Signal Detection Study Focusing on Differences in the Drug Delivery System of Oral 5-Aminosalicylate for Inflammatory Bowel Disease Using the Japanese Pharmacovigilance Database
Abstract
1. Introduction
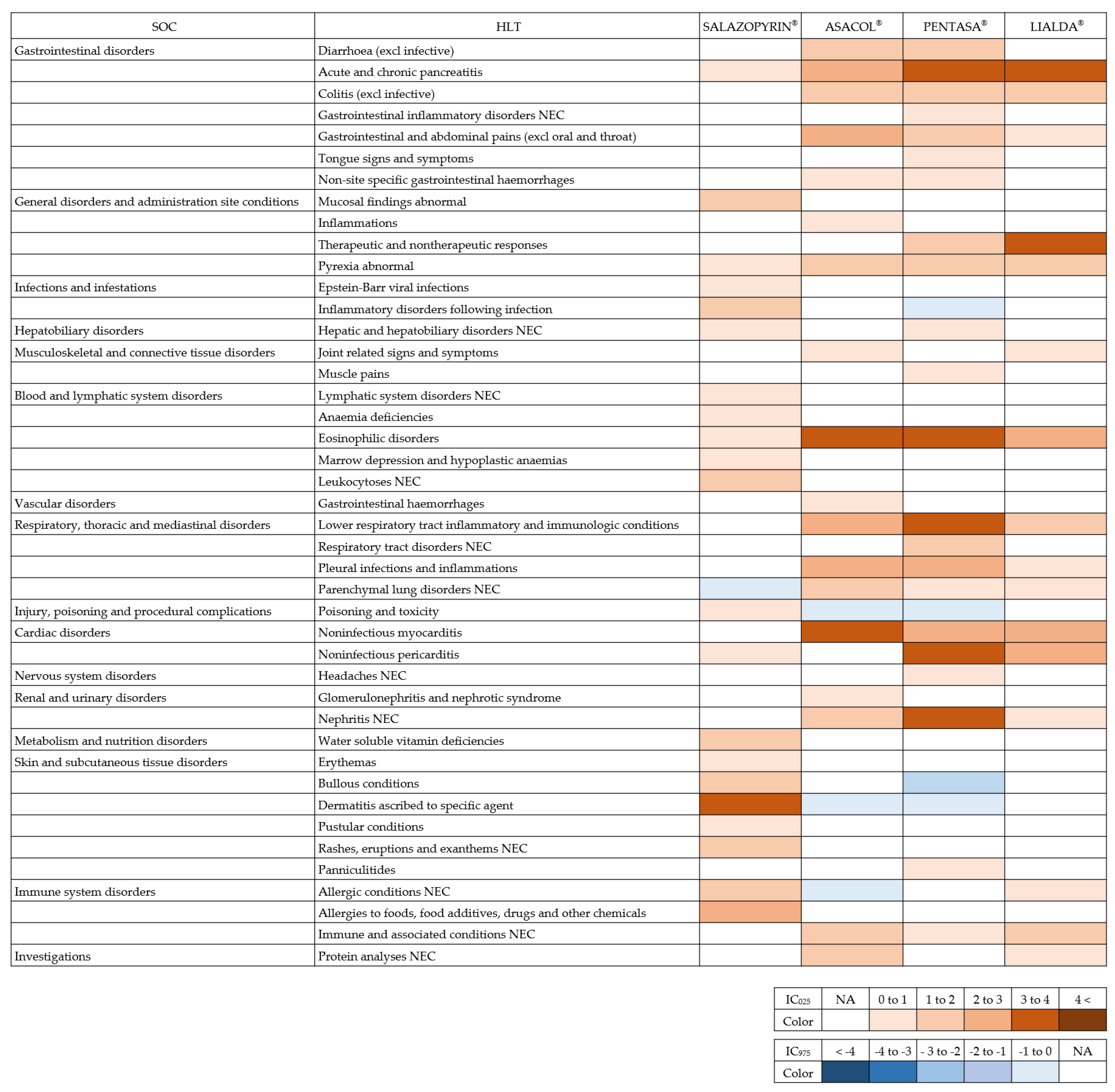
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Drugs and Adverse Events
4.3. Statistical Analysis
4.4. Analysis Software
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colombel, J.F.; Mahadevan, U. Inflammatory bowel disease 2017: Innovations and changing paradigms. Gastroenterology 2017, 152, 309–312. [Google Scholar] [CrossRef]
- Liu, T.C.; Stappenbeck, T.S. Genetics and pathogenesis of inflammatory bowel disease. Annu. Rev. Pathol. 2016, 11, 127–148. [Google Scholar] [CrossRef]
- Fumery, M.; Singh, S.; Dulai, P.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Sandborn, W. Natural history of adult ulcerative colitis in population-based cohorts: A systematic review. Clin. Gastroenterol. Hepatol. 2018, 16, 343–356. [Google Scholar] [CrossRef]
- Frieri, G.; Giacomelli, R.; Pimpo, M.; Palumbo, G.; Passacantando, A.; Pantaleoni, G.; Caprilli, R. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut 2000, 47, 410–414. [Google Scholar] [CrossRef]
- Pfizer. SALAZOPYRIN® Tablets 500 mg Interview Form, 8th ed.; Pfizer Japan Inc.: Tokyo, Japan, 2020; Available online: https://www.info.pmda.go.jp/go/interview/3/672212_6219001F1071_3_1F.pdf (accessed on 25 November 2022).
- ZERIA. ASACOL® Tablets 400 mg Interview Form, 9th ed.; ZERIA Pharmaceutical Co., Ltd.: Tokyo, Japan, 2018; Available online: https://www.info.pmda.go.jp/go/interview/1/380077_2399009F3028_1_010_1F.pdf (accessed on 25 November 2022).
- KYORIN. PENTASA® Tablets 250 mg, 500 mg, and Granules 94%, Interview Form, 24th ed.; KYORIN Pharmaceutical Co., Ltd.: Tokyo, Japan, 2020; Available online: https://www.info.pmda.go.jp/go/interview/1/230109_2399009F1149_1_024_1F.pdf (accessed on 25 November 2022).
- MOCHIDA. LIALDA® Tablets 1200 mg Interview Form, 7th ed.; MOCHIDA Pharmaceutical Co., Ltd.: Tokyo, Japan, 2020; Available online: https://www.info.pmda.go.jp/go/interview/1/790005_2399009F4024_1_M07_1F.pdf (accessed on 25 November 2022).
- Sato, M.; Hirata, J.; Shibahara, Y. Practice Examples of Academic Detailing—Prescription Support Based on Basic Pharmaceutical Science and Evidence. Yakugaku Zasshi 2019, 139, 1085–1089. [Google Scholar] [CrossRef]
- Noguchi, Y.; Esaki, H.; Murayama, A.; Sugioka, M.; Koyama, A.; Tachi, T.; Teramachi, H. Association between dipeptidyl peptidase-4 inhibitor and aspiration pneumonia: Disproportionality analysis using the spontaneous reporting system in Japan. Eur. J. Clin. Pharmacol. 2020, 76, 299–304. [Google Scholar] [CrossRef]
- Noguchi, Y.; Takaoka, M.; Hayashi, T.; Tachi, T.; Teramachi, H. Antiepileptic combination therapy with Stevens-Johnson syndrome and toxic epidermal necrolysis: Analysis of a Japanese pharmacovigilance database. Epilepsia 2020, 61, 1979–1989. [Google Scholar] [CrossRef]
- Suling, M.; Pigeot, I. Signal Detection and Monitoring Based on Longitudinal Healthcare Data. Pharmaceutics 2012, 4, 607–640. [Google Scholar] [CrossRef]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Review of Statistical Methodologies for Detecting Drug-Drug Interactions Using Spontaneous Reporting Systems. Front. Pharmacol. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Kinoshita, S.; Hosomi, K.; Yokoyama, S.; Takada, M. Inverse Association between Metformin and Amiodarone-Associated Extracardiac Adverse Events. Int. J. Med. Sci. 2020, 17, 302–309. [Google Scholar] [CrossRef]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Subset Analysis for Screening Drug–Drug Interaction Signal Using Pharmacovigilance Database. Pharmaceutics 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Aoyama, K.; Kubo, S.; Tachi, T.; Teramachi, H. Improved Detection Criteria for Detecting Drug-Drug Interaction Signals Using the Proportional Reporting Ratio. Pharmaceuticals 2021, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Noguchi, Y.; Otsubo, M.; Tachi, T.; Teramachi, H. Differences in detected safety signals between benzodiazepines and non-benzodiazepine hypnotics: Pharmacovigilance study using a spontaneous reporting system. Int. J. Med. Sci. 2021, 18, 1130–1136. [Google Scholar] [CrossRef]
- Ohyama, K.; Okamoto, T.; Hori, Y. Inverse association between DPP-4 inhibitor use and fracture in older adults: A disproportionality analysis of the FAERS and JADER. Int. J. Clin. Pharmacol. Ther. 2022, 61. [Google Scholar] [CrossRef]
- Khan, A.K.A.; Howes, D.T.; Piris, J.; Truelove, S.C. Optimum dose of sulphasalazine for maintenance treatment in ulcerative colitis. Gut 1980, 21, 232–240. [Google Scholar] [CrossRef]
- Kawai, S. Salazosulfapyridine. Nihon Naika Gakkai Zasshi 2011, 100, 2910–2917. [Google Scholar] [CrossRef]
- Suzuki, K.; Fujii, H.; Yamauchi, T.; Kato-Hayashi, H.; Ishihara, M.; Iihara, H.; Hirose, C.; Nishida, S.; Funato, M.; Kobayashi, R.; et al. Questionnaire survey to identify meal habits which influence adherence to oral 5-aminosalicylic acid regimens in patients with ulcerative colitis. J. Pharm. Pract. Res. 2021, 51, 374–380. [Google Scholar] [CrossRef]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform. 2021, 22, bbab347. [Google Scholar] [CrossRef]
- Weber, J. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. Adv. Inflamm. Res. 1984, 6, 1–7. [Google Scholar]
- Hartnell, N.R.; Wilson, J.P. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004, 24, 743–749. [Google Scholar] [CrossRef]
- Pariente, A.; Gregoire, F.; Fourrier-Reglat, A.; Haramburu, F.; Moore, N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: The notoriety bias. Drug Saf. 2007, 30, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Vogel, U.; van Stekelenborg, J.; Dreyfus, B.; Garg, A.; Habib, M.; Hosain, R.; Wisniewski, A. Investigating Overlap in Signals from EVDAS, FAERS, and VigiBase®. Drug Saf. 2020, 43, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, M.; Stahl, M.; Bate, A.; Edwards, I.R.; Meyboom, R.H.B. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 2000, 23, 533–542. [Google Scholar] [CrossRef] [PubMed]
| Drug | Structural Formula | Property |
|---|---|---|
| SALAZOPYRIN® | 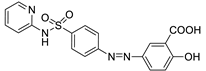 | 5-ASA and sulfapyridine are azo-bonded. |
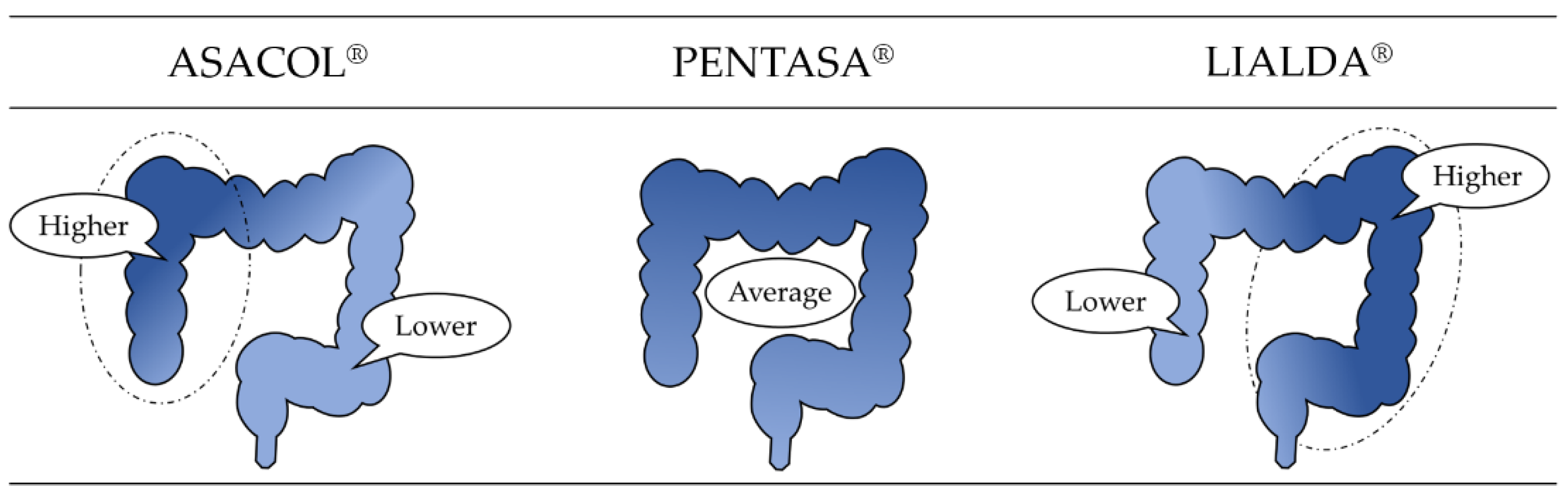
| ASACOL® | 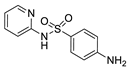 | Delayed release formulation dependent on pH. |
| PENTASA® | Delayed release formulation dependent on time. | |
| LIALDA® | Delayed release formulation dependent on pH and time. |
| Target AE | Other AEs | Total | |
|---|---|---|---|
| Target drug | N11 | N12 | N1+ |
| Other drugs | N21 | N22 | N2+ |
| Total | N+1 | N+2 | N++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, Y.; Yamashita, S.; Tamaki, H.; Osanai, A.; Ino, Y.; Tachi, T.; Iguchi, K.; Teramachi, H. Signal Detection Study Focusing on Differences in the Drug Delivery System of Oral 5-Aminosalicylate for Inflammatory Bowel Disease Using the Japanese Pharmacovigilance Database. Pharmacoepidemiology 2023, 2, 26-34. https://doi.org/10.3390/pharma2010003
Noguchi Y, Yamashita S, Tamaki H, Osanai A, Ino Y, Tachi T, Iguchi K, Teramachi H. Signal Detection Study Focusing on Differences in the Drug Delivery System of Oral 5-Aminosalicylate for Inflammatory Bowel Disease Using the Japanese Pharmacovigilance Database. Pharmacoepidemiology. 2023; 2(1):26-34. https://doi.org/10.3390/pharma2010003
Chicago/Turabian StyleNoguchi, Yoshihiro, Shuji Yamashita, Hirofumi Tamaki, Arihiro Osanai, Yoko Ino, Tomoya Tachi, Kazuhiro Iguchi, and Hitomi Teramachi. 2023. "Signal Detection Study Focusing on Differences in the Drug Delivery System of Oral 5-Aminosalicylate for Inflammatory Bowel Disease Using the Japanese Pharmacovigilance Database" Pharmacoepidemiology 2, no. 1: 26-34. https://doi.org/10.3390/pharma2010003
APA StyleNoguchi, Y., Yamashita, S., Tamaki, H., Osanai, A., Ino, Y., Tachi, T., Iguchi, K., & Teramachi, H. (2023). Signal Detection Study Focusing on Differences in the Drug Delivery System of Oral 5-Aminosalicylate for Inflammatory Bowel Disease Using the Japanese Pharmacovigilance Database. Pharmacoepidemiology, 2(1), 26-34. https://doi.org/10.3390/pharma2010003








