Psilocybin-Assisted Psychotherapy for Chronic Somatoform Pain Disorder: A Case Report
Abstract
1. Introduction
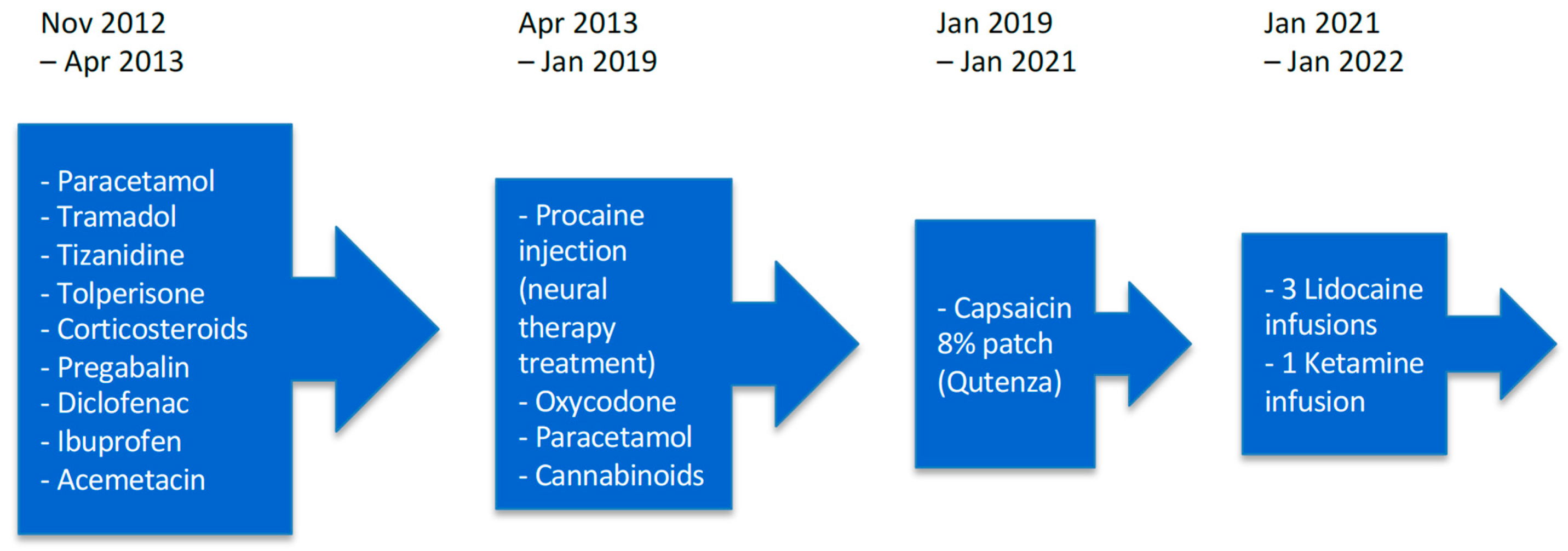
2. Detailed Case Description
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LSD | Lysergic Acid Diethylamide |
| PAP | Psychedelic-Assisted Psychotherapy |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| BPI | Brief Pain Inventory |
| BDI-II | Beck Depression Inventory-II |
| FU | Follow-up |
References
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Hooten, W.M. Chronic Pain and Mental Health Disorders: Shared Neural Mechanisms, Epidemiology, and Treatment. Mayo Clin Proc. 2016, 91, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: an update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Volkow, N.D.; McLellan, A.T. Opioid Abuse in Chronic Pain — Misconceptions and Mitigation Strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. New Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J. Psychopharmacol. 2022, 36, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; O’Donnell, K.; Owens, L.T.; Podrebarac, S.; et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients With Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2022, 79, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Czopek, A.; Jończyk, J.; Fryc, M.; Kluzik, D.; Zagórska, A. Classic Psychedelics in Pain Modulation: Mechanisms, Clinical Evidence, and Future Perspectives. ACS Chem. Neurosci. 2025, 16, 2163–2177. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry. 2018, 30, 363–375. [Google Scholar] [CrossRef]
- Askey, T.; Lasrado, R.; Maiarú, M.; Stephens, G.J. Psilocybin as a novel treatment for chronic pain. Br. J. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Cavarra, M.; Mason, N.L.; Kuypers, K.P.C.; Bonnelle, V.; Smith, W.J.; Feilding, A.; Kryskow, P.; Ramaekers, J.G. Potential analgesic effects of psychedelics on select chronic pain conditions: A survey study. Eur. J. Pain 2023, 28, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, J.; Close, J.B.; Spriggs, M.J.; Carhart-Harris, R.; Roseman, L. Self-Medication for Chronic Pain Using Classic Psychedelics: A Qualitative Investigation to Inform Future Research. Front. Psychiatry 2021, 12, 735427. [Google Scholar] [CrossRef] [PubMed]
- Rucker, J.; Butler, M.; Hambleton, S.; Bird, C.; Seynaeve, M.; Cheema, S.; Campbell-Coker, K.; Maggio, C.; Dunbar, F.; Lambru, G.; et al. Low-dose psilocybin in short-lasting unilateral neuralgiform headache attacks: results from an open-label phase Ib ascending dose study. Headache: J. Head Face Pain 2024, 64, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Lyes, M.; Yang, K.H.; Castellanos, J.; Furnish, T. Microdosing psilocybin for chronic pain: a case series. Pain 2022, 164, 698–702. [Google Scholar] [CrossRef]
- Ramaekers, J.G.; Hutten, N.; Mason, N.L.; Dolder, P.; Theunissen, E.L.; Holze, F.; E Liechti, M.; Feilding, A.; Kuypers, K.P. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J. Psychopharmacol. 2020, 35, 398–405. [Google Scholar] [CrossRef]
- Seragnoli, F.; Thorens, G.; Penzenstadler, L.; Furtado, L.; Buchard, A.; Bachmann, S.; Iuga, R.; Khatcherian, E.; Nowotarski, A.; Sabe, M.; et al. Psychedelic assisted psychotherapy (PAP): The Geneva model. Ann Med Psychol. 2024, 182, 806–813. [Google Scholar] [CrossRef]
- Poquet, N.; Lin, C. The Brief Pain Inventory (BPI). J. Physiother. 2016, 62, 52. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Thompson, T.; Correll, C.U.; Gallop, K.; Vancampfort, D.; Stubbs, B. Is Pain Perception Altered in People With Depression? A Systematic Review and Meta-Analysis of Experimental Pain Research. J. Pain 2016, 17, 1257–1272. [Google Scholar] [CrossRef]
- Scaini, S.; Davies, S.; De Francesco, S.; Pelucchi, A.; Rubino, S.; Battaglia, M. Altered pain perception and nociceptive thresholds in major depression and anxiety disorders: A meta-analysis. Neurosci. Biobehav. Rev. 2025, 169, 106014. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 16, 17002. [Google Scholar] [CrossRef]
- Ramachandran, V.; Chunharas, C.; Marcus, Z.; Furnish, T.; Lin, A. Relief from intractable phantom pain by combining psilocybin and mirror visual-feedback (MVF). Neurocase 2018, 24, 105–110. [Google Scholar] [CrossRef]
- Gonzales, S.A.B.; Alexopoulos, C.; Arkfeld, D.G. Potential Benefits of Psilocybin for Lupus Pain: A Case Report. Curr. Rheumatol. Rev. 2024, 20, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Jevotovsky, D.S.; Chopra, H.; Wing, C.; Spotswood, C.J.; Castellanos, J. Refractory CRPS pain treated with psilocybin: A case report. Clin. Case Rep. 2024, 12, e9421. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Rai, Y.; Sivadas, S.; Diep, C.; Clarke, H.; Shanthanna, H.; Ladha, K.S. Use of Psychedelics for Pain: A Scoping Review. Anesthesiology 2023, 139, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.H.; Whitney, M.; Johnson, B.; Dunn, T.; Attanti, S.; Maloney, J.; Misra, L.; Gomez, D.; Viswanath, O.; Emami, E.; et al. Pain and Perception: Exploring Psychedelics as Novel Therapeutic Agents in Chronic Pain Management. Curr. Pain Headache Rep. 2025, 29, 1–13. [Google Scholar] [CrossRef]
- Meyer, J.H.; Wilson, A.A.; Ginovart, N.; Goulding, V.; Hussey, D.; Hood, K.; Houle, S. Occupancy of Serotonin Transporters by Paroxetine and Citalopram During Treatment of Depression: A [11C]DASB PET Imaging Study. Am. J. Psychiatry 2001, 158, 1843–1849. [Google Scholar] [CrossRef]
- Selvaraj, S.; Walker, C.; Arnone, D.; Cao, B.; Faulkner, P.; Cowen, P.J.; Roiser, J.P.; Howes, O. Effect of Citalopram on Emotion Processing in Humans: A Combined 5-HT1A [11C]CUMI-101 PET and Functional MRI Study. Neuropsychopharmacology 2017, 43, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Vinkers, C.H.; Olivier, B. Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective Receptor Modulators? Adv. Pharmacol. Sci. 2012, 2012, 1–19. [Google Scholar] [CrossRef] [PubMed]

| Time Point | BPI Score | BDI-II Score |
|---|---|---|
| Baseline | 39 | 24 |
| After 1st session | 25 | 15 |
| After 2nd session | 23 | 15 |
| After 3rd session | 32 | 14 |
| After 4th session | 24 | 7 |
| 3-month follow-up | 23 | 9 |
| 9-month follow-up | 20 | 8 |
| Subscore | Baseline | After 1st | After 2nd | After 3rd | After 4th | 3-mo FU | 9-mo FU |
|---|---|---|---|---|---|---|---|
| General activity | 6 | 5 | 4 | 6 | 4 | 5 | 5 |
| Mood | 7 | 4 | 4 | 5 | 4 | 4 | 4 |
| Walking ability | 4 | 3 | 2 | 4 | 2 | 3 | 2 |
| Normal work | 4 | 4 | 4 | 5 | 4 | 4 | 3 |
| Relations with others | 5 | 3 | 3 | 4 | 4 | 3 | 3 |
| Sleep | 6 | 3 | 3 | 4 | 3 | 2 | 1 |
| Enjoyment of life | 7 | 3 | 3 | 4 | 3 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercier, M.; Mabilais, C.; Chytas, V.; Furtado, L.; Seragnoli, F.; Buchard, A.; Aboulafia-Brakha, T.; Thorens, G.; Zullino, D.; Penzenstadler, L. Psilocybin-Assisted Psychotherapy for Chronic Somatoform Pain Disorder: A Case Report. Psychoactives 2025, 4, 30. https://doi.org/10.3390/psychoactives4030030
Mercier M, Mabilais C, Chytas V, Furtado L, Seragnoli F, Buchard A, Aboulafia-Brakha T, Thorens G, Zullino D, Penzenstadler L. Psilocybin-Assisted Psychotherapy for Chronic Somatoform Pain Disorder: A Case Report. Psychoactives. 2025; 4(3):30. https://doi.org/10.3390/psychoactives4030030
Chicago/Turabian StyleMercier, Mathilda, Cedric Mabilais, Vasileios Chytas, Leonice Furtado, Federico Seragnoli, Albert Buchard, Tatiana Aboulafia-Brakha, Gabriel Thorens, Daniele Zullino, and Louise Penzenstadler. 2025. "Psilocybin-Assisted Psychotherapy for Chronic Somatoform Pain Disorder: A Case Report" Psychoactives 4, no. 3: 30. https://doi.org/10.3390/psychoactives4030030
APA StyleMercier, M., Mabilais, C., Chytas, V., Furtado, L., Seragnoli, F., Buchard, A., Aboulafia-Brakha, T., Thorens, G., Zullino, D., & Penzenstadler, L. (2025). Psilocybin-Assisted Psychotherapy for Chronic Somatoform Pain Disorder: A Case Report. Psychoactives, 4(3), 30. https://doi.org/10.3390/psychoactives4030030






