Abstract
(1) Background: Intraperitoneal injections of the endogenous N-acyl amino acid N-Oleoyl alanine (OlAla) effectively reduces both the affective and somatic responses produced by opioid withdrawal in preclinical models. To increase the translational appeal of OlAla in clinical drug applications, the current experiments tested whether oral OlAla pretreatment also attenuates opioid withdrawal in rats. (2) Methods: In Experiment 1, to assess its impact on affective withdrawal behavior, OlAla (0, 5, 20 mg/kg) was orally administered during the conditioning phase of an acute naloxone-precipitated morphine withdrawal conditioned place avoidance task. In Experiment 2, to assess its impact on somatic withdrawal behavior, OlAla (5–80 mg/kg) was orally administered prior to naloxone-precipitated withdrawal from chronic heroin exposure. (3) Results: Pretreatment with oral OlAla at the higher (20 mg/kg), but not lower (5 mg/kg) dose, reduced the establishment of an acute morphine withdrawal-induced conditioned place aversion. Instead, the lower dose of oral OlAla (5 mg/kg) reduced heroin withdrawal-induced abdominal contractions and diarrhea, whereas higher doses were without effect. (4) Conclusions: The results suggest a dose-dependent reduction of opioid withdrawal responses by orally administered OlAla, and further highlight the potential utility of this compound for opioid withdrawal in clinical populations.
1. Introduction
N-acyl amino acids are a class of endogenous lipid-based neuromodulatory molecules, similar to endocannabinoids, which regulate several regulatory processes in the body, ranging from inflammation [1,2,3], energy metabolism [4,5], pain perception [6,7,8], and neuronal injury recovery [9], to behavior and cognition [10,11,12,13]. Given that these molecules are involved in physiological and psychological processes, they may be promising targets for treating disorders with somatic and affective symptoms, such as withdrawal from drugs of abuse.
Within the N-acyl amino acid family, both N-Oleoyl glycine (OlGly) and N-Oleoyl alanine (OlAla) have been shown to participate in the behavioral and neurochemical responses produced by withdrawal from opioids in preclinical animal models [12,14,15,16]. Acute morphine withdrawal (MWD) is readily modelled in rodents by exposure to a high-dose of morphine followed 24-h later by administration of the mu-opioid receptor antagonist naloxone. Using this model in rats, Petrie and colleagues [12] demonstrated that acute naloxone-precipitated MWD elevates OlGly concentrations in the nucleus accumbens and intraperitoneal injection with synthetic OlGly blocked affective withdrawal behavior as measured by an acute MWD-induced conditioned place avoidance (CPA). Given its effect on affective withdrawal, OlGly was subsequently tested against somatic withdrawal responses and was shown to reduce diarrhea, body weight loss, nausea, measures of abdominal discomfort, and mouthing movements following acute MWD [16]. These data suggest that OlGly may be an endogenous neuro-protective molecule that is released in the brain to counteract neurological insult, such as the aversive consequences of acute opioid withdrawal. In further support of this claim, acute traumatic brain injury elevates OlGly in the insular cortex of mice [10], and synthetic OlGly reduces the behavioral indices of brain injury in such models [17]. Similarly, chronic intermittent alcohol consumption increases OlGly in the prefrontal cortex of mice [18].
In vitro studies have revealed that OlGly acts as a peroxisome proliferator-activated receptor alpha (PPARα) agonist, and as an inhibitor of the lipid-degrading enzyme, fatty acid amide hydrolase (FAAH) [10]. Consequently, blocking the activity of either PPARα or the cannabinoid-1 (CB1) receptor reverses OlGly’s effects on acute opioid withdrawal behavior [12,16]. Despite its effects on the CB1 receptor, OlGly does not produce any of the negative side effects associated with cannabimimetic activity [10,19], which is also important to consider during the development of pharmacotherapies. However, OlGly is short-lasting in the body as it is rapidly inactivated by degrading enzymes and may therefore be a poor candidate to develop for human clinical trials. Indeed, systemic administration of the selective and potent inhibitor of FAAH, URB597, leads to a threefold increase of OlGly in the striatum 30 min following its administration [20].
Other bioactive lipid-based compounds that are rapidly hydrolyzed have been stabilized through the addition of a methyl group near the amide linkage of the compound. For example, Abadji et al. [21] created a methylated anandamide, forming methanandamide, which is less susceptible to FAAH degradation and acts as a more potent ligand for the CB1 receptor than anandamide itself. Therefore, a methylated OlGly compound was created, named OlAla (HU595), to assess its impact on the adverse effects of morphine withdrawal in rats [15]. Like OlGly, intraperitoneal OlAla reduces acute naloxone-precipitated MWD-induced CPA and somatic responses in rats through PPARα and CB1 receptor dependent mechanisms. However, when compared to OlGly, OlAla reduces affective MWD-behavior for a longer duration, consistent with the hypothesis that OlAla is a more stable compound. Further, OlAla reduces somatic withdrawal behaviors following chronic opioid exposure, whereas OlGly has no effect [14]. Additionally, OlAla reverses some of the neurochemical changes induced by chronic opioid exposure followed by withdrawal, including altered fatty acid amide composition and gut microbiota [14]. Together, these studies suggest that OlAla is a more suitable therapeutic target for opioid withdrawal and warrants further investigation.
The current study aimed to increase the translational appeal of OlAla as an opioid withdrawal medication by testing whether oral route delivery of OlAla could reduce opioid withdrawal responses in rats. First (Experiment 1), animals were administered oral OlAla prior to naloxone-precipitated MWD place conditioning at doses (0, 5, 20 mg/kg) within the range of effective intraperitoneal (i.p.) doses that prevent this affective withdrawal behavior [15]. Next, the efficacy of oral OlAla was assessed for its ability to reduce somatic withdrawal responses following chronic steady-state (via minipump) opioid exposure. For this experiment, and as previously published [14], heroin was selected due to solubility limits for the desired doses of morphine delivered by minipump. Therefore, in a separate cohort (Experiment 2), oral OlAla was administered at a range of doses (0–80 mg/kg) prior to a naloxone challenge in rats chronically exposed to heroin, then somatic responses were measured. The doses of OlAla used in Experiment 2 included the effective i.p. dose previously found to prevent somatic withdrawal responses following acute and chronic opioid exposure in rats [14,15]. To address pharmacokinetic differences produced by the two routes of administration, pretreatment with oral OlAla occurred 60 min prior to naloxone treatment in contrast to the 10 min pretreatment time reported for i.p. OlAla administration. It was predicted that oral OlAla would reduce opioid-withdrawal induced affective and somatic responses following acute and chronic opioid exposures.
2. Materials and Methods
2.1. Animals
Male Sprague Dawley rats (n = 84; 200–225 g) were purchased from Charles River Laboratories (Saint-Constant, QC, Canada). Animals were pair-housed in standard rat cages located in a colony room with a 12-h reversed light/dark cycle (7:00 off/19:00 on) and consistent room temperature (21 °C). Standard rodent chow (Teklad Global Diets; 14% protein) and water were provided ad libitum at all times. All procedures began at least 2 h into the rat’s dark (active) cycle.
2.2. Drugs
For Experiment 1, morphine was dissolved in saline at 20 mg/mL and administered subcutaneously (s.c.) at a volume of 1 mL/kg. For Experiment 2, osmotic minipumps (Alzet Model 2002, Alzet Osmotic Pumps, Cupertino, CA, USA) were filled with heroin dissolved in saline at a concentration of 175 mg/mL to result in a daily heroin dose of 7 mg/kg.
Oleoyl-alanine (OlAla; HU595) was generously provided by R. Mechoulam, Hebrew University of Jerusalem. For both experiments, OlAla was dissolved in a vehicle (VEH) mixture of ethanol, Tween 80, and physiological saline (1:1:19 ratio), then a nitrogen stream was used to evaporate off the ethanol, resulting in a final VEH consisting of Tween and Saline (1:9 ratio). OlAla was prepared at concentrations ranging from 5–80 mg/mL and administered intragastrically by oral gavage at a volume of 1 mL/kg.
Naloxone was dissolved in physiological saline at 1 mg/mL and administered s.c. at a volume of 1 mL/kg.
2.3. Apparatus
In experiment 1, rectangular black Plexiglas place conditioning boxes (60 × 25 × 25 cm3) with a wire mesh lid were used, as described by Ayoub et al. [15]. During conditioning, grid or hole patterned metal floors were placed in conditioning boxes to serve as contextual cues. During pre-test and test sessions, split floors that were equally divided into half grid/half hole patterns were used to allow animals access to both floor types simultaneously. Sessions were video recorded and uploaded to EthoVision (version 2, Noldus Information Technology, Leesburg, VA, USA) movement tracking software. In experiment 2, somatic withdrawal behaviors were recorded in black opaque Plexiglas an observation chambers (22.5 × 26.0 × 20.0 cm3). Chambers were sat on a clear glass table with a video camera (Panasonic WV-CP484, Panasonic, Newark, NJ, USA) placed below to record the rat’s ventral surface. Recorded videos were uploaded to The Observer Software (version 5, Noldus Information Technology, Leesburg, VA, USA) to score somatic withdrawal behavior.
2.4. Surgery
In experiment 2, osmotic minipumps were implanted into rats while under isoflurane anesthesia. Briefly, a small incision was made near the scapulae and pumps were inserted into the subcutaneous space. Rats were monitored for several days following surgery. To address pain and inflammation animals received carprofen (0.1 mg/kg) prior to surgery, and 24 h following surgery.
2.5. Procedures
2.5.1. Experiment 1
As previously described [15], following one week of habituation to the colony room, rats received a 10 min pre-test using the split grid/hole floor to detect initial floor biases. Rats were then assigned to a pretreatment drug group and drug floor (grid or hole; i.e., the floor paired with MWD), matched on the basis of initial pre-test preferences.
Figure 1 illustrates the place conditioning procedure used to establish an acute naloxone-precipitated MWD-induced CPA. As previously described [15], a 3-day conditioning cycle was used. On day 1 of the cycle, the rats were gavaged intragastrically with VEH 60 min before receiving an s.c. injection of saline. Ten min later, rats were placed on the floor opposite to the assigned MWD floor for 20 min. On day 2, 24 h post-VEH gavage, all rats received a high dose of morphine (20 mg/kg, s.c.) and were placed in an empty shoebox cage. The rats were monitored for signs of respiratory distress and returned to their home cage once fully ambulatory. On day 3, 24 h post-morphine, the rats were gavaged intragastrically with the appropriate pretreatment (n = 12/group: VEH, 5, or 20 mg/kg OlAla) 60 min prior to receiving an s.c. injection of naloxone. Ten min later they were placed in the assigned MWD paired chamber for 20 min.
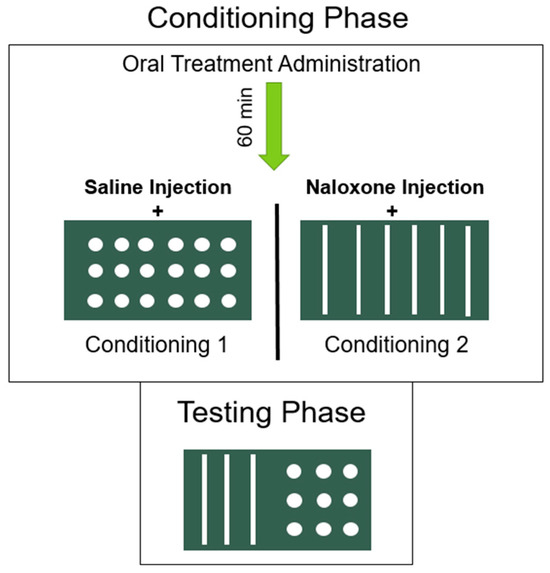
Figure 1.
Place conditioning procedure used to establish an acute naloxone precipitated MWD-induced CPA. On both conditioning days, oral pretreatment was administered 60 minutes prior to floor pairings. Ten minutes prior to floor pairings, rats were injected with saline (on conditioning day 1) or naloxone (on conditioning day 2). Importantly, rats received a high dose of morphine 24-h prior to the naloxone challenge on conditioning day 2, and therefore were in a state of acute withdrawal during floor pairings on this day.
The CPA test occurred 4 days after the naloxone conditioning trial. The rats received an s.c. injection of saline 10 min prior to placement in the test box with the split grid/hole floor for 10 min. Ethovision software tracked movement and measured the time spent on each floor for the duration of the test.
2.5.2. Experiment 2
A total of 48 rats were implanted with osmotic minipumps filled with saline (n = 8) or heroin (n = 40). On Day 12, with the pumps intact, the rats were gavaged intra-gastrically with VEH or OlAla (5, 20, 40, or 80 mg/kg; i.p.), 10 min prior to an injection of naloxone (1 mg/kg; s.c.). Ten min after the final injection, the rats were placed in the somatic withdrawal observation chambers for 30 min and videotaped for a wide range of behaviors. The only withdrawal responses displayed by the heroin group HVN compared with SVN were diarrhea, abdominal contractions, suppressed locomotion and mouthing movements. The groups (n = 8) included: SVN (saline-VEH-naloxone, i.e., saline control), HVN (heroin-VEH-naloxone), and rats that received oral OlAla prior to naloxone-precipitated heroin withdrawal: groups H-OlAla 5 mg/kg-N, H-OlAla 20 mg/kg-N, H-OlAla 40 mg/kg-N, H-OlAla 80 mg/kg-N.
2.6. Data Analysis
In Experiment 1, the time (seconds) spent on the saline paired floor and the drug paired floor was entered into a 3 × 2 mixed factors ANOVA with the between group factor of pretreatment (VEH, 5, or 20 mg/kg OlAla) and the within group factor of floor (saline, drug). In Experiment 2, the duration or frequency of somatic withdrawal behaviors as entered into a one-way ANOVA for each group with subsequent comparisons by Least Significant Difference (LSD) tests, based on the a priori predicted effects.
3. Results
3.1. Experiment 1
Oral OlAla at the 20 mg/kg, but not the 5 mg/kg, dose blocked the establishment of a naloxone-precipitated MWD-induced CPA. Figure 2 presents time spent (seconds) on each floor type during test across drug treatments. The ANOVA revealed a main effect of floor (F(1, 33) = 3.4; p = 0.016] and a pretreatment × floor interaction [F(2, 33) = 8.5; p < 0.001). Separate paired t-tests revealed that VEH pre-treated (p < 0.025) and 5 mg/kg OlAla pre-treated (p < 0.01) rats spent less time on the MWD-paired floor than on the saline paired floor, indicative of a CPA. This CPA was not observed in rats pretreated with 20 mg/kg OlAla (i.e., no difference in time spent between floor types).

Figure 2.
Mean (±SEM) time (seconds) spent on the saline-paired floor and the MWD-paired floor across pretreatment groups (VEH, 5 mg/kg OlAla, 20 mg/kg OlAla). As expected, rats in the oral VEH group exhibited an acute naloxone-precipitated morphine withdrawal induced CPA, as demonstrated by less time spent on the MWD-paired floor. Administration of 20 mg/kg, but not 5 mg/kg, oral OlAla prevented the establishment of this MWD-induced CPA. ** p < 0.01, * p < 0.05 indicates differences between floor types.
3.2. Experiment 2
Naloxone administration in rats chronically exposed to heroin (Group HVN compared to Group SVN), produced somatic withdrawal behaviors of diarrhea, abdominal contractions, suppressed locomotion and mouthing movements, as depicted in Figure 3. Please note that only the far left-hand group (SVN) did not undergo heroin withdrawal; all other groups were treated with heroin prior to naloxone. The ANOVAs revealed a significant effect of group on diarrhea (F(5, 42) = 19.3 p < 0.001), abdominal contractions (F(5, 42) = 9.3; p < 0.001), active locomotion (F(5, 42) = 7.9; p < 0.001) and mouthing movements (F(5, 42) = 12.2; p < 0.001). Following the naloxone challenge, all groups exposed to chronic heroin displayed more instances of diarrhea, increased abdominal contractions, decreased locomotor activity and elevated mouthing movements compared to saline exposed rats (SVN; p’s < 0.001). At a dose of 5 mg/kg, but no other dose, OlAla significantly reduced withdrawal induced instances of diarrhea (p < 0.001) and abdominal contractions (p < 0.01) when compared to VEH treated heroin withdrawal animals (HVN), as indicated by + in Figure 3.
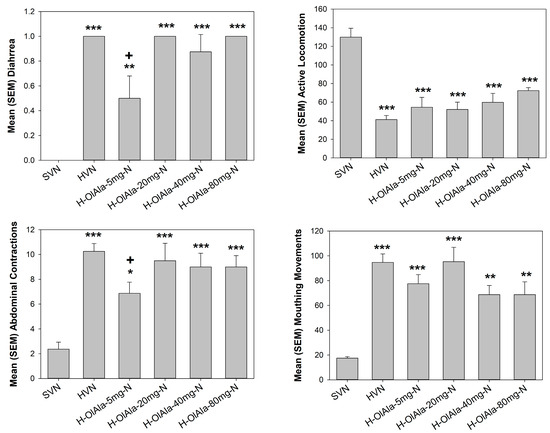
Figure 3.
Mean (±SEM) number, duration or instances of somatic withdrawal symptoms across groups (saline-VEH-naloxone (SVN), heroin-VEH-naloxone (HVN), heroin-5 mg/kg OlAla-naloxone (H-OlAla 5 mg/kg-N), heroin-20 mg/kg OlAla-naloxone (H-OlAla 20 mg-N), heroin-40 mg/kg OlAla-naloxone (H-OlAla 40 mg-N), and heroin-80 mg/kg OlAla-naloxone (H-OlAla-80 mg-N)). As expected, rats that received chronic heroin followed by naloxone (group HVN compared with SVN) exhibited withdrawal responses of diarrhea, abdominal contractions suppressed active locomotion and mouthing movements. Pretreatment with the lowest dose of oral OlAla prevented withdrawal induced diarrhea and abdominal contractions. Asterisks (*) represent significant differences from group SVN (indicating heroin withdrawal responses), crosses (+) represent significant differences among heroin treated rats from HVN (indicating interference with withdrawal by 5 mg/kg of OlAla. *** p < 0.001, ** p < 0.01, * p < 0.05.
4. Discussion
Orally administered OlAla (20 mg/kg) prevented the establishment of an acute naloxone-precipitated MWD-induced conditioned place aversion (CPA) in rats. In addition, orally administered OlAla gavaged at the dose of 5 mg/kg, but not at higher doses tested (20–80 mg/kg), attenuated the somatic withdrawal effects of abdominal contractions and diarrhea produced by naloxone-precipitated withdrawal following chronic heroin exposure. Both experiments suggest that oral OlAla produces dose-dependent effects on opioid withdrawal-related responses in male rats.
In rats that received oral vehicle administration prior to acute naloxone-precipitated MWD place conditioning, a CPA was observed at test, consistent with several previous reports [15,16]. Importantly, 20 mg/kg oral OlAla blocked the establishment of this CPA, though the lower 5 mg/kg was ineffective. Previous work demonstrated that intraperitoneal injections of OlAla blocked the establishment of acute naloxone-precipitated MWD-induced CPA when administered at 1 and 5 mg/kg in rats [15]. Thus, when orally administered, a higher dose of OlAla is necessary to block this affective withdrawal behavior. The establishment of naloxone-precipitated MWD-induced CPA is thought to be modulated by central activity, including areas of the nucleus accumbens and extended amygdala [22,23]. Therefore, if OlAla in the brain is necessary for our observed effects, it is plausible that the low oral dose (5 mg/kg) used in our experiment was insufficient in reaching the brain regions responsible for producing its effects on CPA-behavior. Given the central activity of this withdrawal behavior, future studies using intracranial OlAla delivery should attempt to determine the specific site of OlAla’s action on acute naloxone-precipitated MWD-induced CPA.
The oral gavage itself did not interfere with learning in the conditioning task, since controls (VEH treated rats) demonstrated avoidance of the MWD-paired floor. The increased dose requirement necessary for OlAla to be effective at reducing naloxone-precipitated MWD-induced CPA in rats is likely to be the result of differences in pharmacokinetics between the two routes of administration; however, the pharmacokinetics of OlAla have not yet been evaluated in rats. In general, when compared to injectable routes, lipids administered orally take longer to be absorbed into the bloodstream [24]. To account for this, we tested oral OlAla 60 min following pretreatment time, rather than the 10 min pretreatment time used with intraperitoneal injections in previous studies [14,15]. Additionally, first-pass metabolism is higher in orally administered lipids compared to intraperitoneal injection [24], therefore a higher dose may have been required for OlAla to reach its appropriate central site of action to reduce affective withdrawal behavior in our study.
Interestingly, a lower dose (5 mg/kg) of OlAla attenuated chronic somatic heroin withdrawal responses, while higher doses were ineffective. It appears that the doses necessary to modify somatic opioid withdrawal by OlAla may fall within a narrow window. Future experiments are necessary to determine the reason for the observed differences in the effective OlAla doses required for affective and somatic opioid withdrawal symptoms. One speculative possibility is that lower doses may act on peripheral gut somatic withdrawal reactions than are necessary for affective withdrawal reactions, which may require central activity, but such a distinction has not yet been determined. To support this idea, previous findings from our laboratory and work with collaborators have determined that naloxone-precipitated and spontaneous withdrawal from chronic opioids disturbs gut microbiota, and OlAla pretreatment by injection attenuates some of these disturbances [14]. Additionally, a high level of PPARα and CB1 receptor expression is found in gut tissue [25], and OlAla exerts its effects on opioid withdrawal responses via these receptors. The possibility that OlAla may regulate somatic withdrawal behaviors at the level of the gut would also explain the tendency for i.p. OlAla [15] and oral OlAla to be effective at similar doses (5 mg/kg). That is, because the loci of mechanisms that may underlie OlAla’s effects on somatic withdrawal behaviors may be within the gut, orally administered OlAla could exert its effects on withdrawal without the need for full absorption into the bloodstream.
Our group has determined that intraperitoneally administered OlAla reduces acute naloxone-precipitated MWD through the CB1 receptor and through PPARα, consistent with the ability of OlAla to inhibit FAAH and activate PPARα in vitro [15]. Therefore, oral OlAla is likely to work through similar mechanisms to reduce opioid withdrawal, though future work should confirm these assumptions. Importantly, earlier work has not recorded any adverse side-effect of OlAla pretreatment by itself. In fact, when administered alone, OlAla treatment has never interfered with any of the withdrawal behaviors of interest [14,15], and also produces no effect on body temperature, nociception or activity levels in rats [19]. Taken together, these data highlight the therapeutic potential of OlAla. It is important to note that the therapeutic effects of OlAla and OlGly are not selective to opioids with demonstrated anti-addiction properties towards several other classes of drugs, including cocaine [26], nicotine [10], and alcohol [18]. This accumulation of recent evidence in support of its role in drug addiction underscores the need for additional research probing the ability of OlAla to combat various aspects of drug intake and withdrawal, with the potential that this compound may be suitable for clinical populations in the near future.
These data provide proof of concept support for the effective treatment of opioid withdrawal behaviors by orally administered OlAla, though must be considered alongside several limitations. For one, the exclusive use of male rodents is problematic. While limited studies report sex-based differences, enhanced opioid withdrawal symptoms have been observed in women [27,28]. Preclinical studies instead support inconsistent sex-based differences in withdrawal severity, which may relate to differential time courses of peak withdrawal behaviors between the sexes [29,30]. Therefore, future work should prioritize the extension of these findings in female animals to increase the generalizability of observed results. In addition, future work must define the pharmacokinetics of orally administered OlAla, which is currently unknown, and beyond the scope of this study. Understanding the distribution of orally-dosed OlAla could help further define the mechanisms underlying its ability to reduce affective and somatic opioid withdrawal behaviors and explain the dose-dependent effects observed in this study.
5. Conclusions
These are the first data to suggest that orally administered OlAla is effective at attenuating opioid withdrawal behavior in preclinical models. Overall, these findings expand on previous research demonstrating the efficacy of intraperitoneal OlAla injections in reducing affective and somatic opioid withdrawal-related responses in rats [14,15]. The effectiveness of OlAla in reducing opioid withdrawal-related responses in rats using multiple routes of administration enhances the translational appeal of this compound for the development of opioid withdrawal medication in human populations.
Author Contributions
Conceptualization, S.M.A. and L.A.P.; methodology, S.M.A., E.M.R., C.L.L., M.V.D. and L.A.P.; writing—original draft preparation, S.M.A. and L.A.P.; writing—review and editing, S.M.A. and L.A.P.; funding acquisition, L.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council of Canada (03629).
Institutional Review Board Statement
The animal study protocol was conducted in accordance with guidelines put forth by the Canadian Council on Animal Care and approved by the Institutional Animal Care Committee at the University of Guelph (AUP# 3941, 17 May 2021 approval date).
Data Availability Statement
Please contact parkerl@uoguelph.ca for access to data.
Acknowledgments
We would like to thank Reem Smoum and Raphael Mechoulam, Hebrew University of Jerusalem, for providing the OlAla for use in this study. This paper is dedicated to the late Raphael Mechoulam for inspiring this line of research that led to the identification of N-acyl amino acids as potential “anti-addiction” drugs.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burstein, S.H.; McQuain, C.A.; Ross, A.H.; Salmonsen, R.A.; Zurier, R.E. Resolution of inflammation by N-arachidonoylglycine. J. Cell Biochem. 2011, 112, 3227–3233. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Shabalala, S.C.; Johnson, R.; Louw, J.; et al. N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature. Antioxidants 2020, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Raboune, S.; Stuart, J.M.; Leishman, E.; Takacs, S.M.; Rhodes, B.; Basnet, A.; Jameyfield, E.; McHugh, D.; Widlanski, T.; Bradshaw, H.B. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front. Cell Neurosci. 2014, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Svensson, K.J.; Bateman, L.A.; Lin, H.; Kamenecka, T.; Lokurkar, I.A.; Lou, J.; Rao, R.R.; Chang, M.R.; Jedrychowski, M.P.; et al. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell 2016, 166, 424–435. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Q.; Shu, G.; Wang, L.; Gao, P.; Xi, Q.; Zhang, Y.; Jiang, Q.; Zhu, X. N-Oleoyl glycine, a lipoamino acid, stimulates adipogenesis associated with activation of CB1 receptor and Akt signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2015, 466, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Petros, T.J.; Chang, S.Y.; Zavitsanos, P.A.; Zipkin, R.E.; Sivakumar, R.; Coop, A.; Maeda, D.Y.; De Petrocellis, L.; et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001, 276, 42639–42644. [Google Scholar] [CrossRef] [PubMed]
- Mostyn, S.N.; Wilson, K.A.; Schumann-Gillett, A.; Frangos, Z.J.; Shimmon, S.; Rawling, T.; Ryan, R.M.; O’Mara, M.L.; Vandenberg, R.J. Identification of an allosteric binding site on the human glycine transporter, GlyT2, for bioactive lipid analgesics. Elife 2019, 8, e47150. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.A.; Mitchell, V.A.; Vaughan, C.W. Actions of N-arachidonyl-glycine in a rat neuropathic pain model. Neuropharmacology 2008, 54, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, A.; Cipollone, I.; Verde, R.; Kalkan, H.; Moriello, C.; Iannotti, F.A.; Di Marzo, V.; Piscitelli, F. The endocannabinoidome mediator N-oleoylglycine is a novel protective agent against 1-methyl-4-phenyl-pyridinium-induced neurotoxicity. Front. Aging Neurosci. 2022, 14, 926634. [Google Scholar] [CrossRef]
- Donvito, G.; Piscitelli, F.; Muldoon, P.; Jackson, A.; Vitale, R.M.; D’Aniello, E.; Giordano, C.; Ignatowska-Jankowska, B.M.; Mustafa, M.A.; Guida, F.; et al. N-Oleoyl-glycine reduces nicotine reward and withdrawal in mice. Neuropharmacology 2019, 148, 320–331. [Google Scholar] [CrossRef]
- Lin, Q.; Hai, J.; Yao, L.Y.; Lu, Y. Neuroprotective effects of NSTyr on cognitive function and neuronal plasticity in rats of chronic cerebral hypoperfusion. Brain Res. 2010, 1325, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Petrie, G.N.; Wills, K.L.; Piscitelli, F.; Smoum, R.; Limebeer, C.L.; Rock, E.M.; Humphrey, A.E.; Sheppard-Perkins, M.; Lichtman, A.H.; Mechoulam, R.; et al. Oleoyl glycine: Interference with the aversive effects of acute naloxone-precipitated MWD, but not morphine reward, in male Sprague-Dawley rats. Psychopharmacology 2019, 236, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Egashira, N.; Shirakawa, A.; Abe, M.; Niki, T.; Mishima, K.; Iwasaki, K.; Oishi, R.; Fujiwara, M. N-acetyl-L-cysteine inhibits marble-burying behavior in mice. J. Pharmacol. Sci. 2012, 119, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.M.; Piscitelli, F.; Silvestri, C.; Limebeer, C.L.; Rock, E.M.; Smoum, R.; Farag, M.; de Almeida, H.; Sullivan, M.T.; Lacroix, S.; et al. Spontaneous and Naloxone-Precipitated Withdrawal Behaviors from Chronic Opiates are Accompanied by Changes in N-Oleoylglycine and N-Oleoylalanine Levels in the Brain and Ameliorated by Treatment With These Mediators. Front. Pharmacol. 2021, 12, 706703. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.M.; Smoum, R.; Farag, M.; Atwal, H.; Collins, S.A.; Rock, E.M.; Limebeer, C.L.; Piscitelli, F.; Iannotti, F.A.; Lichtman, A.H.; et al. Oleoyl alanine (HU595): A stable monomethylated oleoyl glycine interferes with acute naloxone precipitated morphine withdrawal in male rats. Psychopharmacology 2020, 237, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Ayoub, S.M.; Limebeer, C.L.; Gene, A.; Wills, K.L.; DeVuono, M.V.; Smoum, R.; Di Marzo, V.; Lichtman, A.H.; Mechoulam, R.; et al. Acute naloxone-precipitated morphine withdrawal elicits nausea-like somatic behaviors in rats in a manner suppressed by N-oleoylglycine. Psychopharmacology 2020, 237, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Guida, F.; Luongo, L.; Iannotti, F.A.; Boccella, S.; Verde, R.; Lauritano, A.; Imperatore, R.; Smoum, R.; Cristino, L.; et al. Protective Effects of N-Oleoylglycine in a Mouse Model of Mild Traumatic Brain Injury. ACS Chem. Neurosci. 2020, 11, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Shahen-Zoabi, S.; Smoum, R.; Bingor, A.; Grad, E.; Nemirovski, A.; Shekh-Ahmad, T.; Mechoulam, R.; Yaka, R. N-oleoyl glycine and N-oleoyl alanine attenuate alcohol self-administration and preference in mice. Transl. Psychiatry 2023, 13, 273. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Sullivan, M.T.; DeVuono, M.V.; Lichtman, A.H.; Di Marzo, V.; Mechoulam, R.; Parker, L.A. N-Oleoylglycine and N-Oleoylalanine Do Not Modify Tolerance to Nociception, Hyperthermia, and Suppression of Activity Produced by Morphine. Front. Synaptic Neurosci. 2021, 13, 620145. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Rimmerman, N.; Hu, S.S.; Burstein, S.; Walker, J.M. Novel endogenous N-acyl glycines identification and characterization. Vitam. Horm. 2009, 81, 191–205. [Google Scholar] [CrossRef]
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994, 37, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Gracy, K.N.; Dankiewicz, L.A.; Koob, G.F. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology 2001, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wills, K.L.; Petrie, G.N.; Millett, G.; Limebeer, C.L.; Rock, E.M.; Niphakis, M.J.; Cravatt, B.F.; Parker, L.A. Double Dissociation of Monoacylglycerol Lipase Inhibition and CB1 Antagonism in the Central Amygdala, Basolateral Amygdala, and the Interoceptive Insular Cortex on the Affective Properties of Acute Naloxone-Precipitated Morphine Withdrawal in Rats. Neuropsychopharmacology 2016, 41, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Sharkey, K.A. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol. Ther. 2010, 126, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Shahen-Zoabi, S.; Smoum, R.; Beiser, T.; Nemirovski, A.; Mechoulam, R.; Yaka, R. N-Oleoyl Glycine and Its Derivatives Attenuate the Acquisition and Expression of Cocaine-Induced Behaviors. Cannabis Cannabinoid Res. 2023, 8, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.E.; Weerts, E.M.; Huhn, A.S.; Schroeder, J.R.; Tompkins, D.A.; Bigelow, G.E.; Strain, E.C. Preliminary evidence of different and clinically meaningful opioid withdrawal phenotypes. Addict. Biol. 2020, 25, e12680. [Google Scholar] [CrossRef] [PubMed]
- Ware, O.D.; Ellis, J.D.; Dunn, K.E.; Hobelmann, J.G.; Finan, P.; Huhn, A.S. The association of chronic pain and opioid withdrawal in men and women with opioid use disorder. Drug Alcohol. Depend. 2022, 240, 109631. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Kest, B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: Central mechanisms of action and roles of gonadal hormones. Horm. Behav. 2010, 58, 72–81. [Google Scholar] [CrossRef]
- Bobzean, S.A.M.; Kokane, S.S.; Butler, B.D.; Perrotti, L.I. Sex differences in the expression of morphine withdrawal symptoms and associated activity in the tail of the ventral tegmental area. Neurosci. Lett. 2019, 705, 124–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).