Intraoperative Flow Measurement as a Quality Control during Carotid Endarterectomy in a Teaching Hospital Setting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Procedures
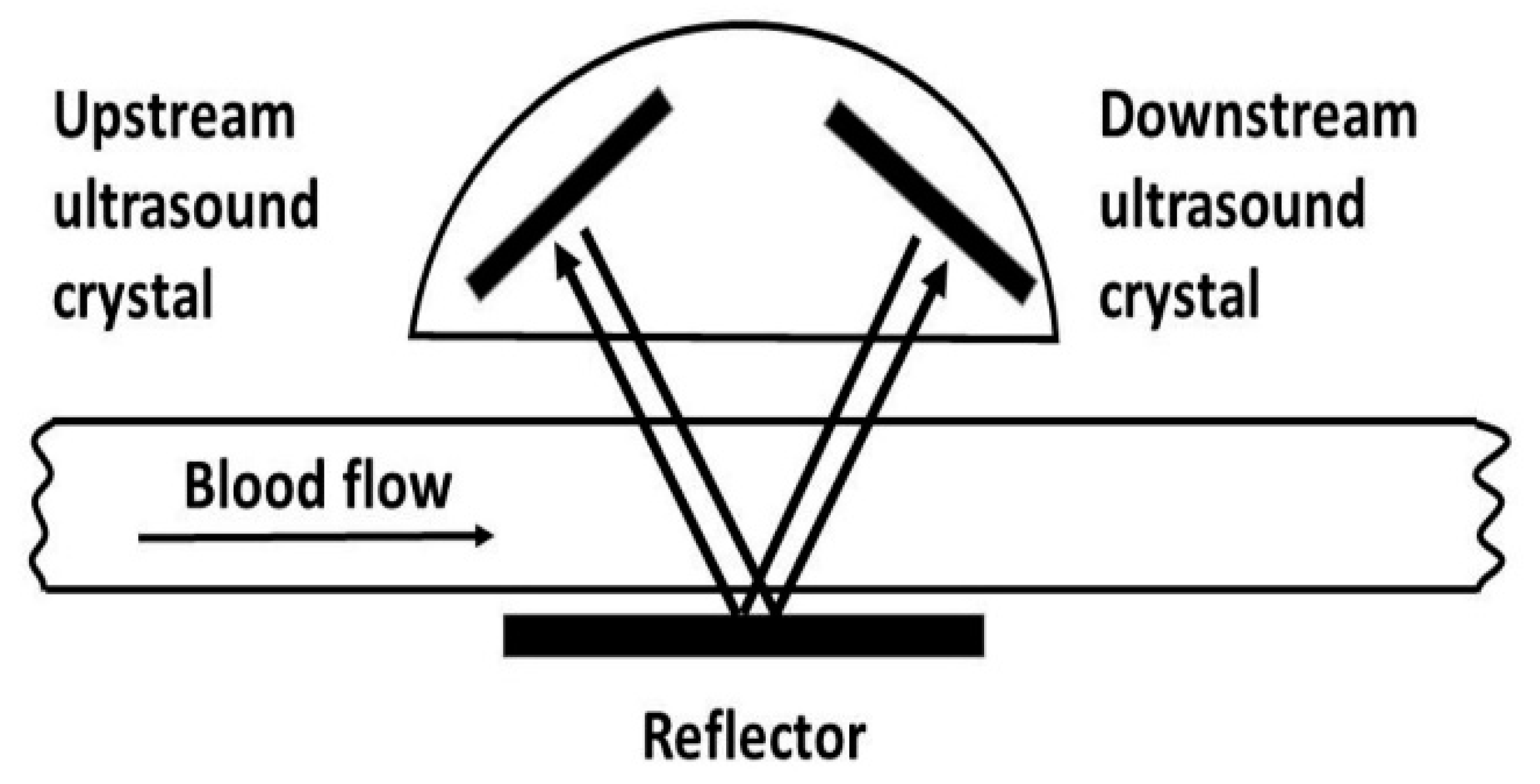
2.3. Intraoperative Assessment
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Intraoperative Assessment
3.3. Postoperative TIA, Stroke, and Death
3.4. Local Complications
3.5. Postoperative Follow-Up
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silvestrini, M.; Vernieri, F.; Troisi, E.; Passarelli, F.; Matteis, M.; Pasqualetti, P.; Rossini, P.M.; Caltagirone, C. Cerebrovascular reactivity in carotid artery occlusion: Possible implications for surgical management of selected groups of patients. Acta Neurol. Scand. 1999, 99, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Hass, W.K.; Fields, W.S.; North, R.R.; Kricheff, I.I.; Chase, N.E.; Bauer, R.B. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA 1968, 203, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.; Goertler, M.; Harms, L.; Diener, H.-C. Distribution and outcome of symptomatic stenoses and occlusions in patients with acute cerebral ischemia. Arch. Neurol. 2006, 63, 1287–1291. [Google Scholar] [CrossRef]
- Riles, T.S.; Imparato, A.M.; Jacobowitz, G.R.; Lamparello, P.J.; Giangola, G.; Adelman, M.A.; Landis, R. The causes of perioperative stroke after carotid endarterectomy. J. Vasc. Surg. 1994, 19, 206–216. [Google Scholar] [CrossRef]
- Pappada, G.; Vergani, F.; Parolin, M.; Cesana, C.; Pirillo, D.; Pirovano, M.; Santoro, P.; Landi, A.; Ferrarese, C. Early acute hemispheric stroke after carotid endarterectomy. Pathogenesis and management. Acta Neurochir. 2010, 152, 579–587. [Google Scholar] [CrossRef]
- Halliday, A.; Harrison, M.; Hayter, E.; Kong, X.; Mansfield, A.; Marro, J.; Pan, H.; Peto, R.; Potter, J.; Rahimi, K.; et al. 10-year stroke prevention after successful carot-id endarterectomy for asymptomatic stenosis (ACST-1): A multicentre ran-domised trial. Lancet 2010, 376, 1074–1084. [Google Scholar] [CrossRef]
- Ascher, E.; Markevich, N.; Hingorani, A.P.; Kallakuri, S.; Gunduz, Y. Internal carotid artery flow volume measurement and other intraoperative duplex scanning parameters as predictors of stroke after carotid endarterectomy. J. Vasc. Surg. 2002, 35, 439–444. [Google Scholar] [CrossRef]
- Lundell, A.; Bergqvist, D. Prediction of early graft occlusion in femoropopliteal and femorodistal reconstruction by measurement of volume flow with a transit time flowmeter and calculation of peripheral resistance. Eur. J. Vasc. Surg. 1993, 7, 704–708. [Google Scholar] [CrossRef]
- Hartman, J.C.; Olszanski, D.A.; Hullinger, T.G.; Brunden, M.N. In vivo validation of a transit time ultrasonic volume flowmeter. J. Pharmacol. Toxicol. Methods 1994, 31, 153–160. [Google Scholar] [CrossRef]
- Laustsen, J.; Pedersen, E.M.; Terp, K.; Steinbrüchel, D.; Kure, H.; Paulsen, P.; Jørgensen, H.; Paaske, W. Validation of a new transit time ultrasound flowmeter in man. Eur. J. Endovasc. Surg. 1996, 12, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Walpoth, B.H.; Bosshard, A.; Genyk, I.; Kipfer, B.; Berdat, P.A.; Hess, O.M.; Althaus, U.; Carrel, T.P. Transit time flow measurement for Detection of early graft failure during myocardial revascularisation. Ann. Thorac. Surg. 1998, 66, 1097–1100. [Google Scholar] [CrossRef]
- Panetton, J.M.; Berger, M.W.; Lewis, B.D.; Hallett, J.W.; Bower, T.C.; Gloviczki, P.; Cherry, K.J. Intraoperative duplex ultrasound during carotid endarterectomy. Vasc. Surg. 2001, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mays, B.W.; Towne, J.B.; Seabrook, G.R.; Cambria, R.A.; Jean-Claude, J. Intraoperative carotid evaluation. Arch. Surg. 2000, 135, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, O.; MacKenzie, K.; Nault, P.; Singher, F.; Dumaine, J. Intraoperative duplex scanning for carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 153–158. [Google Scholar] [CrossRef]
- Dykes, J.R., II; Bergamini, T.M.; Lipski, D.A.; Fulton, R.L.; Garrison, R.N. Intraoperative duplex scanning reduces both residual stenosis and postoperative morbidity of carotid endarterectomy. Am. Surg. 1997, 63, 51–54. [Google Scholar]
- Walker, R.A.; Fox, A.D.; Magee, T.R.; Horrocks, M. Intraoperative duplex scanning as a means of quality control during carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 1996, 11, 364–367. [Google Scholar] [CrossRef]
- Kinney, E.V.; Seabrook, G.R.; Kinney, L.Y.; Bandyk, D.F.; Towne, J.B. The importance of intraoperative detection of residual flow abnormalities after carotid artery endarterectomy. J. Vasc. Surg. 1993, 17, 912–922. [Google Scholar] [CrossRef]
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995, 273, 1421–1428. [Google Scholar] [CrossRef]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Plass, K.G. A New Ultrasonic Flowmeter for Intravascular Application. IEEE Trans. Biomed. Eng. 1964, 11, 154–156. [Google Scholar] [CrossRef]
- Burton, R.G.; Gorewit, R. Ultrasonic flowmeter uses widebeam transit-time technique. Med. Electr. 1984, 15, 68–73. [Google Scholar]
- Bamford, J.; Sandercock, P.; Dennis, M.; Warlow, C.; Jones, L.; McPherson, K.; Vessey, M.; Fowler, G.; Molyneux, A.; Hughes, T. A prospective study of acute cerebrovascular disease in the community: The Oxfordshire Community Stroke Project 1981–1986. Part 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid hemorrhage. J. Neurol. Neurosurg. Psychiatry 1990, 53, 16–22. [Google Scholar] [PubMed] [Green Version]
- Rockman, C.B.; Jacobowitz, G.R.; Lamparello, P.J.; Rockman, C.B.; Jacobowitz, G.R.; Lamparello, P.J.; Adelman, M.A.; Woo, D.; Schanzer, A.; Riles, T.S. Immediate reexploration for the perioperative neurologic event after carotid endarterectomy: Is it worthwhile? J. Vasc. Surg. 2000, 32, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.P.; Zhu, Y.; Matt, C.; Pelz, C.; Roza, A.M.; Adams, M.B. Prognostic value of intraoperative blood flow measurements in vascular acess surgery. Surgery 1998, 124, 729–737. [Google Scholar] [CrossRef]
- Cyrek, A.E.; Bernheim, J.; Juntermanns, B.; Husen, P.; Pacha, A.; Hoffmann, J.N. Intraoperative use of transit time flow measurement improves patency of newly created radiocephalic arteriovenous fistulas in patients requiring hemodialysis. J. Vasc. Access 2020, 21, 990–996. [Google Scholar] [CrossRef]
- Gordon, I.L.; Stemmer, E.A.; Williams, R.A.; Arafi, M.; Wilson, S.E. Changes in internal carotid blood flow after carotid endarterectomy correlate with preoperative stenosis. Am. J. Surg. 1994, 168, 127–130. [Google Scholar] [CrossRef]
- Gordon, I.L.; Stemmer, E.A.; Wilson, S.E. Redistribution of blood flow after carotid endarterectomy. J. Vasc. Surg. 1995, 22, 349–360. [Google Scholar] [CrossRef]
- Zannetti, S.; Cao, P.; De Rango, P.; Giordano, G.; Parlani, G.; Lenti, M.; Nora, A. Intraoperative assessment of technical perfection in carotid endarterectomy: A prospective analysis of 1305 completion procedures—Collaborators of the EVEREST study group. Eversion versus standard carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 1999, 18, 52–58. [Google Scholar] [CrossRef]
- Knappich, C.; Lang, T.; Tsantilas, P.; Schmid, S.; Kallmayer, M.; Haller, B.; Eckstein, H.-H. Intraoperative completion studies in carotid endarterectomy: Systematic review and meta-analysis of techniques and outcomes. Ann. Transl. Med. 2021, 9, 1201. [Google Scholar] [CrossRef]
- Lingenfelter, K.A.; Fuller, B.C.; Sullivan, T.M. Intraoperative Assessment of Carotid Endarterectomy: A Comparison of Techniques. Ann. Vasc. Surg. 1995, 9, 235–240. [Google Scholar] [CrossRef]

| Baseline Characteristics | n = 107 (%) |
|---|---|
| Men | 76 (71%) |
| Women | 31 (29%) |
| Age | 68 ± 4 |
| Hypertension | 94 (87.8%) |
| Diabetes | 89 (83%) |
| Smoking | 92 (86%) |
| Chronic renal insufficiency | 71 (66%) |
| Coronary artery disease (CAD) | 57 (53%) |
| Asymptomatic patients | 41 (38.3%) |
| Bilateral carotid artery stenosis > 70% (NASCET) | 26 (63%) |
| Contralateral closure of internal carotid artery (ICA) | 15 (37%) |
| Symptomatic patients | 66 (61.7%) |
| Stroke | 38 (57.6%) |
| TIA: amaurosis fugax reversible cerebral ischemia minor stroke | 28 (42.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyrek, A.E.; Bernheim, J.; Juntermanns, B.; Burzec, W.; Husen, P.; Radunz, S.; Pacha, A.; Weimar, C.; Treckmann, J.; Hoffmann, J.N. Intraoperative Flow Measurement as a Quality Control during Carotid Endarterectomy in a Teaching Hospital Setting. J. Vasc. Dis. 2022, 1, 71-79. https://doi.org/10.3390/jvd1010008
Cyrek AE, Bernheim J, Juntermanns B, Burzec W, Husen P, Radunz S, Pacha A, Weimar C, Treckmann J, Hoffmann JN. Intraoperative Flow Measurement as a Quality Control during Carotid Endarterectomy in a Teaching Hospital Setting. Journal of Vascular Diseases. 2022; 1(1):71-79. https://doi.org/10.3390/jvd1010008
Chicago/Turabian StyleCyrek, Anna E., Johannes Bernheim, Benjamin Juntermanns, Wieslaw Burzec, Peri Husen, Sonia Radunz, Arkadius Pacha, Christian Weimar, Jürgen Treckmann, and Johannes N. Hoffmann. 2022. "Intraoperative Flow Measurement as a Quality Control during Carotid Endarterectomy in a Teaching Hospital Setting" Journal of Vascular Diseases 1, no. 1: 71-79. https://doi.org/10.3390/jvd1010008
APA StyleCyrek, A. E., Bernheim, J., Juntermanns, B., Burzec, W., Husen, P., Radunz, S., Pacha, A., Weimar, C., Treckmann, J., & Hoffmann, J. N. (2022). Intraoperative Flow Measurement as a Quality Control during Carotid Endarterectomy in a Teaching Hospital Setting. Journal of Vascular Diseases, 1(1), 71-79. https://doi.org/10.3390/jvd1010008






