Cannabis and Palliative Care Utilization among Non-Terminal Cancer Patients in the Illinois Medical Cannabis Program
Abstract
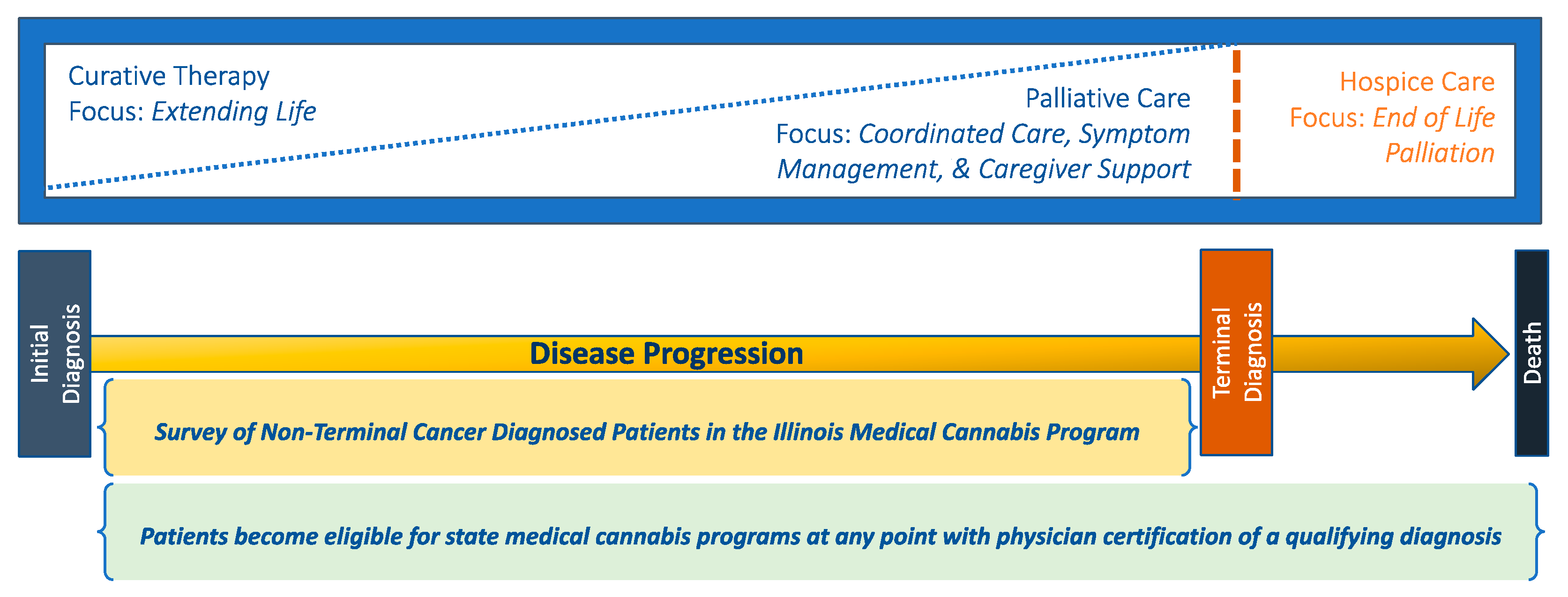
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Sample
2.3. Independent Variables
2.4. Outcome Measures
2.5. Statistical Analyses
3. Results
3.1. Self-Reported Outcome Improvements
3.2. Opioid Use among Non-Terminal Cancer Patients Receiving Palliative Care
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Oliver, D.M. Palliative Care: An Update. Mo. Med. 2017, 114, 110–115. [Google Scholar] [PubMed]
- Morrison, R.S.; Meier, D.E. Clinical practice: Palliative Care. N. Engl. J. Med. 2004, 350, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI). NCI Dictionary of Terms: Supportive Care. 2020. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/supportive-care (accessed on 21 January 2021).
- Center to Advance Palliative Care (CAPC). What is Palliative Care? 2021. Available online: https://getpalliativecare.org/whatis/ (accessed on 4 February 2021).
- National Cancer Institute (NCI). Palliative Care in Cancer. 2021. Available online: https://www.cancer.gov/about-cancer/advanced-cancer/care-choices/palliative-care-fact-sheet#what-is-palliative-care (accessed on 22 February 2023).
- Saunders, C. The Evolution of Palliative Care. J. R. Soc. Med. 2001, 94, 430–432. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Policy Board (NCPB). Improving Palliative Care for Cancer; National Academies Press: Washington, DC, USA; Available online: http://thesispublication.com/wp-content/uploads/National_Cancer_Policy_Board_National_Research_CBookFi.pdf (accessed on 21 January 2021).
- Griffin, J.P.; Nelson, J.E.; Koch, K.; Niell, H.B.; Ackerman, T.F.; Thompson, M.; Cole, F.H. End-of-Life Care in Patients with Lung Cancer. Chest 2003, 123, 312S–331S. [Google Scholar] [CrossRef] [PubMed]
- Breuer, B.; Fleishman, S.B.; Cruciani, R.A.; Portenoy, R.K. Medical Oncologists’ Attitudes and Practice in Cancer Pain Management: A National Survey. J. Clin. Oncol. 2011, 29, 4769–4775. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, D. Effective Pain Management and Improvements in Patients’ Outcomes and Satisfaction. Crit. Care Nurse 2015, 35, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, S.; Montanari, M.; Moja, L.; Apolone, G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008, 19, 1985–1991. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Kleckner, I.R.; Kamen, C.S.; Tejani, M.A.; Janelsins, M.C.; Morrow, G.R.; Peppone, L.J. Opportunities for cannabis in supportive care in cancer. Ther. Adv. Med Oncol. 2019, 11, 1758835919866362. [Google Scholar] [CrossRef]
- Henson, L.A.; Maddocks, M.; Evans, C.; Davidson, M.; Hicks, S.; Higginson, I.J. Palliative Care and the Management of Common Distressing Symptoms in Advanced Cancer: Pain, Breathlessness, Nausea and Vomiting, and Fatigue. J. Clin. Oncol. 2020, 38, 905–914. [Google Scholar] [CrossRef]
- Sizar, O.; Genova, R.; Gupta, M. Opioid Induced Constipation. [Updated 2020 Nov 20]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493184/ (accessed on 21 January 2021).
- Portenoy, R.K.; Thaler, H.T.; Kornblith, A.B.; Lepore, J.M.; Friedlander-Klar, H.; Coyle, N.; Smart-Curley, T.; Kemeny, N.; Norton, L.; Hoskins, W.; et al. Symptom prevalence, characteristics and distress in a cancer population. Qual. Life Res. 1994, 3, 183–189. [Google Scholar] [CrossRef]
- Cheville, A.L.; Alberts, S.R.; Rummans, T.A.; Basford, J.R.; Lapid, M.I.; Sloan, J.A.; Satele, D.V.; Clark, M.M. Improving Adherence to Cancer Treatment by Addressing Quality of Life in Patients with Advanced Gastrointestinal Cancers. J. Pain Symptom Manag. 2015, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Yennurajalingam, S. Palliative Care in Advanced Cancer Patients: How and When? Oncologist 2012, 17, 267–273. [Google Scholar] [CrossRef]
- Faller, H.; Bülzebruck, H.; Drings, P.; Lang, H. Coping, distress, and survival among patients with lung cancer. Arch. Gen. Psychiatry 1999, 56, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Irwin, K.E.; Greer, J.A.; Khatib, J.; Temel, J.S.; Pirl, W.F. Early palliative care and metastatic non-small cell lung cancer: Potential mechanisms of prolonged survival. Chronic Respir. Dis. 2013, 10, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Rowland, K.; Schumann, S.A. PURLs. Palliative care: Earlier is better. J. Fam. Pract. 2010, 59, 695–698. [Google Scholar]
- Prigerson, H.G.; Bao, Y.; Shah, M.A.; Paulk, M.E.; LeBlanc, T.; Schneider, B.J.; Garrido, M.; Reid, M.C.; Berlin, D.A.; Adelson, K.B.; et al. Chemotherapy Use, Performance Status, and Quality of Life at the End of Life. JAMA Oncol. 2015, 1, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Temin, S.; Alesi, E.R.; Abernethy, A.P.; Balboni, T.A.; Basch, E.M.; Ferrell, B.R.; Loscalzo, M.; Meier, D.E.; Paice, J.; et al. American Society of Clinical Oncology Provisional Clinical Opinion: The Integration of Palliative Care into Standard Oncology Care. J. Clin. Oncol. 2012, 30, 880–887. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 5, 96–112. [Google Scholar] [CrossRef]
- Aggarwal, S.K. Use of Cannabinoids in Cancer Care: Palliative Care. Curr. Oncol. 2016, 23, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.K.; Blinderman, C.D. Cannabis for Symptom Control #279. J. Palliat. Med. 2014, 17, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Guzman, M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Neugut, A.I.; Prigerson, H.G. Curative, Life-Extending, and Palliative Chemotherapy: New Outcomes Need New Names. Oncologist 2017, 22, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Vorobeichik, M.; Drawsheh, S.; Omer, A.; Goldberg, V.; Muller, E. The Medical Necessity for Medicinal Cannabis: Prospective, Observational Study Evaluating the Treatment in Cancer Patients on Supportive or Palliative Care. Evid. Based Complement. Altern. Med. 2013, 2013, 510392. [Google Scholar] [CrossRef] [PubMed]
- Casarett, D.J.; Beliveau, J.N.; Arbus, M.S. Benefit of Tetrahydrocannabinol versus Cannabidiol for Common Palliative Care Symptoms. J. Palliat. Med. 2019, 22, 1180–1184. [Google Scholar] [CrossRef]
- Hayes, B.D.; Klein-Schwartz, W.; Barrueto, F. Polypharmacy and the Geriatric Patient. Clin. Geriatr. Med. 2007, 23, 371–390. [Google Scholar] [CrossRef]
- Johannigman, S.; Eschiti, V. Medical Use of Marijuana in Palliative Care. Clin. J. Oncol. Nurs. 2013, 17, 360–362. [Google Scholar] [CrossRef]
- Savage, S.R.; Romero-Sandoval, A.; Schatman, M.; Wallace, M.; Fanciullo, G.; McCarberg, B.; Ware, M. Cannabis in Pain Treatment: Clinical and Research Considerations. J. Pain 2016, 17, 654–668. [Google Scholar] [CrossRef]
- Strouse, T.B. Pot in Palliative Care: What We Need to Know. J. Palliat. Med. 2015, 18, 7–10. [Google Scholar] [CrossRef]
- World Health Organization. Palliative Care. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/palliative-care#:~:text=Pain%20and%20difficulty%20in%20breathing,in%20need%20of%20palliative%20care (accessed on 22 February 2023).
- Bohnert, A.S.; Ilgen, M.A. Understanding Links among Opioid Use, Overdose, and Suicide. N. Engl. J. Med. 2019, 380, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lalovic, B.; Kharasch, E.; Hoffer, C.; Risler, L.; Liu-Chen, L.Y.; Shen, D.D. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites. Clin. Pharmacol. Ther. 2006, 79, 461–479. [Google Scholar] [CrossRef]
- Volkow, N.D.; McLellan, A.T. Opioid Abuse in Chronic Pain—Misconceptions and Mitigation Strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef]
- Hill, K.P. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA 2015, 313, 2474. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Bedi, G.; Ramesh, D.; Balter, R.; Comer, S.D.; Haney, M. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 2018, 43, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Litinas, E.; Clauw, D.J. Medical Cannabis Use Is Associated with Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. J. Pain 2016, 17, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.T.; Flanagan, A.M.; Earleywine, M.; Abrams, D.I.; Aggarwal, S.K.; Grinspoon, L. Cannabis in Palliative Medicine: Improving Care and Reducing Opioid-Related Morbidity. Am. J. Hosp. Palliat. Med. 2011, 28, 297–303. [Google Scholar] [CrossRef]
- Chau, D.; Walker, V.; Pai, L.; Cho, L.M. Opiates and elderly: Use and side effects. Clin. Interv. Aging 2008, 3, 273–278. [Google Scholar] [CrossRef]
- Donovan, K.A.; Chang, Y.D.; Oberoi-Jassal, R.; Rajasekhara, S.; Smith, J.; Haas, M.; Portman, D.G. Relationship of Cannabis Use to Patient-Reported Symptoms in Cancer Patients Seeking Supportive/Palliative Care. J. Palliat. Med. 2019, 22, 1191–1195. [Google Scholar] [CrossRef]
- Ferris, F.D.; Balfour, H.M.; Bowen, K.; Farley, J.; Hardwick, M.; Lamontagne, C.; Lundy, M.; Syme, A.; West, P.J. A Model to Guide Patient and Family Care: Based on Nationally Accepted Principles and Norms of Practice. J. Pain Symptom Manag. 2002, 24, 106–123. [Google Scholar] [CrossRef]
- Croker, J.A.; Bobitt, J.; Sanders, S.; Arora, K.; Mueller, K.; Kaskie, B. Cannabis and End-of-Life Care: A Snapshot of Hospice Planning and Experiences Among Illinois Medical Cannabis Patients With A Terminal Diagnosis. Am. J. Hosp. Palliat. Care 2021, 39, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Croker, J.A.; Bobitt, J.; Arora, K.; Kaskie, B. Medical Cannabis and Utilization of Nonhospice Palliative Care Services: Complements and Alternatives at End of Life. Innov. Aging 2022, 6, igab048. [Google Scholar] [CrossRef] [PubMed]
- Lum, H.D.; Arora, K.; Croker, J.A.; Qualls, S.H.; Schuchman, M.; Bobitt, J.; Milavetz, G.; Kaskie, B. Patterns of Marijuana Use and Health Impact: A Survey Among Older Coloradans. Gerontol. Geriatr. Med. 2019, 5, 2333721419843707. [Google Scholar] [CrossRef] [PubMed]
- Croker, J.A.; Bobitt, J.; Arora, K.; Kaskie, B. Assessing Health-Related Outcomes of Medical Cannabis Use in Older Adults: Findings from Illinois and Colorado. Clin. Gerontol. 2020, 44, 66–79. [Google Scholar] [CrossRef]
- Bobitt, J.; Kang, H.; Croker, J.A.; Silva, L.Q.; Kaskie, B. Use of cannabis and opioids for chronic pain by older adults: Distinguishing clinical and contextual influences. Drug Alcohol Rev. 2020, 39, 753–762. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. J. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Elliott, M.N.; Beckett, M.K.; Chong, K.; Hambarsoomians, K.; Hays, R.D. How Do Proxy Responses and Proxy-Assisted Responses Differ from What Medicare Beneficiaries Might Have Reported about Their Health Care? Healthc. Serv. Res. 2008, 43, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, F.D.; Jones, M.P.; Wehby, G.L. Gathering data from older adults via proxy respondents: Research challenges. J. Comp. Eff. Res. 2012, 1, 467–470. [Google Scholar] [CrossRef]
- Wolinsky, F.D.; Ayres, L.; Jones, M.P.; Lou, Y.; Wehby, G.L.; Ullrich, F.A. A pilot study among older adults of the concordance between their self-reports to a health survey and spousal proxy reports on their behalf. BMC Healthc. Serv. Res. 2016, 16, 485. [Google Scholar] [CrossRef]
- Roydhouse, J.K.; Gutman, R.; Keating, N.L.; Mor, V.; Wilson, I.B. Differences between Proxy and Patient Assessments of Cancer Care Experiences and Quality Ratings. Healthc. Serv. Res. 2017, 53, 919–943. [Google Scholar] [CrossRef]
- Wehby, G.L.; Jones, M.P.; Ullrich, F.; Lou, Y.; Wolinsky, F.D. Does the Relationship of the Proxy to the Target Person Affect the Concordance between Survey Reports and Medicare Claims Measures of Health Services Use? Healthc. Serv. Res. 2016, 51, 314–327. [Google Scholar] [CrossRef]
- Acquati, C.; Hibbard, J.H.; Miller-Sonet, E.; Zhang, A.; Ionescu, E. Patient activation and treatment decision-making in the context of cancer: Examining the contribution of informal caregivers’ involvement. J. Cancer Surviv. 2022, 16, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Thissen, D.; Steinberg, L.; Kuang, D. Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. J. Educ. Behav. Stat. 2001, 81, 332–342. [Google Scholar] [CrossRef]
- Eysenbach, G. Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Kim, A.; Kaufmann, C.N.; Ko, R.; Li, Z.; Han, B.H. Patterns of Medical Cannabis Use among Cancer Patients from a Medical Cannabis Dispensary in New York State. J. Palliat. Med. 2019, 22, 1196–1201. [Google Scholar] [CrossRef]
- Tanco, K.; Dumlao, D.; Kreis, R.; Nguyen, K.; Dibaj, S.; Liu, D.; Marupakula, V.; Shaikh, A.; Baile, W.; Bruera, E. Attitudes and Beliefs About Medical Usefulness and Legalization of Marijuana among Cancer Patients in a Legalized and a Nonlegalized State. J. Palliat. Med. 2019, 22, 1213–1220. [Google Scholar] [CrossRef]
- Rønne, S.T.; Rosenbæk, F.; Pedersen, L.B.; Waldorff, F.B.; Nielsen, J.B.; Riisgaard, H.; Søndergaard, J. Physicians’ experiences, attitudes, and beliefs towards medical cannabis: A systematic literature review. BMC Fam. Pr. 2021, 22, 212. [Google Scholar] [CrossRef]
- Lombardi, E.; Gunter, J.; Tanner, E. Ohio physician attitudes toward medical Cannabis and Ohio’s medical marijuana program. J. Cannabis Res. 2020, 2, 16. [Google Scholar] [CrossRef]
- Fischer, D.J.; Villines, D.; Kim, Y.O.; Epstein, J.B.; Wilkie, D.J. Anxiety, depression, and pain: Differences by primary cancer. Support. Care Cancer 2010, 18, 801–810. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Twaddle, M.L.; Melnick, A.; Meier, D.E. National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. J. Palliat. Med. 2018, 21, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Smith, H. A Comprehensive Review of Rapid-Onset Opioids for Breakthrough Pain. CNS Drugs 2012, 26, 509–535. [Google Scholar] [CrossRef] [PubMed]
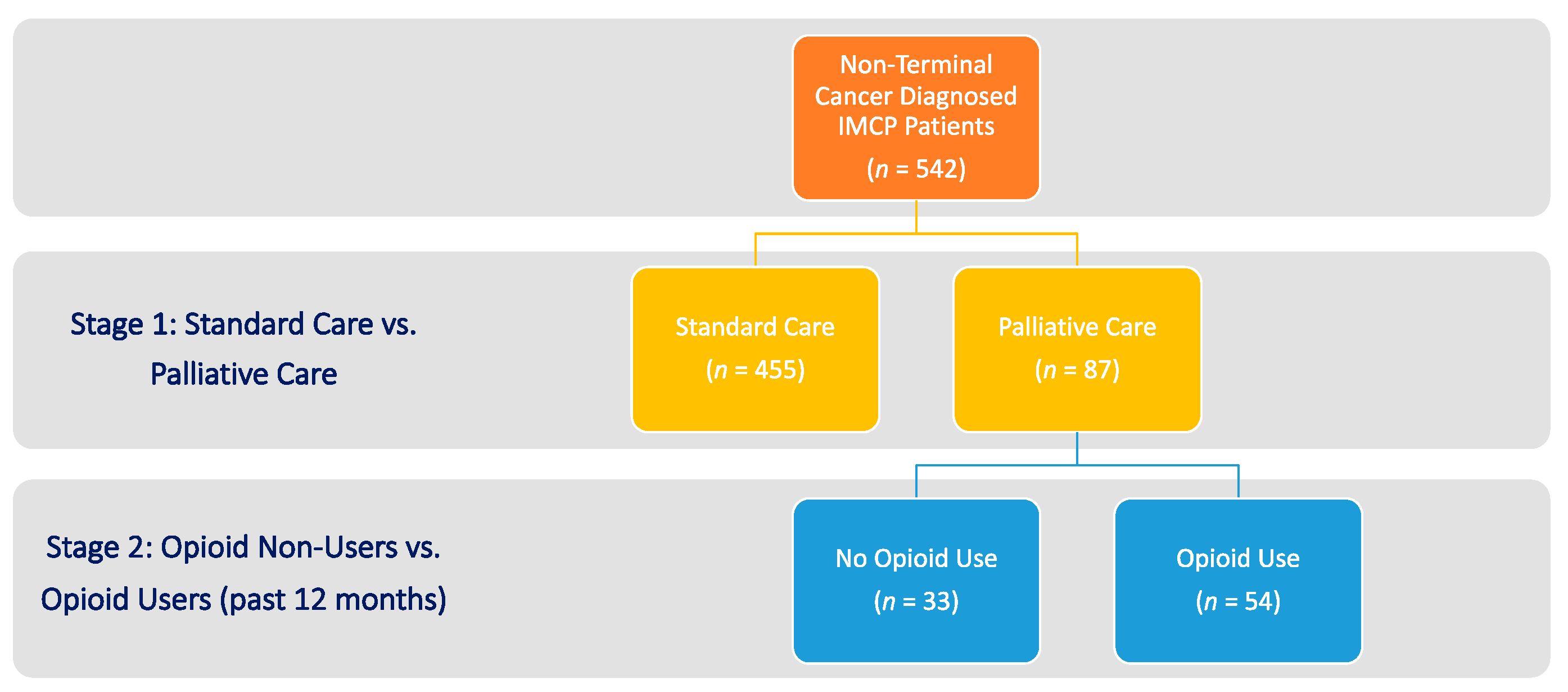
| Characteristics † | Non-PC a | PC | p-Value * |
|---|---|---|---|
| Perc. b (SE) c | Perc. (SE) | ||
| (n = 455) | (n = 87) | ||
| Demographics | |||
| Age in years (Mean (SE)) | 67.37 (0.26) | 67.15 (0.63) | 0.65 |
| Under 60 years old | 0.01 (0.01) | 0.01 (0.01) | 0.41 |
| Age 60–64 years | 0.37 (0.02) | 0.40 (0.05) | 0.49 |
| Age 65–69 years | 0.33 (0.02) | 0.31 (0.05) | 0.69 |
| Age 70–79 years | 0.28 (0.02) | 0.25 (0.05) | 0.59 |
| Age 80 years and older | 0.02 (0.01) | 0.04 (0.02) | 0.86 |
| Female | 0.43 (0.02) | 0.38 (0.05) | 0.19 |
| Non-white | 0.11 (0.02) | 0.18 (0.04) | 0.10 |
| College degree or more | 0.49 (0.02) | 0.44 (0.05) | 0.51 |
| Married | 0.69 (0.02) | 0.62 (0.05) | 0.19 |
| Military veteran | 0.18 (0.02) | 0.20 (0.04) | 0.34 |
| Currently employed | 0.24 (0.02) | 0.19 (0.04) | 0.25 |
| Financially secure | 0.76 (0.02) | 0.67 (0.05) | 0.08 |
| Health status | |||
| Caregiver proxy | 0.05 (0.01) | 0.06 (0.03) | 0.72 |
| Physically disabled | 0.22 (0.02) | 0.30 (0.05) | 0.08 |
| Difficulty managing health status | 0.14 (0.02) | 0.20 (0.04) | 0.21 |
| Low psychological well-being | 0.15 (0.02) | 0.28 (0.05) | <0.001 |
| Average 30-day pain level (0–10) | 4.47 (0.13) | 4.67 (0.26) | 0.46 |
| Frequent emotional problems | 0.21 (0.02) | 0.23 (0.05) | 0.49 |
| Frequent gastrointestinal symptoms | 0.22 (0.02) | 0.35 (0.05) | 0.01 |
| Diagnosed neurological disorder | 0.10 (0.02) | 0.06 (0.03) | 0.35 |
| Diagnosed mental health condition | 0.08 (0.01) | 0.09 (0.03) | 0.74 |
| Diagnosed musculoskeletal disorder | 0.21 (0.02) | 0.17 (0.04) | 0.58 |
| Medically complex | 0.01 (0.01) | 0.01 (0.01) | 0.81 |
| Using cannabis to treat pain | 0.77 (0.02) | 0.84 (0.04) | 0.12 |
| Using cannabis to treat emotional problems | 0.39 (0.03) | 0.46 (0.06) | 0.07 |
| Using cannabis to treat gastrointestinal symptoms | 0.39 (0.03) | 0.54 (0.06) | 0.01 |
| Prescription opioid use in the past year | 0.38 (0.02) | 0.61 (0.05) | <0.001 |
| Cannabis Use and Program Access | |||
| Using cannabis for medical purposes only | 0.66 (0.02) | 0.70 (0.05) | 0.37 |
| Using cannabis for combined medical and recreational purposes | 0.33 (0.02) | 0.29 (0.05) | 0.40 |
| Frequency of cannabis use (0–30 days) (Mean (SE)) | 20.75 (0.49) | 22.22 (1.15) | 0.24 |
| Cannabis dosing via smoke inhalation | 0.47 (0.02) | 0.52 (0.05) | 0.30 |
| Cannabis dosing via oral pill/tablet | 0.20 (0.02) | 0.21 (0.04) | 0.96 |
| Cannabis dosing via edible products | 0.63 (0.02) | 0.52 (0.05) | 0.09 |
| Naïve cannabis user | 0.28 (0.02) | 0.31 (0.05) | 0.67 |
| Knowledge of cannabis program from physician | 0.39 (0.02) | 0.43 (0.05) | 0.55 |
| Insurance coverage of certification visit | 0.42 (0.02) | 0.48 (0.05) | 0.32 |
| Negative cannabis use experience in the past year | 0.11 (0.02) | 0.08 (0.03) | 0.38 |
| Palliative Care Patients | AOR a | [95% CI] | p-Value * |
|---|---|---|---|
| Demographics | |||
| Female | 0.56 | [0.33–0.97] | 0.04 |
| Married | 0.58 | [0.35–0.96] | 0.03 |
| Financially secure | 0.55 | [0.32–0.92] | 0.02 |
| Health status | |||
| Low psychological well-being | 2.28 | [1.03–5.06] | 0.04 |
| Frequent gastrointestinal symptoms | 1.86 | [1.03–3.38] | 0.04 |
| Opioid use in the past year | 1.77 | [1.07–2.90] | 0.03 |
| Instrumental Variables | GI a | Pain | EMO b | PSY c | QOL d |
|---|---|---|---|---|---|
| (n = 193) | (n = 347) | (n = 241) | (n = 278) | (n = 380) | |
| Coef. (SE) | Coef. (SE) | Coef. (SE) | Coef. (SE) | Coef. (SE) | |
| Palliative care service utilization | 12.44 ** | 6.18 | 7.54 | 3.66 | 3.02 |
| (4.67) | (3.80) | (4.17) | (3.62) | (3.40) | |
| Frequency of Cannabis use (0–30 days in past 30 days) | 1.51 *** | 1.58 *** | 1.59 *** | 1.64 *** | 1.65 *** |
| (0.16) | (0.12) | (0.14) | (0.13) | (0.11) | |
| Opioid use in the past year | 6.84 | 7.81 ** | 8.06 * | 11.32 *** | 6.65 * |
| (3.77) | (2.92) | (3.26) | (2.96) | (2.65) | |
| Medically complex classification | 19.61 | 4.28 | 11.52 | 7.40 | 2.71 |
| (26.73) | (15.83) | (17.64) | (13.85) | (12.78) |
| Self-Reported Average Pain Levels | Opioid Non-Users (n = 33) Mean (SE) | Opioid Users (n = 54) Mean (SE) | Difference Mean (SE) | p-Value * |
|---|---|---|---|---|
| Pain level† at initiation of cannabis dosing | 3.89 (0.53) | 6.07 (0.23) | 2.18 (0.58) | <0.001 |
| Average 30-day pain level † | 3.56 (0.38) | 5.38 (0.32) | 1.82 (0.49) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croker, J.A., III; Bobitt, J.; Arora, K.; Kaskie, B. Cannabis and Palliative Care Utilization among Non-Terminal Cancer Patients in the Illinois Medical Cannabis Program. Psychoactives 2023, 2, 52-65. https://doi.org/10.3390/psychoactives2010004
Croker JA III, Bobitt J, Arora K, Kaskie B. Cannabis and Palliative Care Utilization among Non-Terminal Cancer Patients in the Illinois Medical Cannabis Program. Psychoactives. 2023; 2(1):52-65. https://doi.org/10.3390/psychoactives2010004
Chicago/Turabian StyleCroker, James A., III, Julie Bobitt, Kanika Arora, and Brian Kaskie. 2023. "Cannabis and Palliative Care Utilization among Non-Terminal Cancer Patients in the Illinois Medical Cannabis Program" Psychoactives 2, no. 1: 52-65. https://doi.org/10.3390/psychoactives2010004
APA StyleCroker, J. A., III, Bobitt, J., Arora, K., & Kaskie, B. (2023). Cannabis and Palliative Care Utilization among Non-Terminal Cancer Patients in the Illinois Medical Cannabis Program. Psychoactives, 2(1), 52-65. https://doi.org/10.3390/psychoactives2010004






