Timing Matters: Exploring the Role of the Time to Onset in Recall Bias for Adverse Events Following Immunization (AEFIs) of COVID-19 Vaccines from Spontaneous Reports
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics
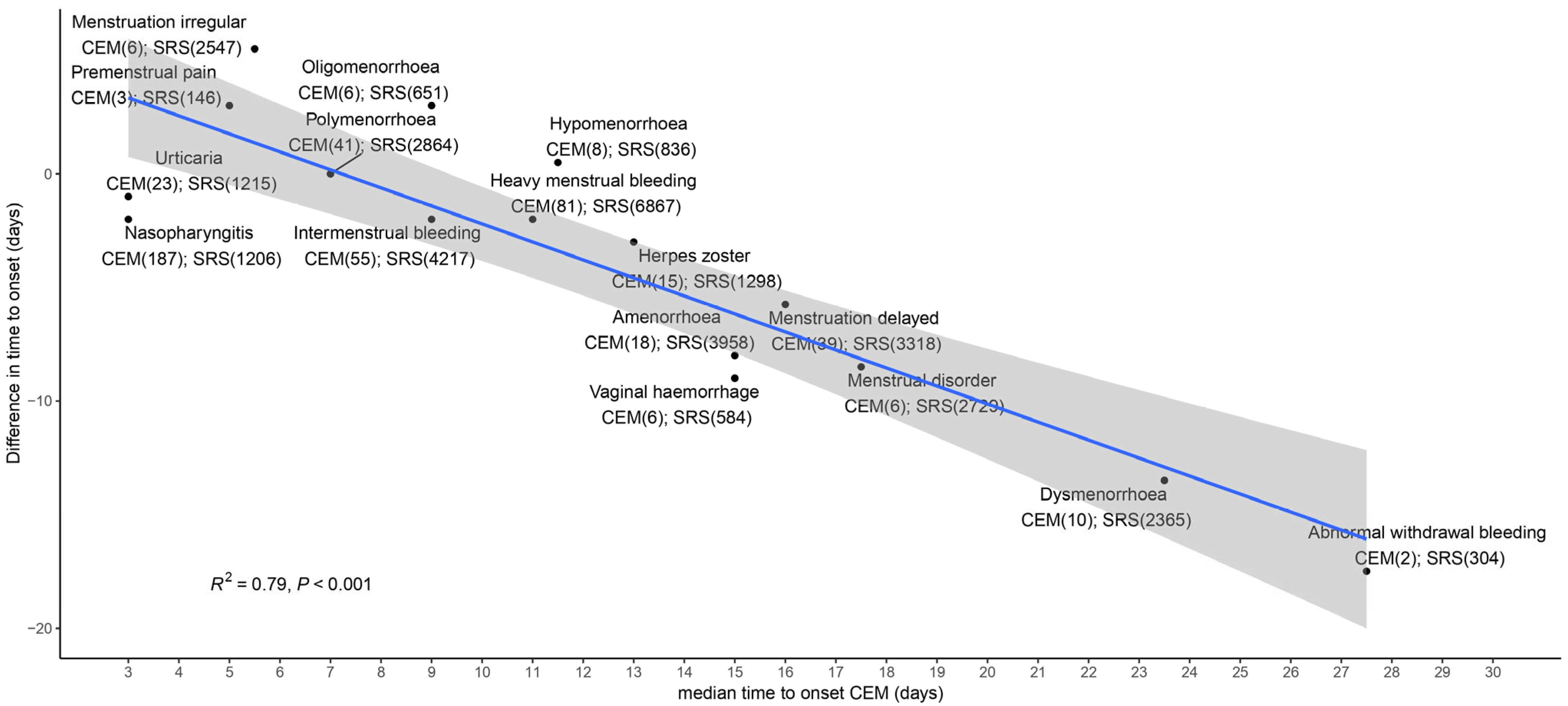
2.2. TTO Analyses (All AEFIs)
2.3. Effect of Media Attention (Menstrual Disorders Only)
3. Discussion
4. Methods
4.1. Data Selection
4.2. Variable Selection
- Solicited vs. unsolicited AEFIs. Solicited AEFIs were those specifically mentioned in the reporting forms and questionnaires and could be selected. Unsolicited reports were those that could be reported in open text-fields and subsequently coded (see Supplementary Table S1 for details).
- Vaccination dose number (1 vs. 2). Since no information on subsequent vaccination dose numbers was available for the CEM studies, only these two were included from the spontaneous reports.
- Perceived burden of the AEFI (high vs. low).
4.3. TTO Analysis
4.4. Effect of Media Attention, Menstrual Disorders as an Example
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| AEFI | Adverse event following immunization |
| CEM | Cohort event monitoring |
| ICSR | Individual case safety reports |
| SRS | Spontaneous reporting system |
| TTO | Time to onset |
References
- Lester, J.; Neyarapally, G.A.; Lipowski, E.; Graham, C.F.; Hall, M.; Dal, P.G. Evaluation of FDA safety-related drug label changes in 2010. Pharmacoepidemiol. Drug Saf. 2013, 22, 302–305. [Google Scholar] [CrossRef]
- Montane, E.; Santesmases, J. Characteristics of drug safety alerts issued by the Spanish Medicines Agency. Front. Pharmacol. 2023, 14, 1090707. [Google Scholar] [CrossRef]
- European Medicines Agency. Safety Signal. Available online: https://www.ema.europa.eu/en/glossary/safety-signal#:~:text=Information%20on%20a%20new%20or,studies%20and%20the%20scientific%20literature (accessed on 10 November 2023).
- Rudolph, A.; Mitchell, J.; Barrett, J.; Skold, H.; Taavola, H.; Erlanson, N.; Melgarejo-Gonzalez, C.; Yue, Q.Y. Global safety monitoring of COVID-19 vaccines: How pharmacovigilance rose to the challenge. Ther. Adv. Drug Saf. 2022, 13, 20420986221118972. [Google Scholar] [CrossRef]
- Rolfes, L.; Harmark, L.; Kant, A.; van Balveren, L.; Hilgersom, W.; van Hunsel, F. COVID-19 vaccine reactogenicity—A cohort event monitoring study in the Netherlands using patient reported outcomes. Vaccine 2022, 40, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.; Jansen, J.; van Balveren, L.; van Hunsel, F. Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands. Drug Saf. 2022, 45, 319–331. [Google Scholar] [CrossRef]
- Duijster, J.W.; Lieber, T.; Pacelli, S.; Van Balveren, L.; Ruijs, L.S.; Raethke, M.; Kant, A.; Van Hunsel, F. Sex-disaggregated outcomes of adverse events after COVID-19 vaccination: A Dutch cohort study and review of the literature. Front. Immunol. 2023, 14, 1078736. [Google Scholar] [CrossRef]
- Ciccimarra, F.; Luxi, N.; Bellitto, C.; L’Abbate, L.; Raethke, M.; van Hunsel, F.; Lieber, T.; Mulder, E.; Riefolo, F.; Dureau-Pournin, C.; et al. Safety Monitoring of COVID-19 Vaccines in Persons with Prior SARS-CoV-2 Infection: A European Multi-Country Study. Vaccines 2024, 12, 241. [Google Scholar] [CrossRef]
- Luxi, N.; Ciccimarra, F.; Bellitto, C.; Raethke, M.; van Hunsel, F.; Lieber, T.; Mulder, E.; L’Abbate, L.; Marques, F.B.; Furci, F.; et al. Safety of COVID-19 Vaccines among People with History of Allergy: A European Active Surveillance Study. Vaccines 2024, 12, 1059. [Google Scholar] [CrossRef]
- Gee, J.; Shimabukuro, T.T.; Su, J.R.; Shay, D.; Ryan, M.; Basavaraju, S.V.; Broder, K.R.; Clark, M.; Buddy Creech, C.; Cunningham, F.; et al. Overview of U.S. COVID-19 vaccine safety surveillance systems. Vaccine 2024, 42 (Suppl. S3), 125748. [Google Scholar] [CrossRef]
- Hazell, L.; Shakir, S.A. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
- Pariente, A.; Gregoire, F.; Fourrier-Reglat, A.; Haramburu, F.; Moore, N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: The notoriety bias. Drug Saf. 2007, 30, 891–898. [Google Scholar] [CrossRef]
- de Boissieu, P.; Kanagaratnam, L.; Abou Taam, M.; Roux, M.P.; Drame, M.; Trenque, T. Notoriety bias in a database of spontaneous reports: The example of osteonecrosis of the jaw under bisphosphonate therapy in the French national pharmacovigilance database. Pharmacoepidemiol. Drug Saf. 2014, 23, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, N.R.; Wilson, J.P. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004, 24, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.H.; Van Puijenbroek, E.P. The value of time-to-onset in statistical signal detection of adverse drug reactions: A comparison with disproportionality analysis in spontaneous reports from the Netherlands. Pharmacoepidemiol. Drug Saf. 2016, 25, 1361–1367. [Google Scholar] [CrossRef]
- Scholl, J.H.G.; van Hunsel, F.; Hak, E.; van Puijenbroek, E.P. Time to onset in statistical signal detection revisited: A follow-up study in long-term onset adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2019, 28, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- van Holle, L.; Bauchau, V. Signal detection on spontaneous reports of adverse events following immunisation: A comparison of the performance of a disproportionality-based algorithm and a time-to-onset-based algorithm. Pharmacoepidemiol. Drug Saf. 2014, 23, 178–185. [Google Scholar] [CrossRef]
- van Holle, L.; Tavares Da Silva, F.; Bauchau, V. Signal detection based on time-to-onset: Extending a new method from spontaneous reports to observational studies. Pharmacoepidemiol. Drug Saf. 2014, 23, 849–858. [Google Scholar] [CrossRef]
- van Holle, L.; Zeinoun, Z.; Bauchau, V.; Verstraeten, T. Using time-to-onset for detecting safety signals in spontaneous reports of adverse events following immunization: A proof of concept study. Pharmacoepidemiol. Drug Saf. 2012, 21, 603–610. [Google Scholar] [CrossRef]
- Norén, G.N.; Hopstadius, J.; Bate, A.; Star, K.; Edwards, I.R. Temporal pattern discovery in longitudinal electronic patient records. Data Min. Knowl. Discov. 2010, 20, 361–387. [Google Scholar] [CrossRef]
- Cornelius, V.R.; Sauzet, O.; Evans, S.J. A signal detection method to detect adverse drug reactions using a parametric time-to-event model in simulated cohort data. Drug Saf. 2012, 35, 599–610. [Google Scholar] [CrossRef]
- Oosterhuis, I.; Scholl, J.; van Puijenbroek, E.; Kant, A.; van Hunsel, F. Optimizing Safety Surveillance for COVID-19 Vaccines at the National Pharmacovigilance Centre Lareb: One Year of COVID-19 Vaccine Experience. Drug Saf. 2023, 46, 65–75. [Google Scholar] [CrossRef]
- Gordillo-Maranon, M.; Szmigiel, A.; Yalmanova, V.; Caplanusi, I.; Genov, G.; Olsen, D.B.; Straus, S. COVID-19 Vaccines and Heavy Menstrual Bleeding: The Impact of Media Attention on Reporting to EudraVigilance. Drug Saf. 2024, 47, 783–798. [Google Scholar] [CrossRef]
- Duijster, J.W.; Schoep, M.E.; Nieboer, T.E.; Jajou, R.; Kant, A.; van Hunsel, F. Menstrual abnormalities after COVID-19 vaccination in the Netherlands: A description of spontaneous and longitudinal patient-reported data. Br. J. Clin. Pharmacol. 2023, 89, 3126–3138. [Google Scholar] [CrossRef] [PubMed]
- Raethke, M.; van Hunsel, F.; Luxi, N.; Lieber, T.; Bellitto, C.; Mulder, E.; Ciccimarra, F.; Riefolo, F.; Thurin, N.H.; Roy, D.; et al. Frequency and timing of adverse reactions to COVID-19 vaccines; A multi-country cohort event monitoring study. Vaccine 2024, 42, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Herve, C.; Laupeze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Kant, A.; van Hunsel, F. Authors’ Reply to Mungmunpuntipantip et al.’s Comment on “Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands”. Drug Saf. 2022, 45, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Comment on “Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands”. Drug Saf. 2022, 45, 923. [Google Scholar] [CrossRef]
- Male, V. Menstrual changes after COVID-19 vaccination. BMJ 2021, 374, n2211. [Google Scholar] [CrossRef]
- Jajou, R.; Lieber, T.; van Puijenbroek, E.P.; Mulder, E.; Overbeek, J.; Hek, K.; van Hunsel, F.; Kant, A. GP consultations for menstrual disorders after COVID-19 vaccination—A self-controlled cohort study based on routine healthcare data from the Netherlands. Vaccine 2024, 42, 126130. [Google Scholar] [CrossRef]
- Smaardijk, V.R.; Jajou, R.; Kant, A.; van Hunsel, F.P.A.M. Menstrual disorders following COVID-19 vaccination: A review using a systematic search. Front. Drug Saf. Regul. 2024, 4, 1338466. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (RIVM). COVID-19. Available online: https://www.rivm.nl/en/coronavirus-covid-19 (accessed on 24 March 2025).
- Huisman, C. Percentage Gevaccineerden Dat Data Wil Delen Met RIVM Daalt Onder de Kritische Grens van 95 Procent. Available online: https://www.volkskrant.nl/nieuws-achtergrond/percentage-gevaccineerden-dat-data-wil-delen-met-rivm-daaltonder-de-kritische-grens-van-95-procentbbe45557/ (accessed on 24 March 2024).
- Rolfes, L.; Haaksman, M.; van Hunsel, F.; van Puijenbroek, E. Insight into the Severity of Adverse Drug Reactions as Experienced by Patients. Drug Saf. 2020, 43, 291–293. [Google Scholar] [CrossRef]


| SRS | CEM | |
|---|---|---|
| Number of reports | 160,613 | 26,730 * |
| Vaccination dose number 1 | 103,127 (64.2%) | 18,177 (68.0%) |
| Number of AEFIs | 755,647 | 103,703 |
| Solicited | 696,965 (92.2%) | 101,466 (97.8%) |
| High burden | 202,399 (26.8%) | 13,910 (13.4%) |
| Median TTO in days (IQR) | 1 (0.1–1) | 1 (0–1) |
| SRS | |
|---|---|
| Number of reports | 22,296 |
| Number of AEFIs | 30,016 |
| Before media attention | 702 (2.4%) |
| During media attention | 29,314 (97.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholl, J.; van Hunsel, F.; van Puijenbroek, E. Timing Matters: Exploring the Role of the Time to Onset in Recall Bias for Adverse Events Following Immunization (AEFIs) of COVID-19 Vaccines from Spontaneous Reports. Pharmacoepidemiology 2025, 4, 8. https://doi.org/10.3390/pharma4020008
Scholl J, van Hunsel F, van Puijenbroek E. Timing Matters: Exploring the Role of the Time to Onset in Recall Bias for Adverse Events Following Immunization (AEFIs) of COVID-19 Vaccines from Spontaneous Reports. Pharmacoepidemiology. 2025; 4(2):8. https://doi.org/10.3390/pharma4020008
Chicago/Turabian StyleScholl, Joep, Florence van Hunsel, and Eugene van Puijenbroek. 2025. "Timing Matters: Exploring the Role of the Time to Onset in Recall Bias for Adverse Events Following Immunization (AEFIs) of COVID-19 Vaccines from Spontaneous Reports" Pharmacoepidemiology 4, no. 2: 8. https://doi.org/10.3390/pharma4020008
APA StyleScholl, J., van Hunsel, F., & van Puijenbroek, E. (2025). Timing Matters: Exploring the Role of the Time to Onset in Recall Bias for Adverse Events Following Immunization (AEFIs) of COVID-19 Vaccines from Spontaneous Reports. Pharmacoepidemiology, 4(2), 8. https://doi.org/10.3390/pharma4020008






