Fact-Finding Survey of Lethal or Fatal Adverse Drug Events in the Japanese Adverse Drug Event Report Database, Fiscal Year 2004–2023 (Adults ≥ 20 Years)
Abstract
1. Introduction
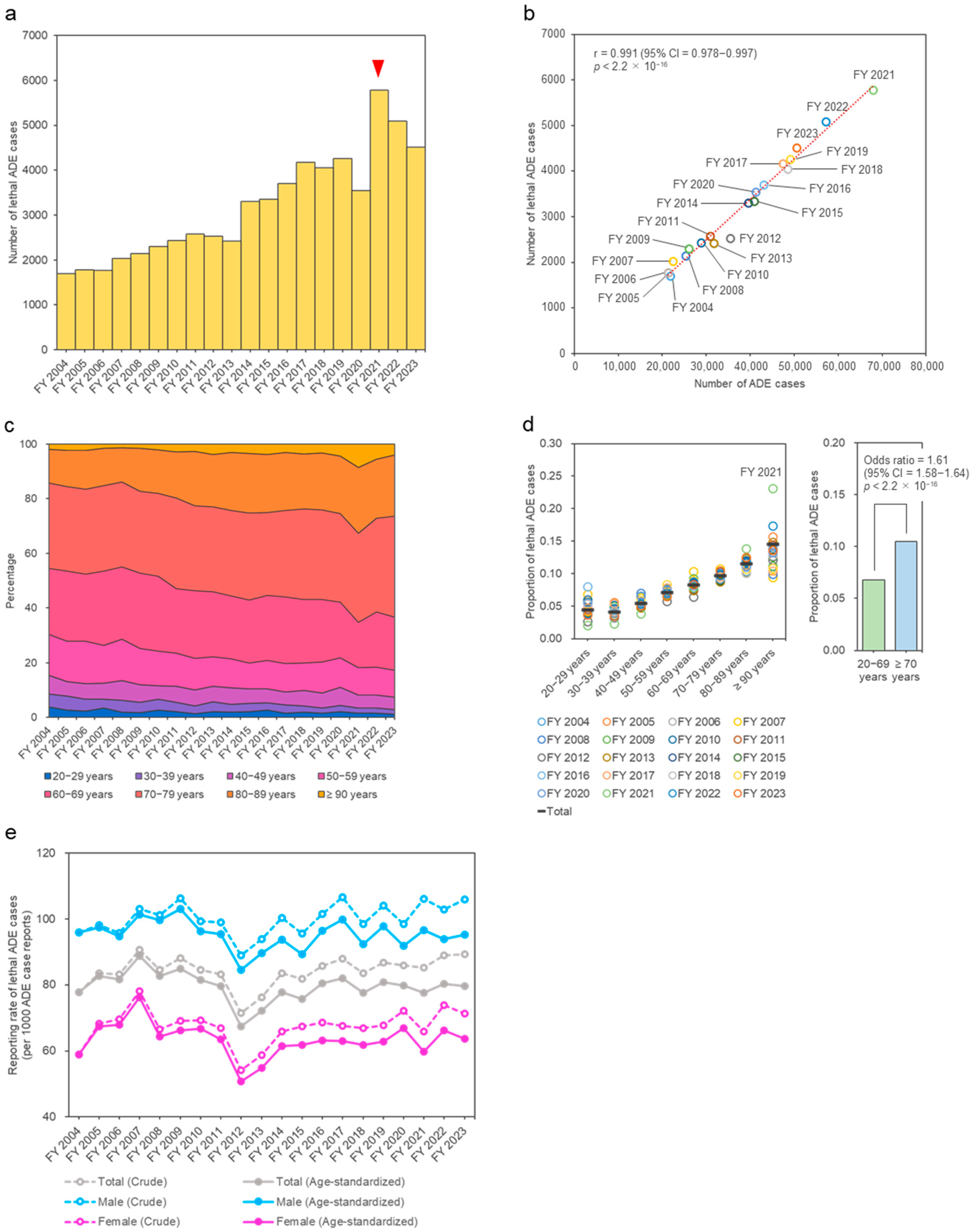
2. Results
3. Discussion
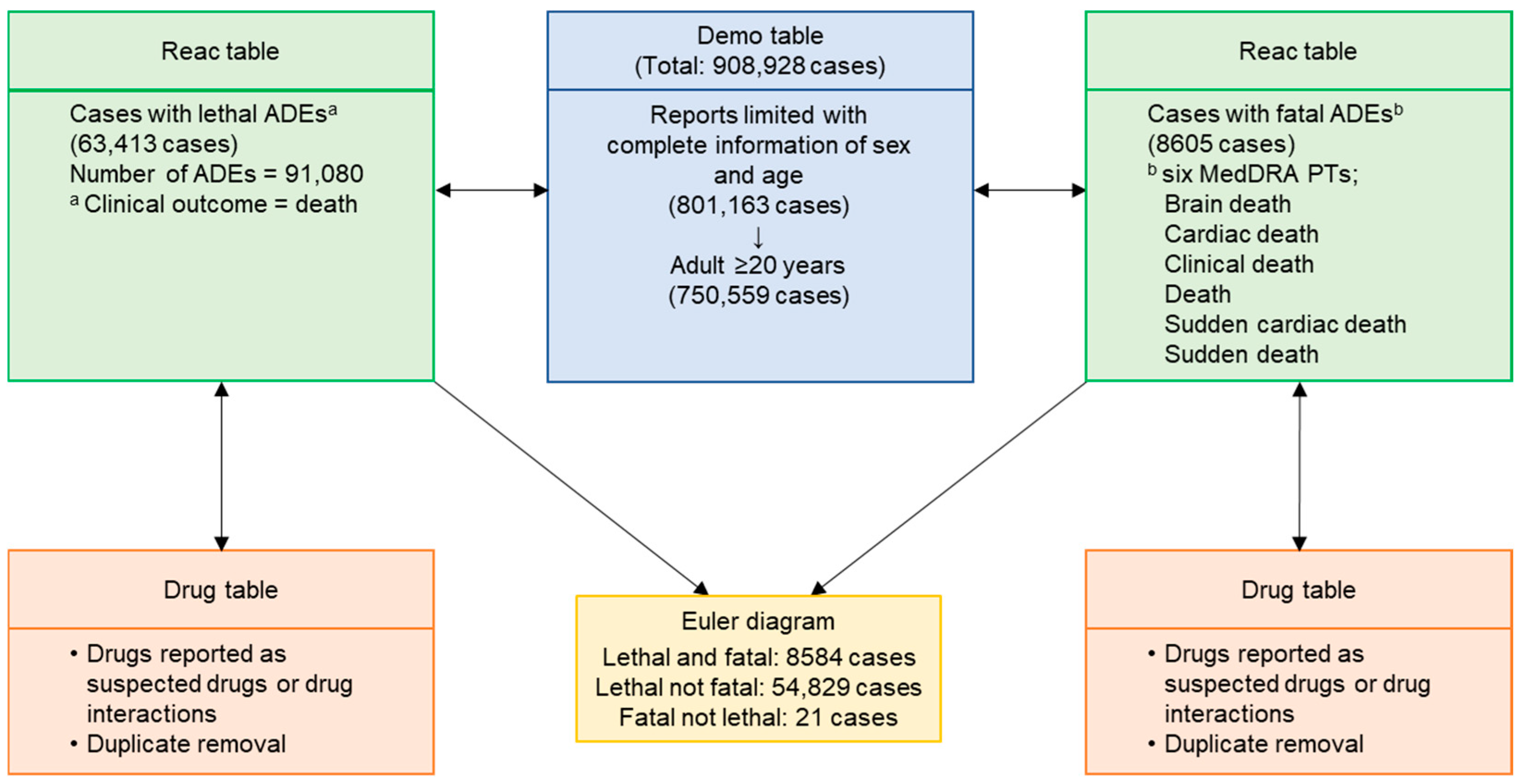
4. Materials and Methods
4.1. Data Source
4.2. Cases of Lethal or Fatal ADEs
4.3. Suspected Drugs in a Broad Sense
4.4. Data Pipeline
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, T.J.; Cohen, M.R.; Furberg, C.D. Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch. Intern. Med. 2007, 167, 1752–1759. [Google Scholar] [CrossRef]
- Bénard-Laribière, A.; Miremont-Salamé, G.; Pérault-Pochat, M.C.; Noize, P.; Haramburu, F.; EMIR Study Group on behalf of the French Network of Pharmacovigilance Centres. Incidence of hospital admissions due to adverse drug reactions in France: The EMIR study. Fundam. Clin. Pharmacol. 2015, 29, 106–111. [Google Scholar] [CrossRef]
- Giardina, C.; Cutroneo, P.M.; Mocciaro, E.; Russo, G.T.; Mandraffino, G.; Basile, G.; Rapisarda, F.; Ferrara, R.; Spina, E.; Arcoraci, V. Adverse drug reactions in hospitalized patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) study. Front. Pharmacol. 2018, 9, 350. [Google Scholar] [CrossRef]
- Patel, T.K.; Patel, P.B. Mortality among patients due to adverse drug reactions that lead to hospitalization: A meta-analysis. Eur. J. Clin. Pharmacol. 2018, 74, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, K.B.; Cheng, N.; Hansen, R.A. Serious adverse drug events reported to the FDA: Analysis of the FDA Adverse Event Reporting System 2006–2014 Database. J. Manag. Care Spec. Pharm. 2018, 24, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Montastruc, J.L.; Lafaurie, M.; de Canecaude, C.; Durrieu, G.; Sommet, A.; Montastruc, F.; Bagheri, H. Fatal adverse drug reactions: A worldwide perspective in the World Health Organization pharmacovigilance database. Br. J. Clin. Pharmacol. 2021, 87, 4334–4340. [Google Scholar] [CrossRef]
- Jo, H.G.; Jeong, K.; Ryu, J.Y.; Park, S.; Choi, Y.S.; Kwack, W.G.; Choi, Y.J.; Chung, E.K. Fatal events associated with adverse drug reactions in the Korean National Pharmacovigilance Database. J. Pers. Med. 2021, 12, 5. [Google Scholar] [CrossRef]
- Holm, L.; Ekman, E.; Jorsäter Blomgren, K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol. Drug Saf. 2017, 26, 335–343. [Google Scholar] [CrossRef]
- Watson, S.; Caster, O.; Rochon, P.A.; Den Ruijter, H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine 2019, 17, 100188. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Tanaka, H.; Endo, K.; Ishii, T. Influence of rapidly increased numbers of reports on adverse events of the COVID-19 vaccine in the Japanese pharmacovigilance database on disproportionality analysis of antineoplastic drug-associated adverse cardiovascular events. Expert Opin. Drug Saf. 2024, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Matsushima, M. The ecology of medical care during the COVID-19 pandemic in Japan: A nationwide survey. J. Gen. Intern. Med. 2022, 37, 1211–1217. [Google Scholar] [CrossRef]
- Kumagai, N. The impact of the COVID-19 pandemic on physician visits in Japan. Front. Public Health 2021, 9, 743371. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Iwagami, M.; Ishiguro, C.; Fujii, D.; Yamamoto, N.; Narisawa, M.; Tsuboi, T.; Umeda, H.; Kinoshita, N.; Iguchi, T.; et al. Safety monitoring of COVID-19 vaccines in Japan. Lancet Reg. Health West. Pac. 2022, 23, 100442. [Google Scholar] [CrossRef]
- Yamaoka, K.; Fujiwara, M.; Uchida, M.; Uesawa, Y.; Shimizu, T. The influence of the rapid increase in the number of adverse event reports for COVID-19 vaccine on the disproportionality analysis using JADER. In Vivo 2023, 37, 345–356. [Google Scholar] [CrossRef]
- Tanaka, H.; Takigawa, M.; Ide, N.; Ishii, T. Characteristics and patterns of adverse event reports in the Japanese Adverse Drug Event Report database over two decades (2004–2023): Exploring findings on sexes and age groups. Drug Discov. Ther. 2025, 19, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Bonniaud, P.; Rossi, G.; Sverzellati, N.; Cottin, V. Drug-induced interstitial lung disease. Eur. Respir. J. 2022, 60, 2102776. [Google Scholar] [CrossRef]
- Dimopoulou, I.; Bamias, A.; Lyberopoulos, P.; Dimopoulos, M.A. Pulmonary toxicity from novel antineoplastic agents. Ann. Oncol. 2006, 17, 372–379. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, S.; Ren, X.; Cui, W.; Wang, Y. Immune-related interstitial lung disease induced by different immune checkpoint inhibitors regimens: A real-world study from 2014 to 2022 based on FAERS databases. Eur. J. Pharmacol. 2023, 946, 175561. [Google Scholar] [CrossRef]
- Iwasa, E.; Fujiyoshi, Y.; Kubota, Y.; Kimura, R.; Chandler, R.E.; Taavola, H.; Norén, G.N.; Wakao, R. Interstitial lung disease as an adverse drug reaction in Japan: Exploration of regulatory actions as a basis for high reporting. Drug Saf. 2020, 43, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Blake, K.; Januskiene, J.; Yue, Q.Y.; Arlett, P. Geographical variation in reporting Interstitial Lung Disease as an adverse drug reaction: Findings from an European Medicines Agency analysis of reports in EudraVigilance. Pharmacoepidemiol. Drug Saf. 2016, 25, 705–712. [Google Scholar] [CrossRef]
- Sato, J.; Sadachi, R.; Koyama, T.; Katsuya, Y.; Okada, M.; Yamamoto, N. Regional diversity in drug-induced lung diseases among the USA, European Union, and Japan. Front. Med. 2024, 11, 1390083. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Jalali, R.K.; Vohora, D. Relevance of the Weber effect in contemporary pharmacovigilance of oncology drugs. Ther. Clin. Risk Manag. 2017, 13, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- MedDRA Maintenance and Support Services Organization. MedDRA® Term Selection: Points to Consider. ICH-Endorsed Guide for MedDRA Users, Release 4.24. March 2024. Available online: https://admin.meddra.org/sites/default/files/guidance/file/001006_termselptc_r4_24_mar2024.pdf (accessed on 21 September 2025).
- Hasegawa, S.; Ikesue, H.; Satake, R.; Inoue, M.; Yoshida, Y.; Tanaka, M.; Matsumoto, K.; Wakabayashi, W.; Oura, K.; Muroi, N.; et al. Osteonecrosis of the jaw caused by denosumab in treatment-naïve and pre-treatment with zoledronic acid groups: A time-to-onset study using the Japanese Adverse Drug Event Report (JADER) database. Drugs Real World Outcomes 2022, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J. Age standardization. IARC Sci. Publ. 2014, 164, 112–115. [Google Scholar]


| Fiscal Year | Rank | Suspected Drug | ATC Classification | Cases (Reporting Rate, %) |
|---|---|---|---|---|
| 2004–2008 (n = 9415) | 1 | Prednisolone | H | 510 (5.4) |
| 2 | Methotrexate | L | 461 (4.9) | |
| 3 | Gefitinib | L | 363 (3.9) | |
| 4 | Fluorouracil | L | 343 (3.6) | |
| 5 | Tacrolimus Hydrate | L | 325 (3.5) | |
| 6 | Cyclosporin | L | 322 (3.4) | |
| 7 | Tegafur/gimeracil/oteracil potassium | L | 313 (3.3) | |
| 8 | Cisplatin | L | 305 (3.2) | |
| 9 | Paclitaxel | L | 245 (2.6) | |
| 10 | Docetaxel hydrate | L | 227 (2.4) | |
| 2009–2013 (n = 12,246) | 1 | Prednisolone | H | 720 (5.9) |
| 2 | Methotrexate | L | 533 (4.4) | |
| 3 | Bevacizumab | L | 445 (3.6) | |
| 4 | Sorafenib tosilate | L | 433 (3.5) | |
| 5 | Tacrolimus Hydrate | L | 338 (2.8) | |
| 6 | Tegafur/gimeracil/oteracil potassium | L | 311 (2.5) | |
| 7 | Cyclosporin | L | 287 (2.3) | |
| 8 | Anti-human thymocyte immunoglobulin, rabbit | L | 281 (2.3) | |
| 9 | Fluorouracil | L | 277 (2.3) | |
| 10 | Cisplatin | L | 267 (2.2) | |
| 2014–2018 (n = 18,563) | 1 | Prednisolone | H | 1067 (5.7) |
| 2 | Nivolumab | L | 675 (3.6) | |
| 3 | Methotrexate | L | 667 (3.6) | |
| 4 | Pembrolizumab | L | 572 (3.1) | |
| 5 | Apixaban | B | 513 (2.8) | |
| 6 | Dexamethasone | H | 467 (2.5) | |
| 7 | Bevacizumab | L | 429 (2.3) | |
| 8 | Tacrolimus hydrate | L | 422 (2.3) | |
| 9 | Lenalidomide hydrate | L | 361 (1.9) | |
| 10 | Rivaroxaban | B | 352 (1.9) | |
| 2019–2023 (n = 23,189) | 1 | Coronavirus (SARS-CoV-2) RNA vaccine (COMIRNATY®) | J | 1863 (8.0) |
| 2 | Nivolumab | L | 1761 (7.6) | |
| 3 | Pembrolizumab | L | 1353 (5.8) | |
| 4 | Ipilimumab | L | 1134 (4.9) | |
| 5 | Prednisolone | H | 977 (4.2) | |
| 6 | Carboplatin | L | 622 (2.7) | |
| 7 | Bevacizumab | L | 602 (2.6) | |
| 8 | Roxadustat | B | 569 (2.5) | |
| 9 | Methotrexate | L | 542 (2.3) | |
| 10 | Atezolizumab | L | 537 (2.3) | |
| 11 a | Dexamethasone | H | 472 (2.0) |
| Fiscal Year | Rank | Suspected Drug | ATC Classification | Cases (Reporting Rate, %) |
|---|---|---|---|---|
| 2004–2008 (n = 510) | 1 | Valsartan | C | 26 (5.1) |
| 2 | Donepezil hydrochloride | N | 23 (4.5) | |
| 3 | Risperidone | N | 19 (3.7) | |
| 4 | Aripiprazole | N | 17 (3.3) | |
| 5 | Olanzapine | N | 16 (3.1) | |
| 5 | Oseltamivir phosphate | J | 16 (3.1) | |
| 7 | Carvedilol | C | 13 (2.5) | |
| 7 | Etanercept | L | 13 (2.5) | |
| 7 | Telmisartan | C | 13 (2.5) | |
| 10 | Ribavirin | J | 12 (2.4) | |
| 10 | Flunitrazepam | N | 12 (2.4) | |
| 2009–2013 (n = 1007) | 1 | Sorafenib tosilate | L | 35 (3.5) |
| 2 | Risperidone | N | 28 (2.8) | |
| 3 | Emulsified influenza HA vaccine (A/H1N1) | J | 27 (2.7) | |
| 4 | Darbepoetin alfa | B | 20 (2.0) | |
| 5 | Valsartan | C | 18 (1.8) | |
| 5 | Tocilizumab | L | 18 (1.8) | |
| 7 | Fludarabine phosphate | L | 17 (1.7) | |
| 8 | Olanzapine | N | 16 (1.6) | |
| 8 | Lamotrigine | N | 16 (1.6) | |
| 8 | Epoetin beta pegol | B | 16 (1.6) | |
| 8 | Donepezil hydrochloride | N | 16 (1.6) | |
| 2014–2018 (n = 2394) | 1 | Pembrolizumab | L | 82 (3.4) |
| 1 | Imatinib mesilate | L | 82 (3.4) | |
| 3 | Nivolumab | L | 76 (3.2) | |
| 4 | Lenalidomide hydrate | L | 63 (2.6) | |
| 5 | Dexamethasone | H | 57 (2.4) | |
| 6 | Risperidone | N | 53 (2.2) | |
| 7 | Deferasirox | V | 52 (2.2) | |
| 8 | Paliperidone palmitate | N | 50 (2.1) | |
| 9 | Darbepoetin alfa | B | 48 (2.0) | |
| 10 | Apixaban | B | 46 (1.9) | |
| 2019–2023 (n = 4694) | 1 | Nivolumab | L | 539 (11.5) |
| 2 | Ipilimumab | L | 397 (8.5) | |
| 3 | Coronavirus (SARS-CoV-2) RNA vaccine (COMIRNATY®) | J | 327 (7.0) | |
| 4 | Pembrolizumab | L | 224 (4.8) | |
| 5 | Lenalidomide hydrate | L | 213 (4.5) | |
| 6 | Bevacizumab | L | 162 (3.5) | |
| 7 | Venetoclax | L | 158 (3.4) | |
| 8 | Atezolizumab | L | 124 (2.6) | |
| 9 | Roxadustat | B | 108 (2.3) | |
| 10 | Durvalumab | L | 107 (2.3) | |
| 11 a | Pomalidomide | L | 105 (2.2) |
| Rank | Adverse Drug Event (PT Code) | Cases (Reporting Rate, %) |
|---|---|---|
| 1 | Death (10011906) | 7232 (11.4) |
| 2 | Interstitial lung disease (10022611) | 5900 (9.3) |
| 3 | Pneumonia (10035664) | 2939 (4.6) |
| 4 | Sepsis (10040047) | 2103 (3.3) |
| 5 | Cerebral hemorrhage (10008111) | 1499 (2.4) |
| 6 | Multiple organ dysfunction syndrome (10077361) | 1434 (2.3) |
| 7 | Cardiac failure (10007554) | 1298 (2.0) |
| 8 | Malignant neoplasm progression (10051398) | 1265 (2.0) |
| 9 | Respiratory failure (10038695) | 1250 (2.0) |
| 10 | Disseminated intravascular coagulation (10013442) | 1242 (2.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, H.; Ishii, T. Fact-Finding Survey of Lethal or Fatal Adverse Drug Events in the Japanese Adverse Drug Event Report Database, Fiscal Year 2004–2023 (Adults ≥ 20 Years). Pharmacoepidemiology 2025, 4, 19. https://doi.org/10.3390/pharma4040019
Tanaka H, Ishii T. Fact-Finding Survey of Lethal or Fatal Adverse Drug Events in the Japanese Adverse Drug Event Report Database, Fiscal Year 2004–2023 (Adults ≥ 20 Years). Pharmacoepidemiology. 2025; 4(4):19. https://doi.org/10.3390/pharma4040019
Chicago/Turabian StyleTanaka, Hiroyuki, and Toshihiro Ishii. 2025. "Fact-Finding Survey of Lethal or Fatal Adverse Drug Events in the Japanese Adverse Drug Event Report Database, Fiscal Year 2004–2023 (Adults ≥ 20 Years)" Pharmacoepidemiology 4, no. 4: 19. https://doi.org/10.3390/pharma4040019
APA StyleTanaka, H., & Ishii, T. (2025). Fact-Finding Survey of Lethal or Fatal Adverse Drug Events in the Japanese Adverse Drug Event Report Database, Fiscal Year 2004–2023 (Adults ≥ 20 Years). Pharmacoepidemiology, 4(4), 19. https://doi.org/10.3390/pharma4040019






