DARE-SAFE: Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation in Pharmaceuticals and Vaccines
Abstract
1. Introduction
1.1. The Problem of Inferring Rates in the Vaccine Adverse Event Reporting System
1.2. Pharmacovigilance Reporting for Pharmaceutical Drugs
2. Results
2.1. VAERS Reporting Rates by Vaccine Type
2.2. VAERS Reporting Rates by COVID-19 Vaccine Manufacturer
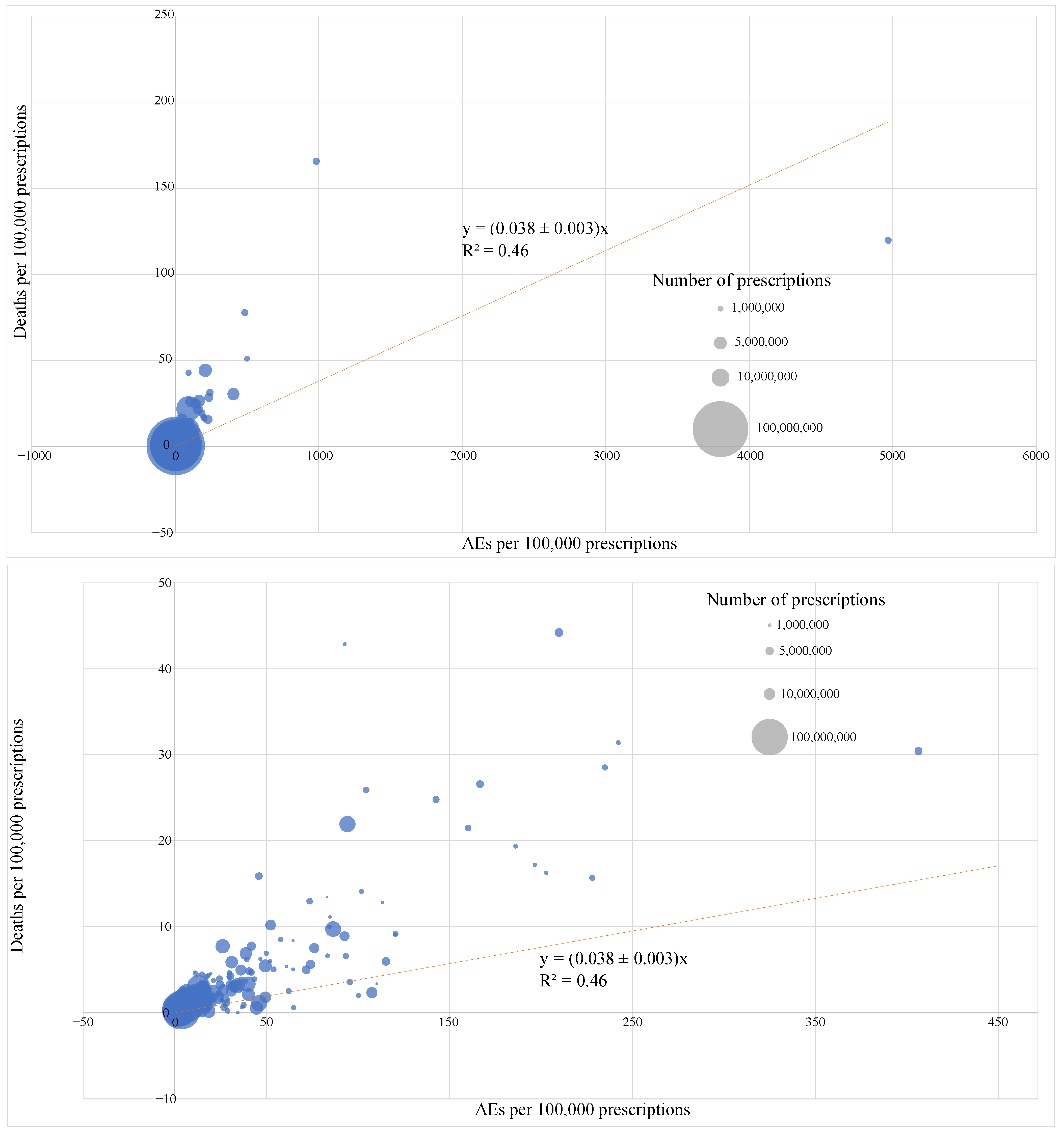
2.3. FAERS Reporting Rates for 250 Most Prescribed Pharmaceuticals in 2022
3. Discussion
4. Methods
4.1. VAERS Reporting Rates by Vaccine Type
4.2. VAERS Reporting Rate by COVID-19 Vaccine Manufacturer
4.3. FAERS Reporting Rates by Drug
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023, 46, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Kazmi, S.K.; Tahir, M.J.; Ullah, I.; Royan, H.A.; Fahriani, M.; Nainu, F.; Rosa, S.G.V. Global Acceptance and Hesitancy of COVID-19 Vaccination: A Narrative Review. Narra J. 2021, 1, e57. [Google Scholar] [CrossRef] [PubMed]
- Staff, N. Strategies to Increase COVID-19 Vaccination Rates in Medicaid Enrollees: Considerations for State Leaders; NASHP: Washington, DC, USA, 2021. [Google Scholar]
- Medi-Cal COVID-19 Vaccine Incentive Program Evaluation Report August 29, 2021–March 6, 2022; California Department of Health Care Services: Sacramento, CA, USA, 2024.
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Moskalets, O.V.; B, M.O. Validity of medical exemptions from vaccination in persons with allergic disorders. Russ. J. Immunol. 2024, 27, 667–672. [Google Scholar] [CrossRef]
- Arlegui, H.; Bollaerts, K.; Salvo, F.; Bauchau, V.; Nachbaur, G.; Bégaud, B.; Praet, N. Benefit–Risk Assessment of Vaccines. Part I: A Systematic Review to Identify and Describe Studies About Quantitative Benefit–Risk Models Applied to Vaccines. Drug Saf. 2020, 43, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, N.; Schwarz, K.; Chan, I.; Kobau, R.; Alexander, R.; Kollar, L.; Rodriguez, L.; Mansergh, G.; Repetski, T.; Gandhi, P.; et al. COVID-19 Vaccine Hesitancy Among US Adults: Safety and Effectiveness Perceptions and Messaging to Increase Vaccine Confidence and Intent to Vaccinate. Public Health Rep. 2024, 139, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Decoteau, C.L.; Sweet, P.L. Vaccine Hesitancy and the Accumulation of Distrust. Social. Problems 2024, 71, 1231–1246. [Google Scholar] [CrossRef]
- SteelFisher, G.K.; Findling, M.G.; Caporello, H.L.; Stein, R.I. Has COVID-19 Threatened Routine Childhood Vaccination? Insights From US Public Opinion Polls. Health Aff. Forefr. 2023. [Google Scholar] [CrossRef]
- Putri, R.A.; Ikawati, Z.; Rahmawati, F.; Yasin, N.M. An Awareness of Pharmacovigilance Among Healthcare Professionals Due to an Underreporting of Adverse Drug Reactions Issue: A Systematic Review of the Current State, Obstacles, and Strategy. Curr. Drug Saf. 2024, 19, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J. In Silico Prediction of Drug Side Effects. In Antitargets and Drug Safety; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 19–44. ISBN 978-3-527-67364-3. [Google Scholar]
- Skardal, A.; Aleman, J.; Forsythe, S.; Rajan, S.; Murphy, S.; Devarasetty, M.; Pourhabibi Zarandi, N.; Nzou, G.; Wicks, R.; Sadri-Ardekani, H.; et al. Drug Compound Screening in Single and Integrated Multi-Organoid Body-on-a-Chip Systems. Biofabrication 2020, 12, 025017. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti-Montano, B.; Demuru, G.; Laconi, E.; Musio, M. A Comparative Analysis on Serious Adverse Events Reported for COVID-19 Vaccines in Adolescents and Young Adults. Front. Public Health 2023, 11, 1145645. [Google Scholar] [CrossRef]
- FAQ. Available online: https://openvaers.com/ (accessed on 9 January 2025).
- Total COVID-19 Vaccine Doses Administered. Available online: https://ourworldindata.org/grapher/cumulative-covid-vaccinations?country=~USA (accessed on 13 January 2025).
- National Vaccine Injury Compensation Program Monthly Statistics Report; Health Resources and Services Administration: Rockville, MD, USA, 2025. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/vaccines/vicp-stats-01-01-25.pdf (accessed on 9 January 2025).
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Vaccinations. Our World Data 2020. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 9 January 2025).
- ClinCalc DrugStats Database. Available online: https://clincalc.com/DrugStats/ (accessed on 9 January 2025).
- US Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) Public Dashboard. FDA 2023. Available online: https://www.fda.gov/drugs/fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard (accessed on 9 January 2025).
- Lazar, V.; Zhang, B.; Magidi, S.; Le Tourneau, C.; Raymond, E.; Ducreux, M.; Bresson, C.; Raynaud, J.; Wunder, F.; Onn, A.; et al. A Transcriptomics Approach to Expand Therapeutic Options and Optimize Clinical Trials in Oncology. Ther. Adv. Med. Oncol. 2023, 15, 17588359231156382. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, B.; Fakri, F.; Odigie, J.; Nnabuife, L.; Isitua, C.C.; Chiari, W. Chemometric-Empowered Spectroscopic Techniques in Pharmaceutical Fields: A Bibliometric Analysis and Updated Review. Narra X 2023, 1, 1–16. [Google Scholar] [CrossRef]
- Lima, B.S.; Videira, M.A. Toxicology and Biodistribution: The Clinical Value of Animal Biodistribution Studies. Mol. Ther. Methods Clin. Dev. 2018, 8, 183–197. [Google Scholar] [CrossRef]
- CDC. Accessing and Using Data from the Vaccine Safety Datalink (VSD). Vaccine Safety Systems. 2024. Available online: https://www.cdc.gov/vaccine-safety-systems/vsd/access-use.html (accessed on 15 March 2025).
- Thompson, W.W.; Price, C.; Goodson, B.; Shay, D.K.; Benson, P.; Hinrichsen, V.L.; Lewis, E.; Eriksen, E.; Ray, P.; Vaccine Safety Datalink Team; et al. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N. Engl. J. Med. 2007, 357, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Price, C.S.; Thompson, W.W.; Goodson, B.; Weintraub, E.S.; Croen, L.A.; Hinrichsen, V.L.; Marcy, M.; Robertson, A.; Eriksen, E.; Lewis, E.; et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010, 126, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Novavax COVID-19 Vaccine Emergency Use Authorization. 2022. Available online: https://www.fda.gov/media/159902/download (accessed on 2 February 2025).
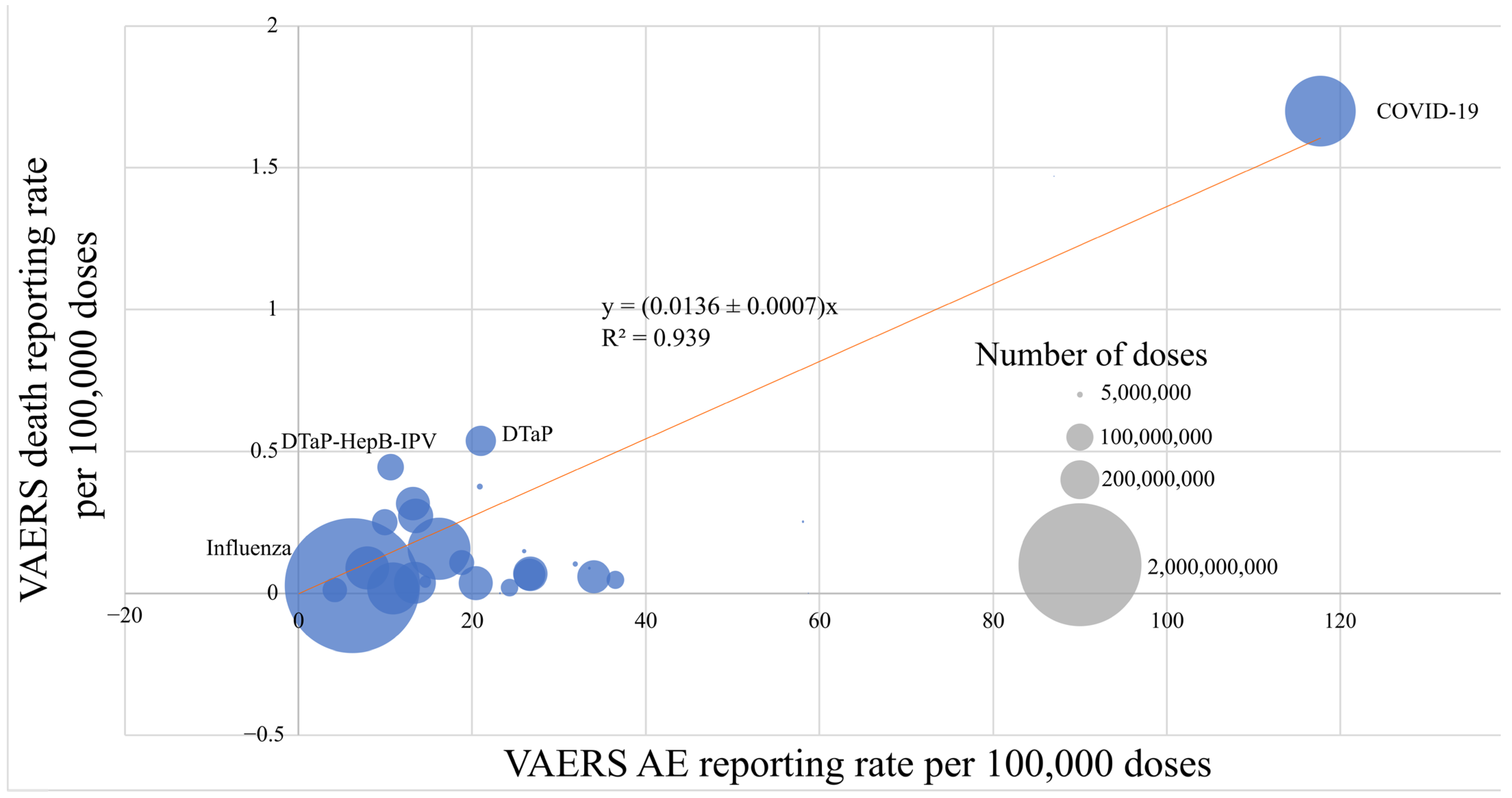
| Vaccine Name | Number of Doses Distributed (2006–2022) | Number of VAERs Reports | VAERS Reporting Rate per 100,000 Doses | VAERS Deaths | VAERS Deaths Reporting Rate per 100,000 Doses |
|---|---|---|---|---|---|
| DT | 794,777 | 462 | 58.1 | 2 | 0.252 |
| DTaP | 122,237,653 | 25,629 | 21 | 656 | 0.537 |
| DTaP-Hep B-IPV | 94,331,585 | 9990 | 10.6 | 419 | 0.444 |
| DTaP-HiB | 1,135,474 | 380 | 33.5 | 1 | 0.0881 |
| DTaP-IPV | 40,456,384 | 9818 | 24.3 | 8 | 0.0198 |
| DTaP-IPV-HiB | 89,568,786 | 8906 | 9.94 | 224 | 0.250 |
| DTaP-IPV-HiB-Hep B | 2,021,770 | 526 | 26.0 | 3 | 0.148 |
| DTP | 0 | 556 | N/A | 3 | N/A |
| DTP-HiB | 0 | 57 | N/A | 2 | N/A |
| Hep A+Hep B | 19,811,507 | 2893 | 14.6 | 8 | 0.0404 |
| Hep B-HiB | 4,787,457 | 1000 | 20.9 | 18 | 0.376 |
| Hepatitis A (Hep A) | 231,034,565 | 30,930 | 13.4 | 85 | 0.0368 |
| Hepatitis B (Hep B) | 248,816,802 | 19,737 | 7.93 | 222 | 0.0892 |
| HiB | 159,451,493 | 21,526 | 13.5 | 435 | 0.273 |
| HPV | 158,878,541 | 42,464 | 26.7 | 109 | 0.0686 |
| Influenza | 2,407,000,000 | 149,512 | 6.21 | 650 | 0.0270 |
| IPV | 85,815,525 | 16,104 | 18.8 | 93 | 0.108 |
| Measles | 135,660 | 118 | 87.0 | 2 | 1.47 |
| Meningococcal | 152,565,553 | 31,050 | 20.4 | 54 | 0.0354 |
| MMR | 134,424,338 | 35,743 | 26.6 | 88 | 0.0655 |
| MMR-Varicella | 42,936,444 | 15,668 | 36.5 | 20 | 0.0466 |
| Mumps | 110,749 | 65 | 58.7 | 0 | 0 |
| OPV | 0 | 188 | N/A | 5 | N/A |
| Pneumococcal | 517,159,908 | 83,537 | 16.2 | 810 | 0.157 |
| Rotavirus | 150,866,652 | 19,899 | 13.2 | 476 | 0.316 |
| Rubella | 422,548 | 98 | 23.2 | 0 | 0 |
| Td | 79,443,263 | 3322 | 4.18 | 9 | 0.0113 |
| Tdap | 358,134,237 | 39,153 | 10.9 | 59 | 0.0165 |
| Tetanus | 3,838,993 | 1226 | 31.9 | 4 | 0.104 |
| Varicella | 143,906,028 | 48,863 | 34 | 84 | 0.0584 |
| COVID-19 | 663,000,000 * | 781,075 | 117.7 | 11,288 | 1.70 |
| COVID-19 Vaccine Manufacturer | Doses (Cumulative to 31 December 2022 in USA) | Number of VAERS Reports | Number of VAERS Death Reports | VAERS Reporting Rate per 100,000 Doses | VAERS Death Reporting Rate per 100,000 Doses |
|---|---|---|---|---|---|
| Pfizer/BioNTech | 395,801,679 | 398,648 | 6530 | 101 | 1.65 |
| Moderna | 248,752,253 | 371,774 | 6056 | 150 | 2.43 |
| Johnson & Johnson | 18,953,653 | 61,262 | 1093 | 323 | 5.77 |
| Novavax | 69,623 | 627 | 0 | 901 | 0 |
| Drug Name | Total Prescriptions (2022, Millions) | Total Patients (2022, Millions) | Total AEs | Serious AEs | Deaths | AEs per 100,000 Prescriptions | Serious AEs per 100,000 Prescriptions | Deaths per 100,000 Prescriptions | AEs per 100,000 Patients | Serious AEs per 100,000 Patients | Deaths per 100,000 Patients |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atorvastatin | 109.583 | 27.936 | 3834 | 3601 | 305 | 3.50 | 3.29 | 0.28 | 26.4 | 23.7 | 3.39 |
| Metformin | 86.748 | 19.536 | 5164 | 4631 | 663 | 5.95 | 5.34 | 0.76 | 12.6 | 6.83 | 1.41 |
| Lisinopril | 82.514 | 20.314 | 2564 | 1387 | 286 | 3.11 | 1.68 | 0.35 | 9.69 | 7.56 | 1.39 |
| Levothyroxine | 82.432 | 18.130 | 1756 | 1370 | 252 | 2.13 | 1.66 | 0.31 | 21.9 | 20.7 | 4.08 |
| Amlodipine | 70.766 | 17.790 | 3903 | 3682 | 726 | 5.52 | 5.20 | 1.03 | 15.2 | 13.5 | 2.57 |
| Metoprolol | 65.245 | 15.543 | 2360 | 2096 | 400 | 3.62 | 3.21 | 0.61 | 12.0 | 10.8 | 0.57 |
| Albuterol | 59.075 | 19.265 | 2305 | 2073 | 109 | 3.90 | 3.51 | 0.19 | 9.08 | 7.45 | 1.19 |
| Losartan | 53.556 | 13.150 | 1194 | 980 | 157 | 2.23 | 1.83 | 0.29 | 35.1 | 31.9 | 3.88 |
| Omeprazole | 52.133 | 13.802 | 4844 | 4405 | 536 | 9.29 | 8.45 | 1.03 | 53.2 | 42.0 | 12.1 |
| Gabapentin | 40.141 | 9.890 | 5263 | 4149 | 1195 | 13.1 | 10.3 | 2.98 | 26.4 | 23.7 | 3.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halma, M.; Varon, J. DARE-SAFE: Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation in Pharmaceuticals and Vaccines. Pharmacoepidemiology 2025, 4, 7. https://doi.org/10.3390/pharma4020007
Halma M, Varon J. DARE-SAFE: Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation in Pharmaceuticals and Vaccines. Pharmacoepidemiology. 2025; 4(2):7. https://doi.org/10.3390/pharma4020007
Chicago/Turabian StyleHalma, Matthew, and Joseph Varon. 2025. "DARE-SAFE: Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation in Pharmaceuticals and Vaccines" Pharmacoepidemiology 4, no. 2: 7. https://doi.org/10.3390/pharma4020007
APA StyleHalma, M., & Varon, J. (2025). DARE-SAFE: Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation in Pharmaceuticals and Vaccines. Pharmacoepidemiology, 4(2), 7. https://doi.org/10.3390/pharma4020007






