coll. Cardiol. 2006, 47, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Mu, G.; Wei, S.; Liu, Z.; Wang, Z.; Xiang, Q.; Cui, Y. Associations Between Polymorphisms of Endothelial Nitric Oxide Synthase, Matrix Metalloproteinase 3, Angiotensinogen, and Angiotensin II Type 1 Receptor and Risk of Restenosis after Percutaneous Coronary Intervention: A Meta-Analysis. Clin. Ther. 2020, 42, 458–474. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Nam, B.-H.; D’Agostino, R.; Levy, D.; Wang, T.J.; Wilson, P.W.F.; Donnell, C.J.O. Parental Cardiovascular Disease as a Risk Factor for Cardiovascular Disease in Middle-Aged Adults A Prospective Study of Parents and Offspring. JAMA 2004, 291, 2204–2211. [Google Scholar] [CrossRef]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Venkatason, P.; Salleh, N.M.; Zubairi, Y.; Hafidz, I.; Ahmad, W.A.W.; Han, S.K.; Zuhdi, A.S.M. The Bizzare Phenomenon of Smokers’ Paradox in the Immediate Outcome Post Acute Myocardial Infarction: An Insight into the Malaysian National Cardiovascular Database-Acute Coronary Syndrome (NCVD-ACS) Registry Year 2006–2013. Springerplus 2016, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Chauhan, M.S.; Baim, D.S.; Ho, K.K.L.; Popma, J.J.; Carrozza, J.P.; Cohen, D.J.; Kuntz, R.E. Clinical Restenosis after Coronary Stenting: Perspectives from Multicenter Clinical Trials. J. Am. Coll. Cardiol. 2002, 40, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.T.; Liu, J.; Zhou, Y.; Hu, B.L. Association of Smoking with Restenosis and Major Adverse Cardiac Events after Coronary Stenting: A Meta-Analysis. Pak. J. Med. Sci. 2015, 31, 1002–1008. [Google Scholar] [CrossRef]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Balachander, J. Predictor of In-Stent Restenosis in Patients with Drug-Eluting Stent (PRIDE)- a Retrospective Cohort Study. Clín. Investig. Arterioscler. 2021, 33, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, H.; Okada, K.; Kimura, T.; Yock, P.G.; Lansky, A.J.; Popma, J.J.; Yeung, A.C.; Fitzgerald, P.J.; Honda, Y. Impact of Stent Size Selection on Acute and Long-Term Outcomes after Drug-Eluting Stent Implantation in de Novo Coronary Lesions. Circ. Cardiovasc. Interv. 2017, 10, e004795. [Google Scholar] [CrossRef]
- Applegate, R.J.; Sacrinty, M.T.; Kutcher, M.A.; Santos, R.M.; Gandhi, S.K.; Little, W.C. Effect of Length and Diameter of Drug-Eluting Stents versus Bare-Metal Stents on Late Outcomes. Circ. Cardiovasc. Interv. 2009, 2, 35–42. [Google Scholar] [CrossRef]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-Eluting Stents versus Standard Stents in Patients with Stenosis in a Native Coronary Artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Holmes, D.R.; Leon, M.B.; Moses, J.W.; Popma, J.J.; Cutlip, D.; Fitzgerald, P.J.; Brown, C.; Fischell, T.; Wong, S.C.; Midei, M.; et al. Analysis of 1-Year Clinical Outcomes in the SIRIUS Trial. Circulation 2004, 109, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Koh, Y.S.; Lim, S.; Kim, J.J.; Chang, M.; Kang, M.; Hwang, B.H.; Kim, C.J.; Kim, T.H.; Seo, S.M.; et al. Impact of the Stent Length on Long-Term Clinical Outcomes Following Newer-Generation Drug-Eluting Stent Implantation. Am. J. Cardiol. 2014, 113, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Song, H.G.; Kang, S.J.; Ahn, J.M.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Park, S.W.; et al. Intravascular Ultrasound Assessment of Optimal Stent Area to Prevent In-Stent Restenosis after Zotarolimus-, Everolimus-, and Sirolimus-Eluting Stent Implantation. Catheter. Cardiovasc. Interv. 2014, 83, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, L.; Song, Y.; Chen, L.; Xue, Q.; Tian, J.; Wang, Y.; Chen, Y. Efficacy and Safety of Limus-Eluting versus Paclitaxel-Eluting Coronary Artery Stents in Patients with Diabetes Mellitus: A Meta-Analysis. Int. J. Cardiol. 2015, 184, 680–691. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Shiomi, H.; Natsuaki, M.; Morimoto, T.; Igarashi, K.; Kadota, K.; Muramatsu, T.; Tanabe, K.; Morino, Y.; Akasaka, T.; et al. Second-Generation versus First-Generation Drug-Eluting Stents in Patients with and without Diabetes Mellitus: Pooled Analysis from the RESET and NEXT Trials. Cardiovasc. Interv. Ther. 2018, 33, 125–134. [Google Scholar] [CrossRef] [PubMed]
- De Innocentiis, C.; Zimarino, M.; De Caterina, R. Is Complete Revascularisation Mandated for All Patients with Multivessel Coronary Artery Disease? Interv. Cardiol. Rev. 2018, 13, 45–50. [Google Scholar] [CrossRef]
- Mikhail, G.W.; Airoldi, F.; Tavano, D.; Chieffo, A.; Rogacka, R.; Carlino, M.; Montorfano, M.; Sangiorgi, G.; Corvaja, N.; Michev, I.; et al. The Use of Drug Eluting Stents in Single and Multivessel Disease: Results from a Single Centre Experience. Heart 2004, 90, 990–994. [Google Scholar] [CrossRef]
- TsiGkas, G.G.G.; Karantalis, V.; Hahalis, G.; Alexopoulos, D. Stent Restenosis, Pathophysiology and Treatment Options: A 2010 Update. Hell. J. Cardiol. 2011, 52, 149–157. [Google Scholar]
- Kastrati, A.; Schoemig, A.; Elezi, S.; Dirschinger, J.; Mehilli, J.; Schuhlen, H.; Blasini, R.; Neumann, F.J. Prognostic Value of the Modified American College of Cardiology/American Heart Association Stenosis Morphology Classification for Long-Term Angiographic and Clinical Outcome after Coronary Stent Placement. Circulation 1999, 100, 1285–1290. [Google Scholar] [CrossRef]
- Alraies, M.; Darmoch, F.; Tummala, R.; Waksman, R. Diagnosis and Management Challenges of In-Stent Restenosis in Coronary Arteries. World J. Cardiol. 2017, 9, 640–647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).