Probiotics Alter the Microbial and Behavioral Consequences of Methamphetamine Exposure in a Sex-Selective Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Groups and Experimental Timeline
2.3. Methamphetamine Administration
2.4. Probiotics
2.5. Syringe Feeding
2.6. Behavioral Testing
2.7. Data Analysis for Behavioral Assessments
2.8. Fecal Sample Collection
2.9. 16S rRNA Gene Sequencing and Compositional Analysis
2.10. Gut Microbiome Analysis
3. Results
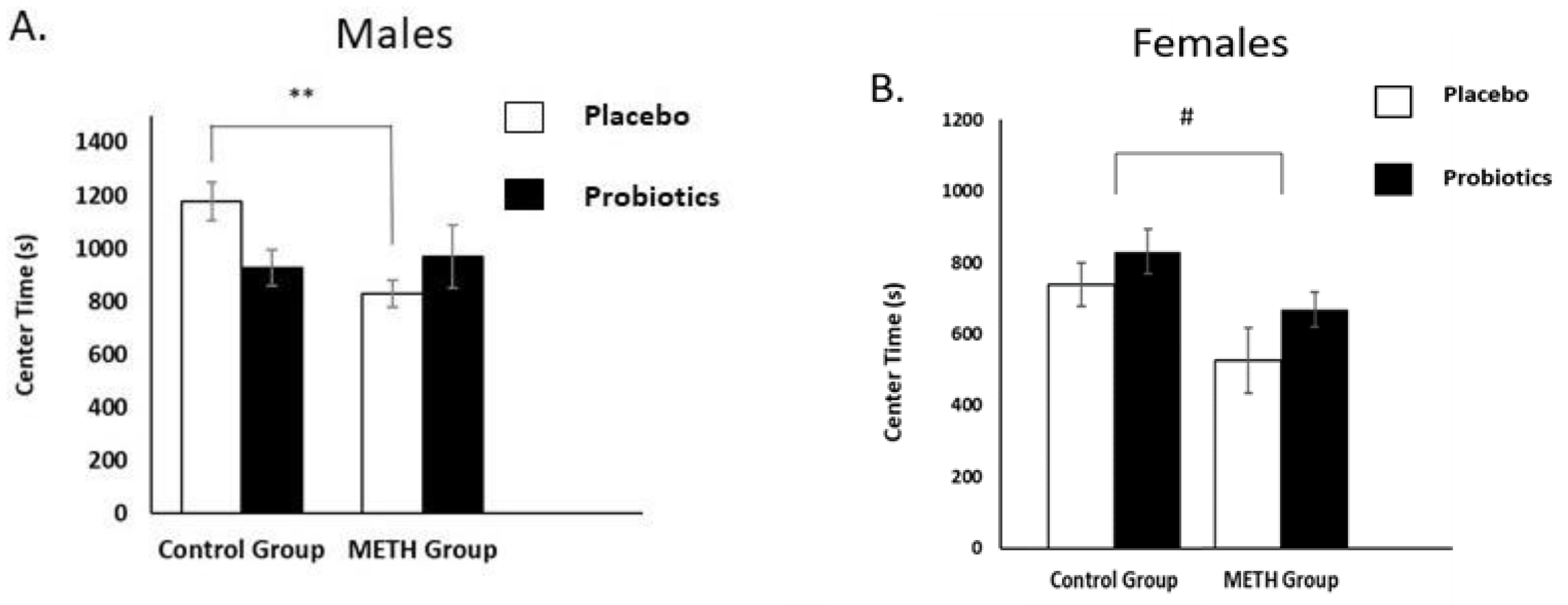
3.1. Open-Field Test (OFT)
3.1.1. Male Rats
3.1.2. Female Rats
3.2. Forced-Swim Test (FST)
3.3. Microbial Diversity
3.3.1. Male Rats
3.3.2. Female Rats
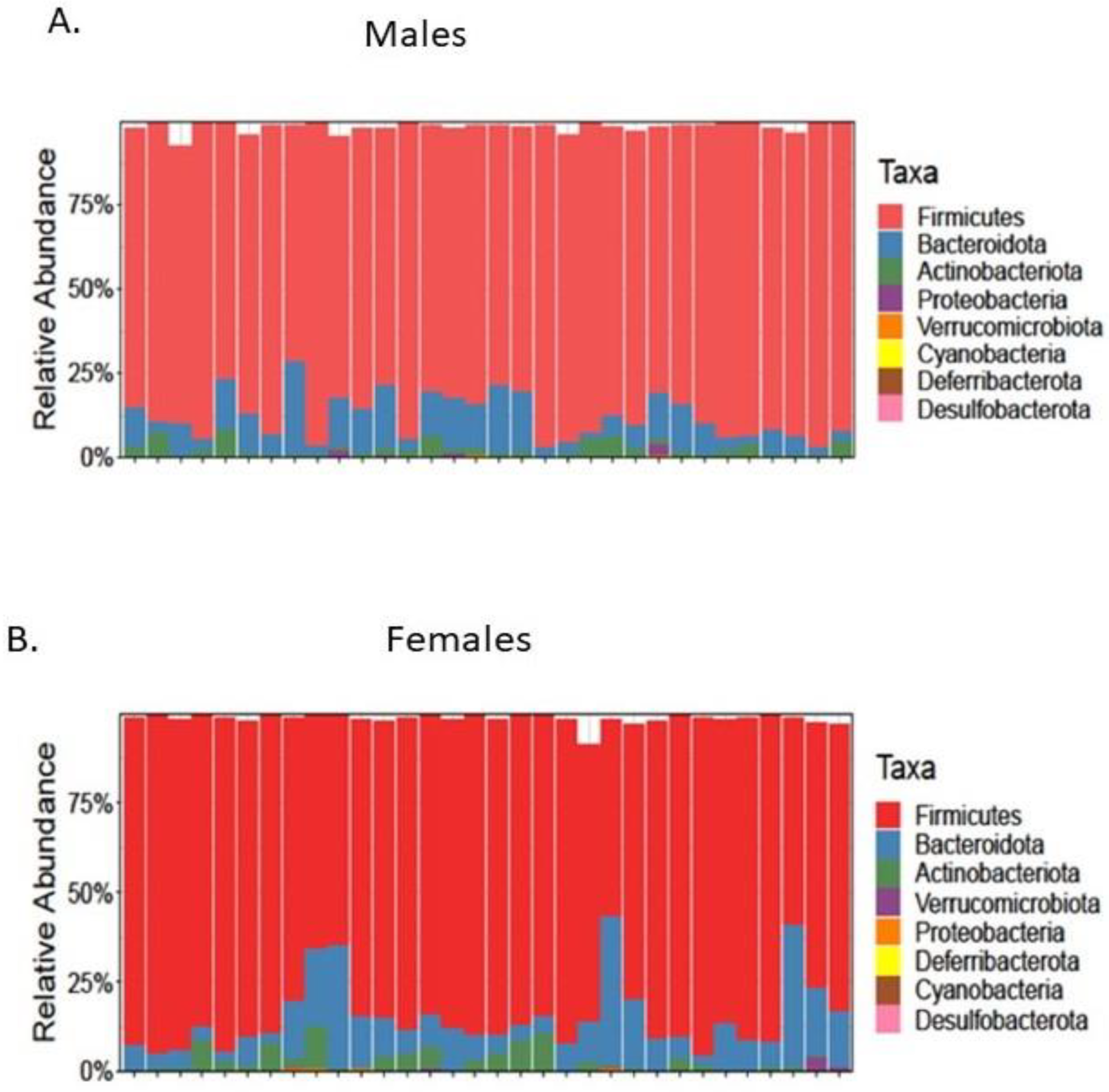
3.4. Microbial Composition
3.4.1. Male Rats
3.4.2. Female Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIDA. What Is Methamphetamine? Available online: https://nida.nih.gov/publications/research-reports/methamphetamine/what-methamphetamine (accessed on 1 June 2023).
- McLellan, A.T.; Lewis, D.C.; O’Brien, C.P.; Kleber, H.D. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA 2000, 284, 1689–1695. [Google Scholar] [CrossRef]
- Novak, S.P.; Kral, A.H. Comparing injection and non-injection routes of administration for heroin, methamphetamine, and cocaine users in the United States. J. Addict. Dis. 2011, 30, 248–257. [Google Scholar] [CrossRef] [PubMed]
- London, E.D.; Simon, S.L.; Berman, S.M.; Mandelkern, M.A.; Lichtman, A.M.; Bramen, J.; Shinn, A.K.; Miotto, K.; Learn, J.; Dong, Y.; et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch. Gen. Psychiatry 2004, 61, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zorick, T.; Nestor, L.; Miotto, K.; Sugar, C.; Hellemann, G.; Scanlon, G.; Rawson, R.; London, E.D. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction 2010, 105, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, J.; Ren, W.; Xie, Y.; Tao, J.; Zhang, X.; He, J. Anxiety level and correlates in methamphetamine-dependent patients during acute withdrawal. Medicine 2017, 96, e6434. [Google Scholar] [CrossRef]
- Brecht, M.L.; O’Brien, A.; von Mayrhauser, C.; Anglin, M.D. Methamphetamine use behaviors and gender differences. Addict. Behav. 2004, 29, 89–106. [Google Scholar] [CrossRef]
- Newton, T.; Kalechstein, A.; Duran, S.; Vansluis, N.; Ling, W. Methamphetamine abstinence syndrome: Preliminary findings. Am. J. Addict. 2004, 13, 248–255. [Google Scholar] [CrossRef]
- McGregor, C.; Srisurapanont, M.; Jittiwutikarn, J.; Laobhripatr, S.; Wongtan, T.; White, J.M. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005, 100, 1320–1329. [Google Scholar] [CrossRef]
- Khoramizadeh, M.; Effatpanah, M.; Mostaghimi, A.; Rezaei, M.; Mahjoub, A.; Shishehgar, S. Treatment of amphetamine abuse/use disorder: A systematic review of a recent health concern. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2019, 27, 743–753. [Google Scholar] [CrossRef]
- Ray, L.A.; Meredith, L.R.; Kiluk, B.D.; Walthers, J.; Carroll, K.M.; Magill, M. Combined Pharmacotherapy and Cognitive Behavioral Therapy for Adults With Alcohol or Substance Use Disorders: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e208279. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.; Cowan, C.S.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making sense of… the microbiome in psychiatry. Int. J. Neuropsychopharmacol. 2018, 22, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Skosnik, P.D.; Cortes-Briones, J.A. Targeting the ecology within: The role of the gut–brain axis and human microbiota in drug addiction. Med. Hypotheses 2016, 93, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.; Thompson, D.; Fowler, J.C.; Ajami, N.J.; Salas, R.; Frueh, B.C.; Bradshaw, M.R.; Weinstein, B.L.; Oldham, J.M.; Petrosino, J.F. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J. Affect. Disord. 2020, 264, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef]
- Mason, B.L.; Li, Q.; Minhajuddin, A.; Czysz, A.H.; Coughlin, L.A.; Hussain, S.K.; Koh, A.Y.; Trivedi, M.H. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J. Affect. Disord. 2020, 266, 394–401. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fan, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, 43859. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Arseneault-Breard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef]
- Forouzan, S.; Hoffman, K.L.; Kosten, T.A. Methamphetamine exposure and its cessation alter gut microbiota and induce depressive-like behavioral effects on rats. Psychopharmacology 2021, 238, 281–292. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef] [PubMed]
- Karakula-Juchnowicz, H.; Rog, J.; Juchnowicz, D.; Łoniewski, I.; Skonieczna-Żydecka, K.; Krukow, P.; Futyma-Jedrzejewska, M.; Kaczmarczyk, M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): A 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr. J. 2019, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Bulik-Sullivan, E.C.; Glenny, E.M.; Zerwas, S.C.; Huh, E.Y.; Tsilimigras, M.C.; Fodor, A.A.; Bulik, C.M.; Carroll, I.M. The Gut-Brain Axis in Healthy Females: Lack of Significant Association between Microbial Composition and Diversity with Psychiatric Measures. PLoS ONE 2017, 12, e0170208. [Google Scholar] [CrossRef] [PubMed]
- National_Research_Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S.; Wegener, G. Syringe-feeding as a novel delivery method for accurate individual dosing of probiotics in rats. Benef. Microbes 2018, 9, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Myles, E.M.; O’Leary, M.E.; Romkey, I.D.; Piano, A.; de Carvalho, V.; Tompkins, T.A.; Perrot, T.S. Guidelines for best practice in placebo-controlled experimental studies on probiotics in rodent animal models. Benef. Microbes 2020, 11, 245–254. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, K.L.; Merriweather, M.Y.; Yuva-Paylor, L.A.; Paylor, R. The use of behavioral test batteries: Effects of training history. Physiol. Behav. 2001, 73, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S. Emotional behavior in the rat. III. The relationship between emotionality and ambulatory activity. J. Comp. Physiol. Psychol. 1936, 22, 345–352. [Google Scholar] [CrossRef]
- Weiss, J.M.; Bailey, W.H.; Pohrecky, L.A.; Korzeniowski, D.; Grillione, G. Stress-induced depression of motor activity correlates with regional changes in brain norepinephrine but not in dopamine. Neurochem. Res. 1980, 5, 9–22. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McCaly, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. De Pharmacodyn. Et. De Ther. 1977, 229, 327–336. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Dwass, M. Modified tests for nonparametric hypotheses. Ann. Math. Stat. 1957, 28, 181–187. [Google Scholar] [CrossRef]
- Benjamin, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFracL: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 828–8235. [Google Scholar] [CrossRef]
- Uys, J.D.; Stein, D.J.; Daniels, W.M.; Harvey, B.H. Animal models of anxiety disorders. Curr. Psychiatry Rep. 2003, 5, 274–281. [Google Scholar] [CrossRef]
- Vesga-López, O.; Schneier, F.R.; Wang, S.; Heimberg, R.G.; Liu, S.M.; Hasin, D.S.; Blanco, C. Gender differences in generalized anxiety disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J. Clin. Psychiatry 2008, 69, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Su, H.; Song, Y.; Chen, T.; Sun, Q.; Jiang, H.; Zhao, M. Altered Fecal Microbiota Correlated With Systemic Inflammation in Male Subjects With Methamphetamine Use Disorder. Front. Cell. Infect. Microbiol. 2021, 11, 783917. [Google Scholar] [CrossRef]
- Yang, C.; Fu, X.; Hao, W.; Xiang, X.; Liu, T.; Yang, B.Z.; Zhang, X. Gut dysbiosis associated with the rats’ responses in methamphetamine-induced conditioned place preference. Addict. Biol. 2021, 26, e12975. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zheng, P.; Liu, Y.Y.; Zhong, X.G.; Wang, H.Y.; Guo, Y.J.; Xie, P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahar, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
- Alonso, S.J.; Castellano, M.A.; Afonso, D.; Rodriguez, M. Sex differences in behavioral despair: Relationships between behavioral despair and open field activity. Physiol. Behav. 1991, 49, 69–72. [Google Scholar] [CrossRef]
- Abderrahim, L.; Hicham, E.M.; Aboubaker, E.; Fatima, A.; Tarik, T.; Soufiane, B.; Abdelhalim, M. Sex differences in behavioral, cognitive and voluntary ethanol-intake effects in Dexamethasone-induced depression-like state in Wistar rat. AIMS Neurosci. 2022, 9, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Cortez, I.; Rodgers, S.P.; Kosten, T.A.; Leasure, J.L. Sex and Age Effects on Neurobehavioral Toxicity Induced by Binge Alcohol. Brain Plast. 2020, 6, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Meckel, K.R.; Simpson, S.S.; Godino, A.; Peck, E.G.; Sens, J.P.; Leonard, M.Z.; George, O.; Calipari, E.S.; Hofford, R.S.; Kiraly, D.D. Microbial short-chain fatty acids regulate drug seeking and transcriptional control in a model of cocaine seeking. Neuropsychopharmacology 2024, 49, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Acuña, A.M.; Olive, M.F. Influence of gut microbiome metabolites on cocaine demand and cocaine-seeking behavior. Neuropsychopharmacology 2024, 49, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Peterson, V.L.; Richards, J.B.; Meyer, P.J.; Cabrera-Rubio, R.; Tripi, J.A.; King, C.P.; Polesskaya, O.; Baud, A.; Chitre, A.S.; Bastiaanssen, T.F.S.; et al. Sex-dependent associations between addiction-related behaviors and the microbiome in outbred rats. EBioMedicine 2020, 55, 102769. [Google Scholar] [CrossRef] [PubMed]
- Forouzan, S.; McGrew, K.; Kosten, T.A. Drugs and bugs: Negative affect, psychostimulant use and withdrawal, and the microbiome. Am. J. Addict. 2021, 30, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Hofford, R.S.; Kiraly, D.D. Clinical and preclinical evidence for gut microbiome mechanisms in substance use disorder. Biol. Psychiatry 2024, 95, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Sil, S.; Kumar, M.; Buch, S. Substance use, microbiome and psychiatric disorders. Pharmacol. Biochem. Behav. 2022, 219, 173432. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, T.; Cai, J.; Liu, B.; Zeng, Y.; Zhang, X. The Microbiome-Gut-Brain Axis, a Potential Therapeutic Target for Substance-Related Disorders. Front. Microbiol. 2021, 12, 738401. [Google Scholar] [CrossRef]
- Russell, J.T.; Zhou, Y.; Weinstock, G.M.; Bubier, J.A. The Gut Microbiome and Substance Use Disorder. Front. Neurosci. 2021, 15, 725500. [Google Scholar] [CrossRef]



| METH and Probiotic Groups | Males (n = 8/Group) | Females (n = 8/Group) |
|---|---|---|
| Control-Placebo | 11,260 ± 589 | 10,482 ± 995 |
| Control-Probiotic | 8087 ± 841 | 11,091 ± 626 |
| METH-Placebo | 9915 ± 1277 | 11,387 ± 1022 |
| METH-Probiotic | 10,027 ± 353 | 10,419 ± 632 |
| Phylum | Group | Baseline | Day 14 | Day 17 | Day 40 |
|---|---|---|---|---|---|
| Firmacutes | Control-Placebo | 0.83 | 0.87 | 0.9 | 0.89 |
| METH-Placebo | 0.83 | 0.9 | 0.92 | 0.86 | |
| Control-Probiotics | 0.85 | 0.85 | 0.88 | 0.83 | |
| METH-Probiotics | 0.9 | 0.86 | 0.85 | 0.84 | |
| Bacteroidota | Control-Placebo | 0.111 | 0.074 | 0.073 | 0.052 |
| METH-Placebo | 0.118 | 0.051 | 0.081 | 0.052 | |
| Control-Probiotics | 0.091 | 0.096 | 0.104 | 0.07 | |
| METH-Probiotics | 0.059 | 0.075 | 0.095 | 0.089 | |
| Actinobacteria | Control-Placebo | 0.029 | 0.026 | 0.017 | 0.015 |
| METH-Placebo | 0.019 | 0.018 | 0.014 | 0.006 | |
| Control-Probiotics | 0.024 | 0.027 | 0.021 | 0.024 | |
| METH-Probiotics | 0.018 | 0.037 | 0.026 | 0.019 | |
| Proteobacteria | Control-Placebo | 0.002 | 0.003 | 0.002 | 0.001 |
| METH-Placebo | 0.006 | 0.004 | 0.004 | 0.002 | |
| Control-Probiotics | 0.006 | 0.003 | 0.004 | 0.002 | |
| METH-Probiotics | 0.001 | 0.001 | 0.001 | 0.001 | |
| Genus | |||||
| Bifidobacterium | Control-Placebo | 0.025 | 0.022 | 0.013 | 0.011 |
| METH-Placebo | 0.011 | 0.014 | 0.008 | 0.002 | |
| Control-Probiotics | 0.016 | 0.022 | 0.019 | 0.016 | |
| METH-Probiotics | 0.013 | 0.036 | 0.022 | 0.017 | |
| Lactobacillus | Control-Placebo | 0.114 | 0.147 | 0.142 | 0.118 |
| METH-Placebo | 0.129 | 0.227 | 0.157 | 0.123 | |
| Control-Probiotics | 0.178 | 0.141 | 0.132 | 0.087 | |
| METH-Probiotics | 0.161 | 0.171 | 0.143 | 0.082 |
| Phylum | Group | Baseline | Day 14 | Day 17 | Day 40 |
|---|---|---|---|---|---|
| Firmacutes | Control-Placebo | 0.892 | 0.907 | 0.86 | 0.918 |
| METH-Placebo | 0.798 | 0.85 | 0.883 | 0.882 | |
| Control-Probiotics | 0.805 | 0.8 | 0.82 | 0.8 | |
| METH-Probiotics | 0.823 | 0.87 | 0.769 | 0.835 | |
| Bacteroidota | Control-Placebo | 0.061 | 0.047 | 0.086 | 0.029 |
| METH-Placebo | 0.145 | 0.092 | 0.084 | 0.062 | |
| Control-Probiotics | 0.128 | 0.137 | 0.113 | 0.147 | |
| METH-Probiotics | 0.139 | 0.084 | 0.178 | 0.116 | |
| Actinobacteria | Control-Placebo | 0.032 | 0.03 | 0.035 | 0.039 |
| METH-Placebo | 0.039 | 0.045 | 0.01 | 0.038 | |
| Control-Probiotics | 0.035 | 0.028 | 0.008 | 0.018 | |
| METH-Probiotics | 0.011 | 0.032 | 0.028 | 0.017 | |
| Proteobacteria | Control-Placebo | 0.0016 | 0.00072 | 0.0016 | 0.0011 |
| METH-Placebo | 0.0039 | 0.0021 | 0.0006 | 0.0009 | |
| Control-Probiotics | 0.0028 | 0.0039 | 0.0018 | 0.0053 | |
| METH-Probiotics | 0.0014 | 0.00077 | 0.0029 | 0.0021 | |
| Genus | |||||
| Bifidobacterium | Control-Placebo | 0.0276 | 0.034 | 0.032 | 0.037 |
| METH-Placebo | 0.035 | 0.042 | 0.008 | 0.034 | |
| Control-Probiotics | 0.032 | 0.024 | 0.005 | 0.015 | |
| METH-Probiotics | 0.008 | 0.029 | 0.025 | 0.011 | |
| Lactobacillus | Control-Placebo | 0.101 | 0.0716 | 0.111 | 0.072 |
| METH-Placebo | 0.151 | 0.2277 | 0.151 | 0.092 | |
| Control-Probiotics | 0.101 | 0.1251 | 0.108 | 0.066 | |
| METH-Probiotics | 0.114 | 0.2003 | 0.0969 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forouzan, S.; Hoffman, K.L.; Kosten, T.A. Probiotics Alter the Microbial and Behavioral Consequences of Methamphetamine Exposure in a Sex-Selective Manner. Psychoactives 2024, 3, 318-336. https://doi.org/10.3390/psychoactives3030020
Forouzan S, Hoffman KL, Kosten TA. Probiotics Alter the Microbial and Behavioral Consequences of Methamphetamine Exposure in a Sex-Selective Manner. Psychoactives. 2024; 3(3):318-336. https://doi.org/10.3390/psychoactives3030020
Chicago/Turabian StyleForouzan, Shadab, Kristi L. Hoffman, and Therese A. Kosten. 2024. "Probiotics Alter the Microbial and Behavioral Consequences of Methamphetamine Exposure in a Sex-Selective Manner" Psychoactives 3, no. 3: 318-336. https://doi.org/10.3390/psychoactives3030020
APA StyleForouzan, S., Hoffman, K. L., & Kosten, T. A. (2024). Probiotics Alter the Microbial and Behavioral Consequences of Methamphetamine Exposure in a Sex-Selective Manner. Psychoactives, 3(3), 318-336. https://doi.org/10.3390/psychoactives3030020






