Suspected Adverse Drug Reactions Associated with Leukotriene Receptor Antagonists Versus First-Line Asthma Medications: A National Registry–Pharmacology Approach
Abstract
1. Introduction
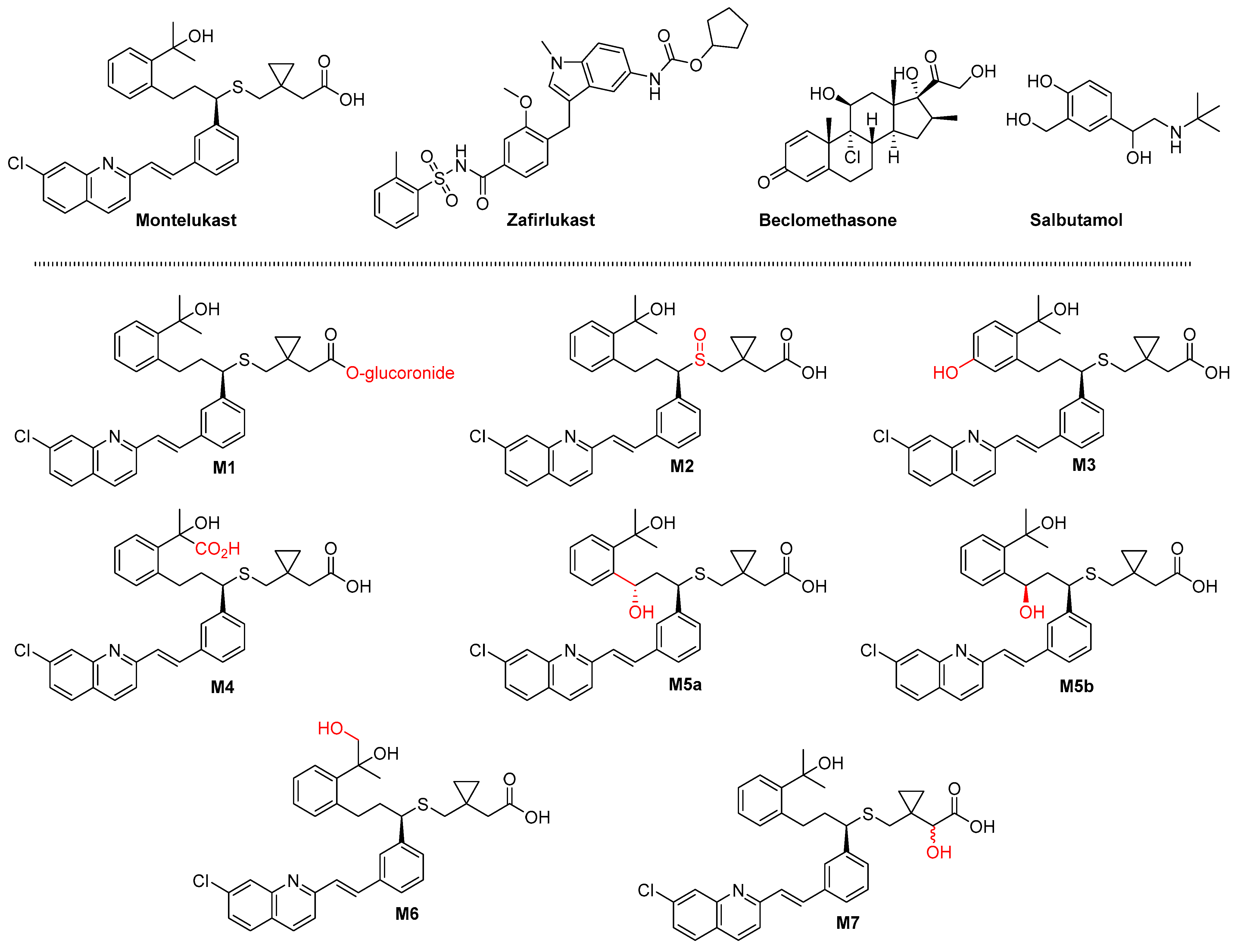
2. Results
2.1. Molecular Properties
2.2. Pharmacokinetic Properties
2.3. Pharmacology Properties
2.4. ADRs
3. Discussion
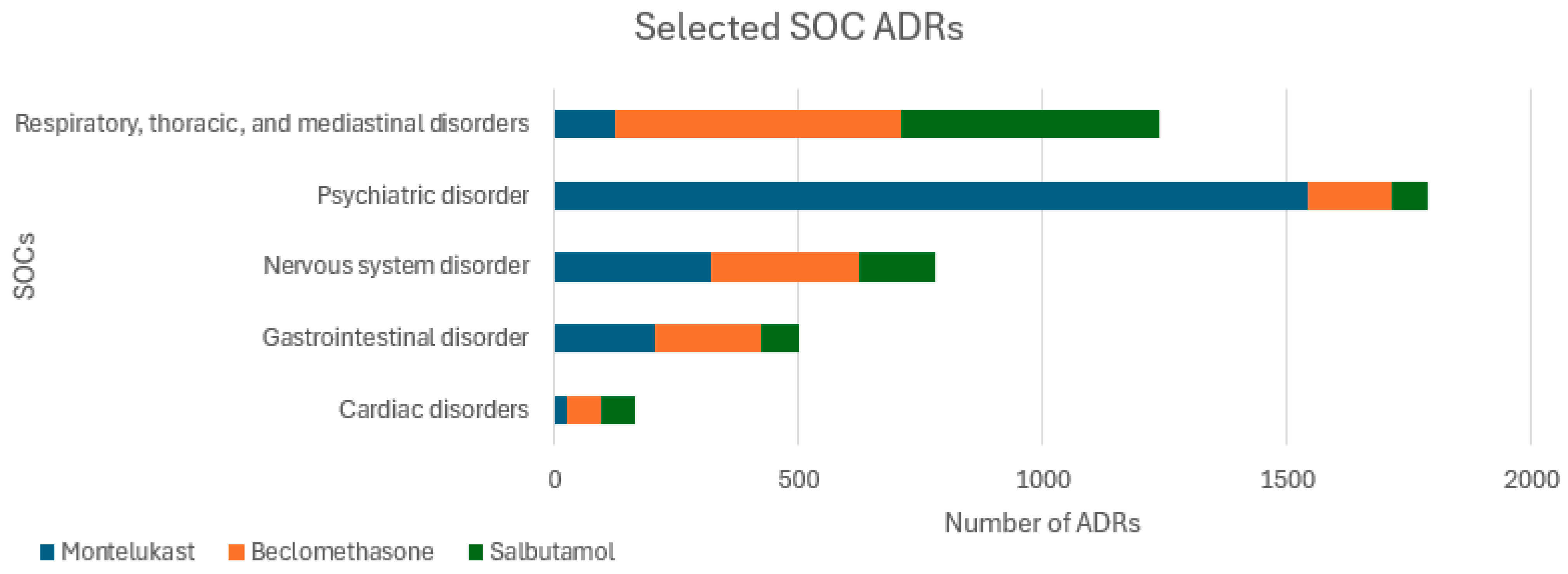
3.1. Total ADRs and Fatalities
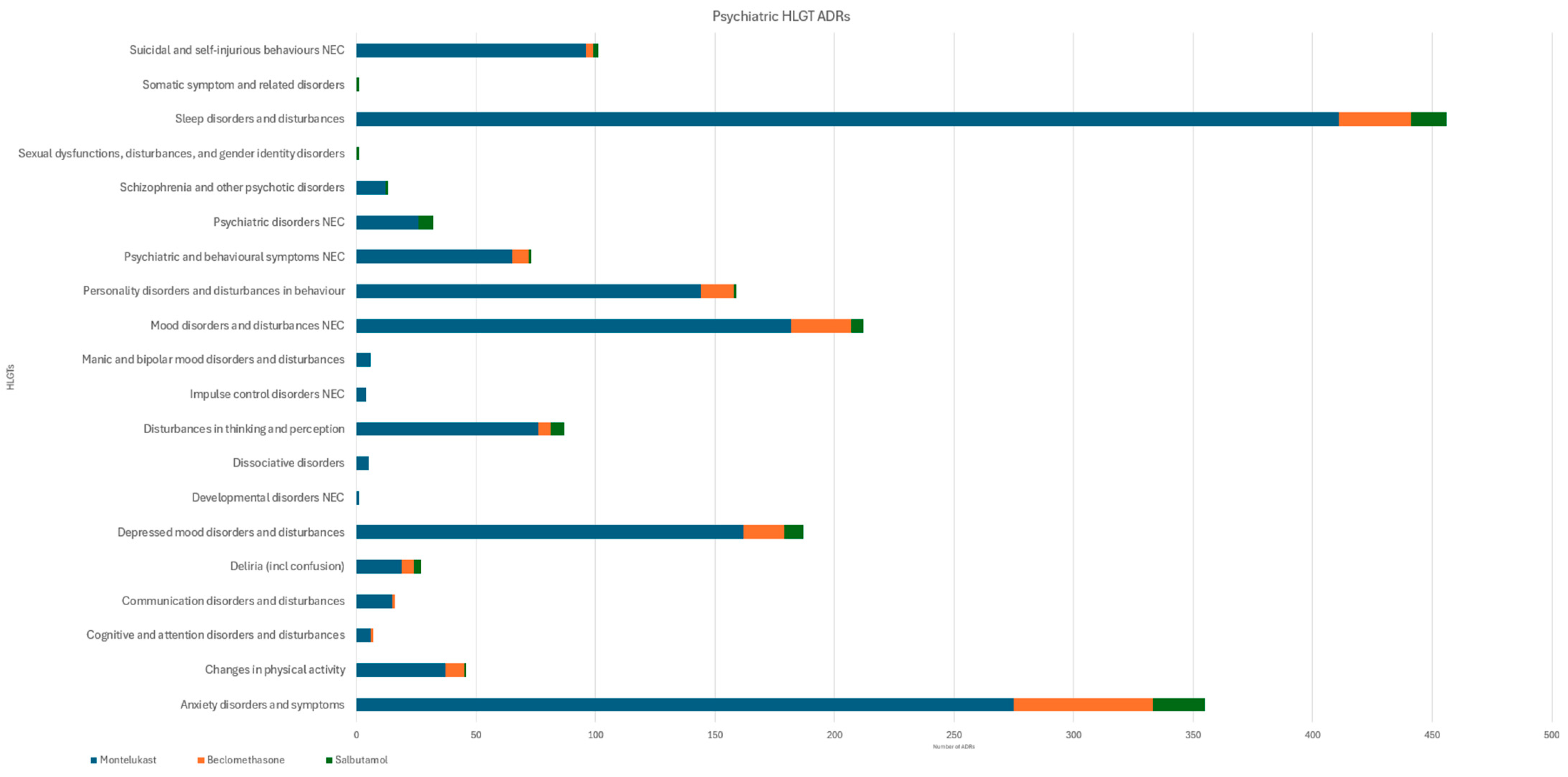
3.2. Psychiatric ADRs
3.3. Cardiac ADRs and Fatalities
3.4. Gastrointestinal Disorders and Site of Administration ADRs
3.5. Nervous System ADRs
4. Limitations
5. Methods
5.1. Method Databases
5.2. Physiochemical Properties
5.3. Pharmacokinetic Properties
5.4. Pharmacological Properties
5.5. Prescribing Data
5.6. Adverse Drug Reactions
5.7. Ethics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asthma and Lung UK. What is Asthma?|Asthma + Lung UK. Available online: https://www.asthmaandlung.org.uk/conditions/asthma/what-asthma#:~:text=Asthma%20is%20a%20very%20common (accessed on 10 December 2023).
- NICE. What Is the Prevalence of Asthma? NICE. May 2022. Available online: https://cks.nice.org.uk/topics/asthma/background-information/prevalence/ (accessed on 10 December 2023).
- BTS/NICE/SIGN Joint Guideline on Asthma: Diagnosis, Monitoring and Chronic Asthma Management. Available online: https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed on 12 June 2024).
- Mukherjee, M.; Stoddart, A.; Gupta, R.P.; Nwaru, B.I.; Farr, A.; Heaven, M.; Fitzsimmons, D.; Bandyopadhyay, A.; Aftab, C.; Simpson, C.R.; et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: Analyses of standalone and linked national databases. BMC Med. 2016, 14, 113. [Google Scholar] [CrossRef]
- NICE. Available online: https://www.nice.org.uk/guidance/ng80/resources/algorithm-f-pharmacological-treatment-of-chronic-asthma-in-adults-aged-17-and-over-pdf-4656176754 (accessed on 12 June 2024).
- NICE. Algorithm C: Pharmacological Management of Asthma in People Aged 12 Years and Over. Available online: https://www.nice.org.uk/guidance/ng245/resources/algorithm-c-pharmacological-management-of-asthma-in-people-aged-12-years-and-over-bts-nice-pdf-13556516367 (accessed on 17 September 2025).
- Sampson, A.; Holgate, S. Leukotriene modifiers in the treatment of asthma. BMJ 1998, 316, 1257–1258. [Google Scholar] [CrossRef]
- Coleman, J.J.; Pontefract, S.K. Adverse drug reactions. Clin. Med. 2016, 16, 481–485. [Google Scholar] [CrossRef] [PubMed]
- King, C.; McKenna, A.; Farzan, N.; Vijverberg, S.J.; van der Schee, M.P.; Maitland-van der Zee, A.H.; Arianto, L.; Bisgaard, H.; BØnnelykke, K.; Berce, V.; et al. Pharmacogenomic associations of adverse drug reactions in asthma: Systematic review and research prioritisation. Pharmacogenom. J. 2020, 20, 621–628. [Google Scholar] [CrossRef]
- Paljarvi, T.; Forton, J.; Luciano, S.; Herttua, K.; Fazel, S. Analysis of Neuropsychiatric Diagnoses After Montelukast Initiation. JAMA Netw. Open 2022, 5, e2213643. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, M.; Seo, M.S.; Shin, J.Y. Risk of neuropsychiatric adverse events associated with montelukast use in children and adolescents: A population-based case-crossover study. BMJ Paediatr. Open 2024, 8, e002483. [Google Scholar] [CrossRef]
- Wintzell, V.; Brenner, P.; Halldner, L.; Rhedin, S.; Gong, T.; Almqvist, C. Montelukast Use and the Risk of Neuropsychiatric Adverse Events in Children. JAMA Pediatr. 2025, 179, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Kaminsky, E.; Taavola, H.; Attalla, M.; Yue, Q.Y. Montelukast and Nightmares: Further Characterisation Using Data from VigiBase. Drug Saf. 2022, 45, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, C.K. Montelukast use over the past 20 years: Monitoring of its effects and safety issues. Clin. Exp. Pediatr. 2020, 63, 376–381. [Google Scholar] [CrossRef]
- Umetsu, R.; Tanaka, M.; Nakayama, Y.; Kato, Y.; Ueda, N.; Nishibata, Y.; Hasegawa, S.; Matsumoto, K.; Takeyama, N.; Iguchi, K.; et al. Neuropsychiatric Adverse Events of Montelukast: An Analysis of Real-World Datasets and drug-gene Interaction Network. Front. Pharmacol. 2021, 12, 764279. [Google Scholar] [CrossRef]
- Lo, C.W.H.; Pathadka, S.; Qin, S.X.; Fung, L.W.Y.; Yan, V.K.C.; Yiu, H.H.E.; Bloom, C.I.; Wong, I.C.K.; Chan, E.W.Y. Neuropsychiatric events associated with montelukast in patients with asthma: A systematic review. Eur. Respir. Rev. 2023, 32, 230079. [Google Scholar] [CrossRef] [PubMed]
- Montelukast (Singulair): Reminder of the Risk of Neuropsychiatric Reactions. Available online: https://www.gov.uk/drug-safety-update/montelukast-singulair-reminder-of-the-risk-of-neuropsychiatric-reactions (accessed on 16 December 2023).
- Yao, T.C.; Huang, J.L.; Wu, C.S.; Lu, H.H.S.; Chang, Y.C.; Chen, W.Y.; Kao, H.F.; Wu, A.C.; Tsai, H.J. Comparative Risk of Neuropsychiatric Adverse Events Associated With Leukotriene-Receptor Antagonists Versus Inhaled Corticosteroids. J. Allergy Clin. Immunol. Pract. 2025, 13, 903–911.e5. [Google Scholar] [CrossRef] [PubMed]
- Kovesi, T. Neuropsychiatric side effects of montelukast. J. Pediatr. 2019, 212, 248. [Google Scholar] [CrossRef]
- Mou, Y.; Song, Q.; Zhao, C.; Fang, H.; Ren, C.; Song, X. Meta-analysis of the relationship between montelukast use and neuropsychiatric events in patients with allergic airway disease. Heliyon 2023, 9, e21842. [Google Scholar] [CrossRef]
- Marques, C.F.; Marques, M.M.; Justino, G.C. The mechanisms underlying montelukast’s neuropsychiatric effects-new insights from a combined metabolic and multiomics approach. Life Sci. 2022, 310, 121056. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, N.P.; Tatonetti, N.P. A database of pediatric drug effects to evaluate ontogenic mechanisms from child growth and development. Med. 2022, 3, 579–595.e7. [Google Scholar] [CrossRef]
- Costello, J.F. Leukotriene Antagonists. Pulm. Pharamacol. Ther. 1998, 11, 393–395. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Nakatsuji, T.; Kambara, H.; Niinomi, I.; Oyama, S.; Inada, A.; Ueno, S.; Uchida, M.; Iwanaga, K.; Iida, T.; et al. Drug-induced Neuropsychiatric Adverse Events Using Post-Marketing Surveillance. Curr. Rev. Clin. Exper. Pharmacol. 2022, 17, 144–148. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, J.; Oliveira, R.V.; Lu, J.B.; Desta, Z. In Vitro Metabolism of Montelukast by Cytochrome P450s and UDP-Glucuronosyltransferases. Drug Metab. Dispos. 2015, 43, 1905–1916. [Google Scholar] [CrossRef]
- Balani, S.K.; Xu, X.; Pratha, V.; Koss, M.A.; Amin, R.D.; Dufresne, C.; Miller, R.R.; Arison, B.H.; Doss, G.A.; Chiba, M.; et al. Metabolic profiles of montelukast sodium (Singulair), a potent cysteinyl leukotriene1 receptor antagonist, in human plasma and bile. Drug Metab. Dispos. 1997, 25, 1282–1287. [Google Scholar] [PubMed]
- Filppula, A.M.; Laitila, J.; Neuvonen, P.J.; Backman, J.T. Reevaluation of the microsomal metabolism of montelukast: Major contribution by CYP2C8 at clinically relevant concentrations. Drug Metab. Dispos. 2011, 39, 904–911. [Google Scholar] [CrossRef]
- Jones, L.; Jones, A.M. Suspected adverse drug reactions of the type 2 antidiabetic drug class dipeptidyl-peptidase IV inhibitors (DPP4i): Can polypharmacology help explain? Pharmacol. Res. Perspect. 2022, 10, e01029. [Google Scholar] [CrossRef]
- Zafirlukast discontinued. Prescribing Advice for GPs. Available online: https://www.prescriber.org.uk/2018/04/zafirlukast-discontinued/comment-page-1/#:~:text=Commercial%20reasons%20are%20cited%20for (accessed on 16 January 2024).
- Michael, J.; Bessa de Sousa, D.; Conway, J.; Gonzalez-Labrada, E.; Obeid, R.; Tevini, J.; Felder, T.; Hutter-Paier, B.; Zerbe, H.; Paiement, N.; et al. Improved Bioavailability of Montelukast through a Novel Oral Mucoadhesive Film in Humans and Mice. Pharmaceutics 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, J.; Schäffner, I.; Klein, B.; Gelfert, R.; Rivera, F.J.; Illes, S.; Grassner, L.; Janssen, M.; Rotheneichner, P.; Schmuckermair, C.; et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 2015, 6, 8466. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cho, Y.J.; Yun, J.Y.; Lee, H.J.; Yu, J.; Yang, H.J.; Suh, D.I. Leukotriene receptor antagonists and risk of neuropsychiatric events in children, adolescents, and young adults: A self-controlled case series. Eur. Respir. J. 2022, 60, 2102467. [Google Scholar] [CrossRef]
- Henderson, I.; Caiazzo, E.; McSharry, C.J.; Guzik, T.; Maffia, P.M. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol. Res. 2020, 160, 105189. [Google Scholar] [CrossRef]
- Paggiaro, P.; Bacci, E. Montelukast in asthma: A review of its efficacy and place in therapy. Ther. Adv. Chronic Dis. 2010, 2, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef]
- Nishat, S.; Khan, L.A.; Ansari, Z.M.; Basir, S.F. Adenosine A3 Receptor: A promising therapeutic target in cardiovascular disease. Curr. Cardiol. Rev. 2016, 12, 18–26. [Google Scholar] [CrossRef]
- Electronic Medicines Compendium (EMC). Available online: https://www.medicines.org.uk/emc/ (accessed on 10 December 2023).
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. Drugbank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- ChEMBL Database. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 16 December 2023).
- Calhoun, W.J. Summary of Clinical Trials with Zafirlukast. Am. J. Respir. Crit. Care Med. 1998, 157, S238–S246. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Bajaj, T. Zafirlukast. PubMed. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557844/#:~:text=Zafirlukast%20belongs%20to%20the%20leukotriene (accessed on 16 September 2025).
- SciFinder. Available online: https://scifinder-n.cas.org/searchDetail/reference/6543cd23f2a71734588a5251/referenceDetails (accessed on 10 December 2023).
- Roberts, J.K.; Moore, C.D.; Ward, R.M.; Yost, G.S.; Reilly, C.A. Metabolism of Beclomethasone Dipropionate by Cytochrome P450 3A Enzymes. J. Pharmacol. Exp. Ther. 2013, 345, 308–316. [Google Scholar] [CrossRef]
- Hindle, M.; Chrystyn, H. Determination of the relative bioavailability of salbutamol to the lung following inhalation [see comments]. Br. J. Clin. Pharmacol. 1992, 34, 311–315. [Google Scholar] [CrossRef]
- Kruizinga, M.D.; Birkhoff, W.A.; van Esdonk, M.J.; Klarenbeek, N.B.; Cholewinski, T.; Nelemans, T.; Dröge, M.J.; Cohen, A.F.; Zuiker, R.G. Pharmacokinetics of intravenous and inhaled salbutamol and tobramycin: An exploratory study to investigate the potential of exhaled breath condensate as a matrix for pharmacokinetic analysis. Br. J. Clin. Pharmacol. 2020, 86, 175–181. [Google Scholar] [CrossRef]
- Open Prescribing. Available online: https://openprescribing.net/ (accessed on 20 October 2023).
- Medicines and Healthcare Products Regulatory Agency. Available online: https://yellowcard.mhra.gov.uk/idaps (accessed on 16 September 2025).
- Ferro, C.J.; Solkhon, F.; Jalal, Z.; Al-Hamid, A.M.; Jones, A.M. Relevance of physicochemical properties and functional pharmacology data to predict the clinical safety profile of direct oral anticoagulants. Pharmacol. Res. Perspect. 2020, 8, e00603. [Google Scholar] [CrossRef]
- Matharu, K.; Chana, K.; Ferro, C.; Jones, A.M. Polypharmacology of clinical sodium glucose co-transport protein 2 inhibitors and relationship to suspected adverse drug reactions. Pharmacol. Res. Perspect. 2021, 9, e00867. [Google Scholar] [CrossRef]
- Sandhu, D.; Antolin, A.A.; Cox, A.R.; Jones, A.M. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. Brit. J. Clin. Pharmacol. 2022, 88, 742–752. [Google Scholar] [CrossRef]
- Salim, H.; Jones, A.M. Angiotensin II Receptor Blockers (ARBs) and Manufacturing Contamination: A Retrospective National Register Study into suspected associated adverse drug reactions. Brit. J. Clin. Pharmacol. 2022, 88, 4812–4827. [Google Scholar] [CrossRef]
- Yousaf, H.; Jones, A.M. The Relationship Between Suspected Adverse Drug Reactions Of HMG-Coa Reductase Inhibitors and Polypharmacology Using a National Registry Approach. MedRxiv 2024. [Google Scholar] [CrossRef]
- Phillips, B.; Evans, I.; Skerrett, V.; Jones, A.M. United Kingdom National Register Study of Anti-Epileptic Medications: Suspected Foetal Congenital and Pregnancy-Associated Side Effects. MedRxiv 2024. [Google Scholar] [CrossRef]
| Short-Acting Beta-2 Agonist | Inhaled Corticosteroid | Leukotriene Receptor Antagonist |
|---|---|---|
| Arrythmias | Arrythmias | Aggressive behaviour |
| Fine tremor | Fine tremor | Anxiety |
| Headache | Headache | Headache |
| Tachycardia | Tachycardia | Hallucinations |
| Sleeping issues Abdominal pain |
| Variables | Montelukast | Zafirlukast | Beclomethasone | Salbutamol |
|---|---|---|---|---|
| pIC50 | 8.64 | 8.74 | 8.08 | 6.01 |
| clog10P | 8.49 | 6.4 | 2.15 | 0.34 |
| LLE | 0.15 | 2.34 | 5.93 | 5.67 |
| MW (Da) | 586.2 | 575.69 | 408.92 | 239.31 |
| pKa | 4.4 (acidic) | 4.29 (acidic) | 12.44 (neutral) | 9.4 (basic) |
| tPSA (Å) | 70.42 | 115.73 | 94.83 | 72.72 |
| HB acceptors | 4 | 7 | 5 | 4 |
| HB donors | 2 | 2 | 3 | 4 |
| clog10D7.4 | 5.8 | 5.46 | 2.15 | −1.32 |
| P-glycoprotein Substrate | Yes | Yes | Yes | Yes |
| No. of BBB requirements met | 2 | 1 | 4 | 4 |
| Variables | Montelukast | Zafirlukast | Beclomethasone | Salbutamol |
|---|---|---|---|---|
| Bioavailability (%F) | 64% | 100% | 41% | 24.8% |
| Half-life (h) | 2.7–5.5 | 8–16 | 5.7 | 2.7–5.5 |
| Tmax (h) | 3 | 3 | 0.7 | 0.17 |
| Cmax (nM) | 656.77 | 442.95 | 86.46 | 1678.83 |
| CYP metabolism | CYP3A4, CYP28C, CYP28B | CYP2C9 | CYP430 3A | CYP 450 |
| Renal excretion | <0.2% | 10% | negligible | 272 ± 38 mL/min |
| Volume of distribution (L) | 8–11 | 70 | 20 | 156 ± 38 (IV) |
| Clearance (L/h) | 2.7 | 20 | 150 | 25.22 |
| PPB (%) | 99% | 99% | 87% | 10% |
| Protein Group | Protein Compound | Montelukast | Zafirlukast | Beclomethasone | Salbutamol |
|---|---|---|---|---|---|
| G Protein coupled receptors | Cysteinyl leukotriene receptor 1 | 2.3 | 8.7 | ||
| Cysteinyl leukotriene receptor 2 | 27,000 | 7397 | |||
| Alpha-2a adrenergic receptor | 3919 | ||||
| Alpha-2c adrenergic receptor | 5279 | ||||
| Adenosine A1 receptor | >10,000 | ||||
| Adenosine A3 receptor | 434 | 1363 | |||
| Beta-2 adrenergic receptor | 3488 | 980 | |||
| Beta-3 adrenergic receptor | 4300 | ||||
| Dopamine D1 receptor | >10,000 | ||||
| Dopamine D3 receptor | 7747 | ||||
| Delta opioid receptor | 4795 | ||||
| Mu opioid receptor | >10,000 | ||||
| Norepinephrine receptor | 2689 | ||||
| Histamine H1 receptor | >10,000 | ||||
| Neurokinin 2 receptor | 3822 | ||||
| Muscarinic acetylcholine receptor M1 | 8045 | ||||
| Muscarinic acetylcholine receptor M3 | 6626 | ||||
| Serotonin 2b (5T-HT2b) receptor | 6256 | ||||
| Transmembrane protein | Dopamine transporter | 2601 | |||
| Epidermal growth factor 1 erbB1 | 3197 | 5751 | |||
| MAPEG | Leukotriene C4 synthase | <5000 | |||
| Prostaglandin E synthase | 18,100 | ||||
| MAP | MAP kinase p38 alpha | 856 | 6.4 | ||
| MAP kinase ERK1 | 4376 | ||||
| MAP kinase ERK2 | 538 | ||||
| CYP5 | Thromboxane A synthase | 1525 | 3810 | ||
| Src family kinases (SFKs) | Tyrosine-protein kinase FYN | 4702 | |||
| CYP 450 | Cytochrome P450 2C8 | 1000 | |||
| SLC10 family of solute carrier proteins | Bile acid transporter | 6500 | |||
| ABC superfamily transport proteins | Bile salt export pump | 11,100 | 16,985 | >1,000,000 | |
| APC transport family | Multidrug resistance-associated protein 1 | 1300 | |||
| Multidrug resistance-associated protein 2 | 7600 | ||||
| Multidrug resistance-associated protein 4 | 12,100 | >133,000 | |||
| Canalicular multispecific organic anion transporter 1 | 58,800 | >133,000 | |||
| Canalicular multispecific organic anion transporter 2 | >133,000 | >133,000 | |||
| Group IVA cytosolic | Cytosolic phospholipase A2 | 85,000 | |||
| Nuclear receptor family | Glucocorticoid receptor | 8.2 | |||
| Protein superfamily | Thiosulfate sulfur transferase | >100,000 | |||
| Other | Epoxidase hydratase | 2000 | |||
| C max (nM) | 657 | 443 | 86 | 1679 | |
| Threshold (×1.5) | 985 | 664 | 130 | 2518 | |
| Legend | blank = unknown | Green = Clinically significant |
| Variables | Montelukast | Zafirlukast | Beclomethasone | Salbutamol | p |
|---|---|---|---|---|---|
| Total prescriptions | 18,757,214 | 1327 | 70,250,968 | 107,619,145 | - |
| Total ADRs | 2935 (15.64) | 9 (678.22) | 2327 (3.37) | 1342 (1.24) | <0.001 |
| Fatalities | 2 (0.01) | 0 (0) | 1 (0) | 15 (0.01) | 0.95 |
| Cardiac disorder | 26 (0.13) | 0 (0) | 71 (0.1) | 68 (0.06) | 0.98 |
| Fatalities | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| Palpitations | 23 (0.12) | 0 (0) | 59 (0.08) | 24 (0.02) | 0.96 |
| Gastrointestinal disorder | 204 (1.08) | 0 (0) | 221 (0.31) | 75 (0.06) | 0.56 |
| Fatalities | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| Abdominal pain | 17 (0.09) | 0 (0) | 3 (0.00) | 1 (0) | 0.92 |
| Nausea | 39 (0.20) | 0 (0) | 37 (0.05) | 9 (0) | 0.88 |
| Nervous system disorder | 322 ((1.71) | 1 (72.88) | 303 (0.43) | 154 (0.14) | 0.39 |
| Fatalities | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| Headache | 72 (0.38) | 0 (0) | 67 (0.09) | 19 (0.01) | 0.79 |
| Psychiatric disorder | 1542 (8.22) | 0 (0) | 174 (0.24) | 73 (0.06) | <0.001 |
| Fatalities | 2 (0.01) | 0 (0) | 0 (0) | 1 (0) | 0.99 |
| Agitation | 34 (0.18) | 0 (0) | 6 (0) | 1 (0) | 0.84 |
| Anxiety | 165 (0.87) | 0 (0) | 35 (0.04) | 7 (0) | 0.46 |
| Depression | 82 (0.43) | 0 (0) | 7 (0) | 4 (0) | 0.66 |
| Hallucinations | 42 (0.22) | 0 (0) | 1 (0) | 3 (0) | 0.80 |
| Nightmare | 162 (0.86) | 0 (0) | 1 (0) | 0 (0) | 0.42 |
| Suicidal ideation | 62 (0.33) | 0 (0) | 2 (0) | 2 (0) | 0.72 |
| Completed suicide | 3 (0.01) | 0 (0) | 0 (0) | 0 (0) | - |
| Respiratory, thoracic, and mediastinal disorders | 125 (0.66) | 0 (0) | 587 (0.83) | 528 (0.24) | 0.85 |
| Fatalities | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| HLGT | Montelukast | Beclomethasone | Salbutamol | p |
|---|---|---|---|---|
| Anxiety disorders and symptoms | 275 (1.47) | 58 (0.08) | 22 (0.02) | 0.28 |
| Changes in physical activity | 37 (0.20) | 8 (0.01) | 1 (0.00) | - |
| Cognitive and attention disorders and disturbances | 6 (0.03) | 1 (0.00) | 0 | - |
| Communication disorders and disturbances | 15 (0.08) | 1 (0.00) | 0 | - |
| Deliria (including confusion) | 19 (0.10) | 5 (0.01) | 3 (0.00) | 0.92 |
| Depressed mood disorders and disturbances | 162 (0.86) | 17 (0.02) | 8 (0.01) | 0.45 |
| Developmental disorders NEC | 1 (0.01) | 0 | 0 | - |
| Dissociative disorders | 5 (0.03) | 0 | 0 | - |
| Disturbances in thinking and perception | 76 (0.41) | 5 (0.01) | 6 (0.01) | 0.69 |
| Impulse control disorders NEC | 4 (0.02) | 0 | 0 | - |
| Manic and bipolar mood disorders and disturbances | 6 (0.03) | 0 | 0 | - |
| Mood disorders and disturbances NEC | 182 (0.97) | 25 (0.04) | 5 (0.00) | 0.41 |
| Personality disorders and disturbances in behaviour | 144 (0.77) | 14 (0.02) | 1 (0.00) | - |
| Psychiatric and behavioural symptoms NEC | 65 (0.35) | 7 (0.01) | 1 (0.00) | - |
| Psychiatric disorders NEC | 26 (0.14) | 0 | 6 (0.01) | - |
| Schizophrenia and other psychotic disorders | 12 (0.06) | 0 | 1 (0.00) | - |
| Sexual dysfunctions, disturbances, and gender identity disorders | 0 | 0 | 1 (0.00) | - |
| Sleep disorders and disturbances | 411 (2.19) | 30 (0.04) | 15 (0.01) | 0.12 |
| Somatic symptoms and related disorders | 0 | 0 | 1 (0.00) | - |
| Suicidal and self-injurious behaviours NEC | 96 (0.51) [2] | 3 (0.00) | 2 (0.00) | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Hirsch, C.; Jones, A.M. Suspected Adverse Drug Reactions Associated with Leukotriene Receptor Antagonists Versus First-Line Asthma Medications: A National Registry–Pharmacology Approach. Pharmacoepidemiology 2025, 4, 18. https://doi.org/10.3390/pharma4030018
Khan M, Hirsch C, Jones AM. Suspected Adverse Drug Reactions Associated with Leukotriene Receptor Antagonists Versus First-Line Asthma Medications: A National Registry–Pharmacology Approach. Pharmacoepidemiology. 2025; 4(3):18. https://doi.org/10.3390/pharma4030018
Chicago/Turabian StyleKhan, Mohammed, Christine Hirsch, and Alan M. Jones. 2025. "Suspected Adverse Drug Reactions Associated with Leukotriene Receptor Antagonists Versus First-Line Asthma Medications: A National Registry–Pharmacology Approach" Pharmacoepidemiology 4, no. 3: 18. https://doi.org/10.3390/pharma4030018
APA StyleKhan, M., Hirsch, C., & Jones, A. M. (2025). Suspected Adverse Drug Reactions Associated with Leukotriene Receptor Antagonists Versus First-Line Asthma Medications: A National Registry–Pharmacology Approach. Pharmacoepidemiology, 4(3), 18. https://doi.org/10.3390/pharma4030018







