Azygos Lobe in a 38-Year-Old Male Donor Diagnosed with Ogilvie’s Syndrome
Abstract
1. Introduction
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Levitzky, M.G. Pulmonary Physiology, 10th ed.; McGraw Hill Medical: New York, NY, USA, 2022. [Google Scholar]
- West, J.B.; Luks, A. West’s Respiratory Physiology: The Essentials, 11th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021. [Google Scholar]
- Suresh, K.; Shimoda, L.A. Lung Circulation. Compr. Physiol. 2016, 6, 897–943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cudkowicz, L.; Armstrong, J.B. Observations on the normal anatomy of the bronchial arteries. Thorax 1951, 6, 343–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyden, E.A. The distribution of bronchi in gross anomalies of the right upper lobe particularly lobes subdivided by the azygos vein and those containing pre-eparterial bronchi. Radiology 1952, 58, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Al-Mnayyis, A.; Al-Alami, Z.; Altamimi, N.; Alawneh, K.Z.; Aleshawi, A. Azygos Lobe: Prevalence of an Anatomical Variant and Its Recognition Among Postgraduate Physicians. Diagnostics 2020, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Xu, Y.; Liu, L. Pulmonary azygous lobe. QJM 2018, 111, 137. [Google Scholar] [CrossRef] [PubMed]
- Melnick, S.; Loynd, R.; Khateeb, D. Azygous lobe: A normal variant of pulmonary anatomy. J. Community Hosp. Intern. Med. Perspect. 2016, 6, 33776. [Google Scholar] [CrossRef] [PubMed]
- Yurasakpong, L.; Yammine, K.; Limpanuparb, T.; Janta, S.; Chaiyamoon, A.; Kruepunga, N.; Meemon, K.; Suwannakhan, A. The prevalence of the azygos lobe: A meta-analysis of 1,033,083 subjects. Clin. Anat. 2021, 34, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Mata, J.; Cáceres, J.; Alegret, X.; Coscojuela, P.; De Marcos, J.A. Imaging of the azygos lobe: Normal anatomy and variations. AJR Am. J. Roentgenol. 1991, 156, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Hikspoors, J.P.; Soffers, J.H.; Mekonen, H.K.; Cornillie, P.; Köhler, S.E.; Lamers, W.H. Development of the human infrahepatic inferior caval and azygos venous systems. J. Anat. 2015, 226, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, J.E.; Godwin, J.D. Left azygos lobe. Radiology 1989, 171, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Stibbe, E.P. The Accessory Pulmonary Lobe of the Vena Azygos. J. Anat. 1919, 53 Pt 4, 305–314. [Google Scholar] [PubMed] [PubMed Central]
- Rasband, W.S. ImageJ Version 1.51q; U.S. National Institutes of Health: Bethesda, MD, USA, 2018; Available online: https://imagej.net/ij/ (accessed on 1 June 2025).
- Godwin, J.D.; Tarver, R.D. Accessory fissures of the lung. AJR Am. J. Roentgenol. 1985, 144, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kalchiem-Dekel, O.; Galvin, J.R.; Burke, A.P.; Atamas, S.P.; Todd, N.W. Interstitial Lung Disease and Pulmonary Fibrosis: A Practical Approach for General Medicine Physicians with Focus on the Medical History. J. Clin. Med. 2018, 7, 476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, C.T.; Miao, K.H.; Lui, F. Anatomy, Thorax, Lung Azygos Lobe. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518977/ (accessed on 1 June 2025).
- Baumgartner, F.J. Thoracoscopic surgery for hyperhidrosis in the presence of congenital azygous lobe and its suspensory web. Tex. Heart Inst. J. 2009, 36, 44–47. [Google Scholar] [PubMed]
- Gill, A.J.; Cavanagh, S.P.; Gough, M.J. The azygos lobe: An anatomical variant encountered during thoracoscopic sympathectomy. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lemme, J.D.; Small, J.E. Cervical lung herniation of the azygous lobe: A case report and literature review. Surg. Radiol. Anat. 2024, 46, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Gorenstein, L.A.; Putnam, J.B., Jr. Resection of pulmonary metastasis to the azygous lobe from a malignant fibrous histiocytoma. Chest 1992, 101, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Maloney, N.; Vargas, H.D. Acute intestinal pseudo-obstruction (Ogilvie’s syndrome). Clin. Colon Rectal Surg. 2005, 18, 96–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dewey, J.; Prahlow, J.A. Acute colonic pseudo-obstruction (Ogilvie syndrome) leading to respiratory compromise and death. J. Forensic Sci. 2021, 66, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Tarry, D.; Powell, M. Hypoxic Pulmonary Vasoconstriction. BJA Educ. 2017, 6, 208–213. [Google Scholar] [CrossRef]
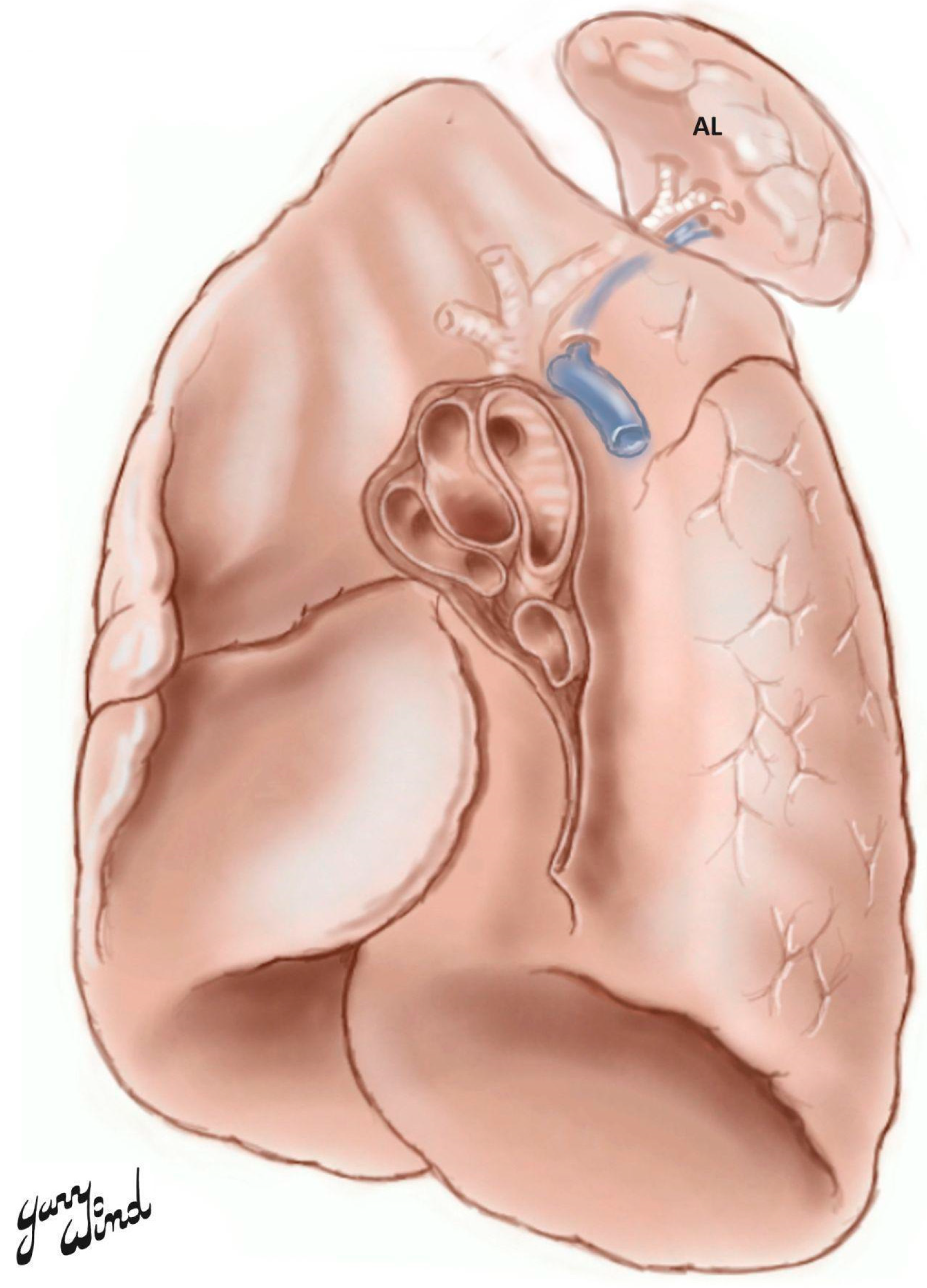
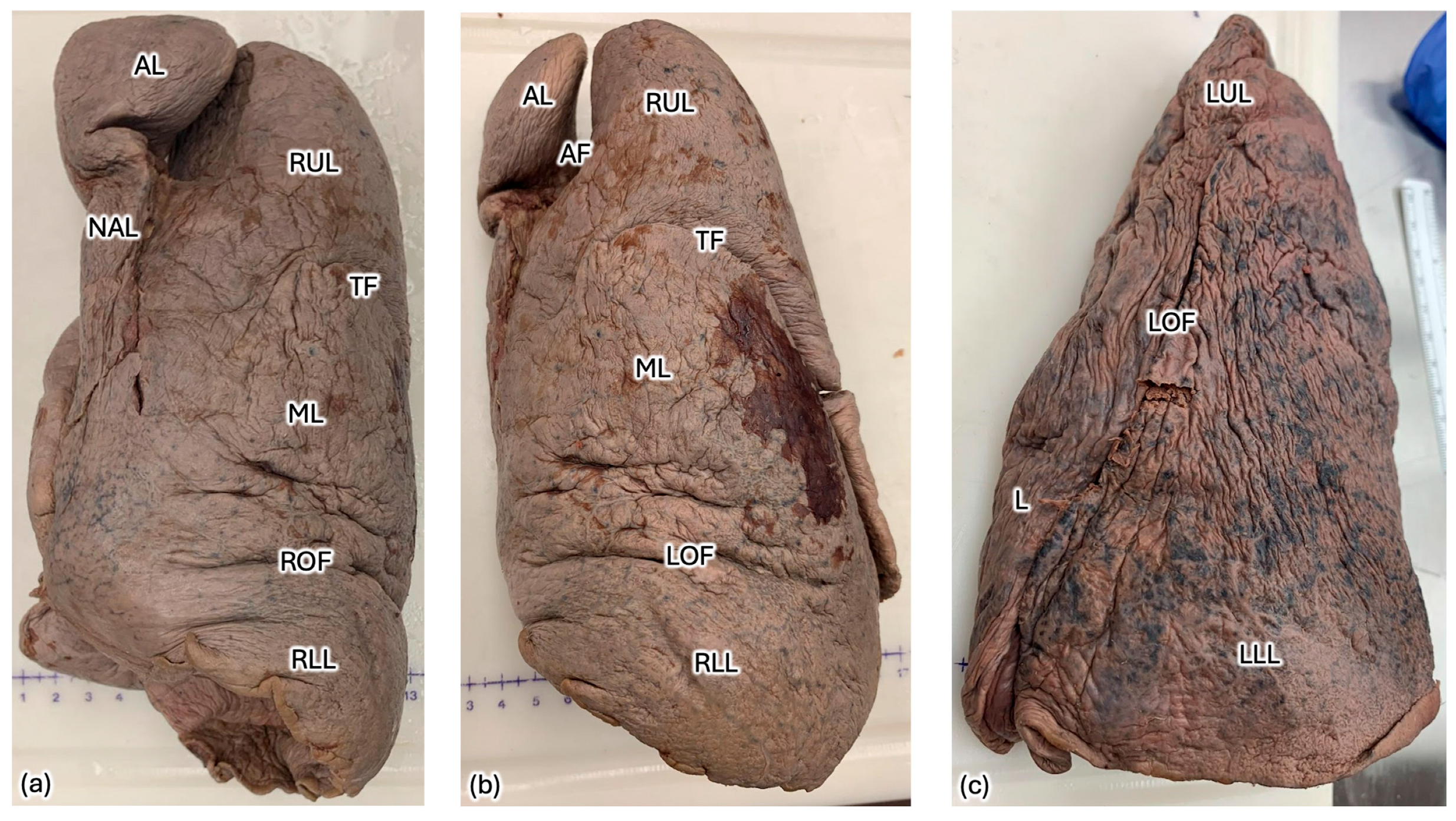
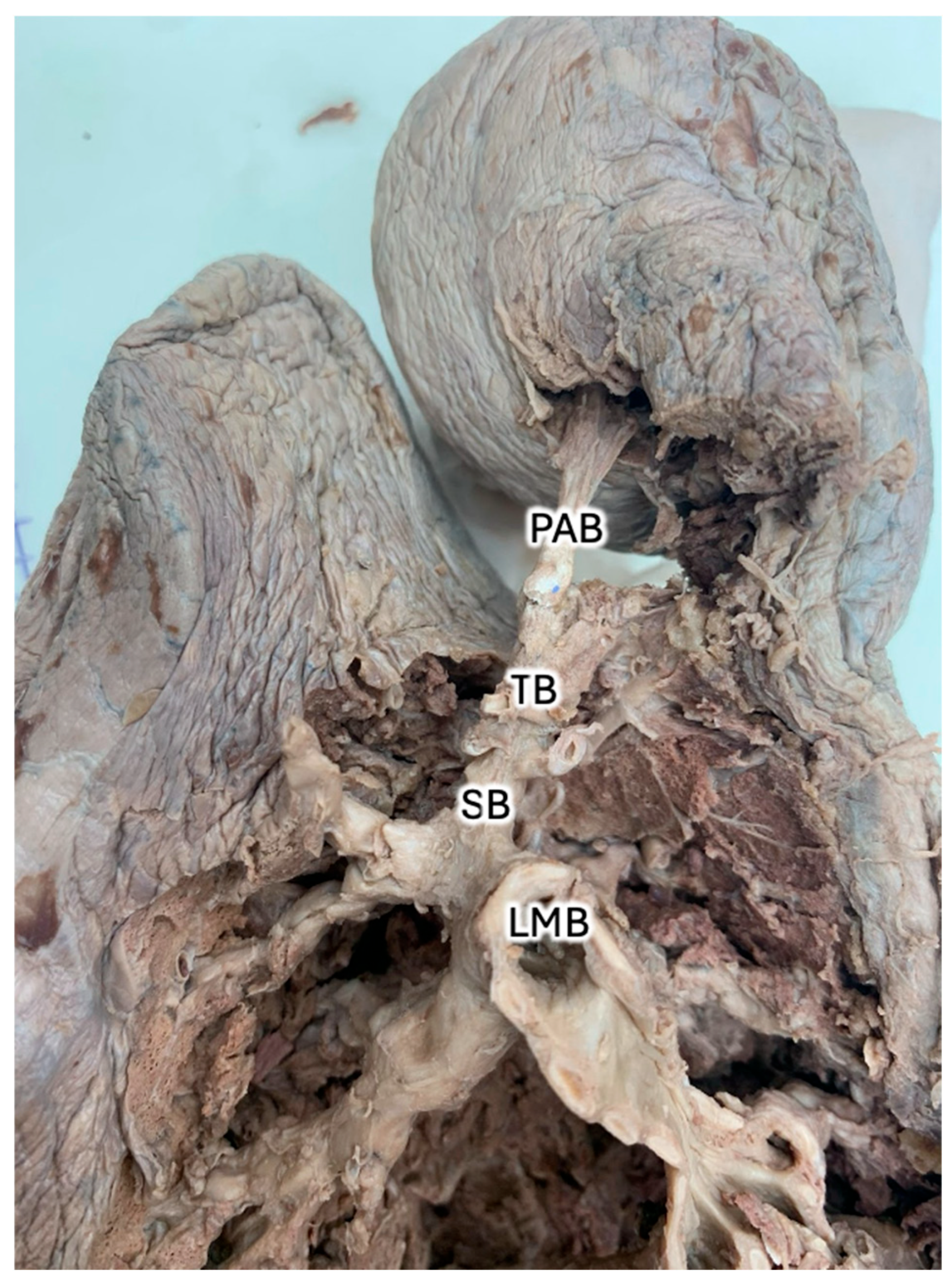
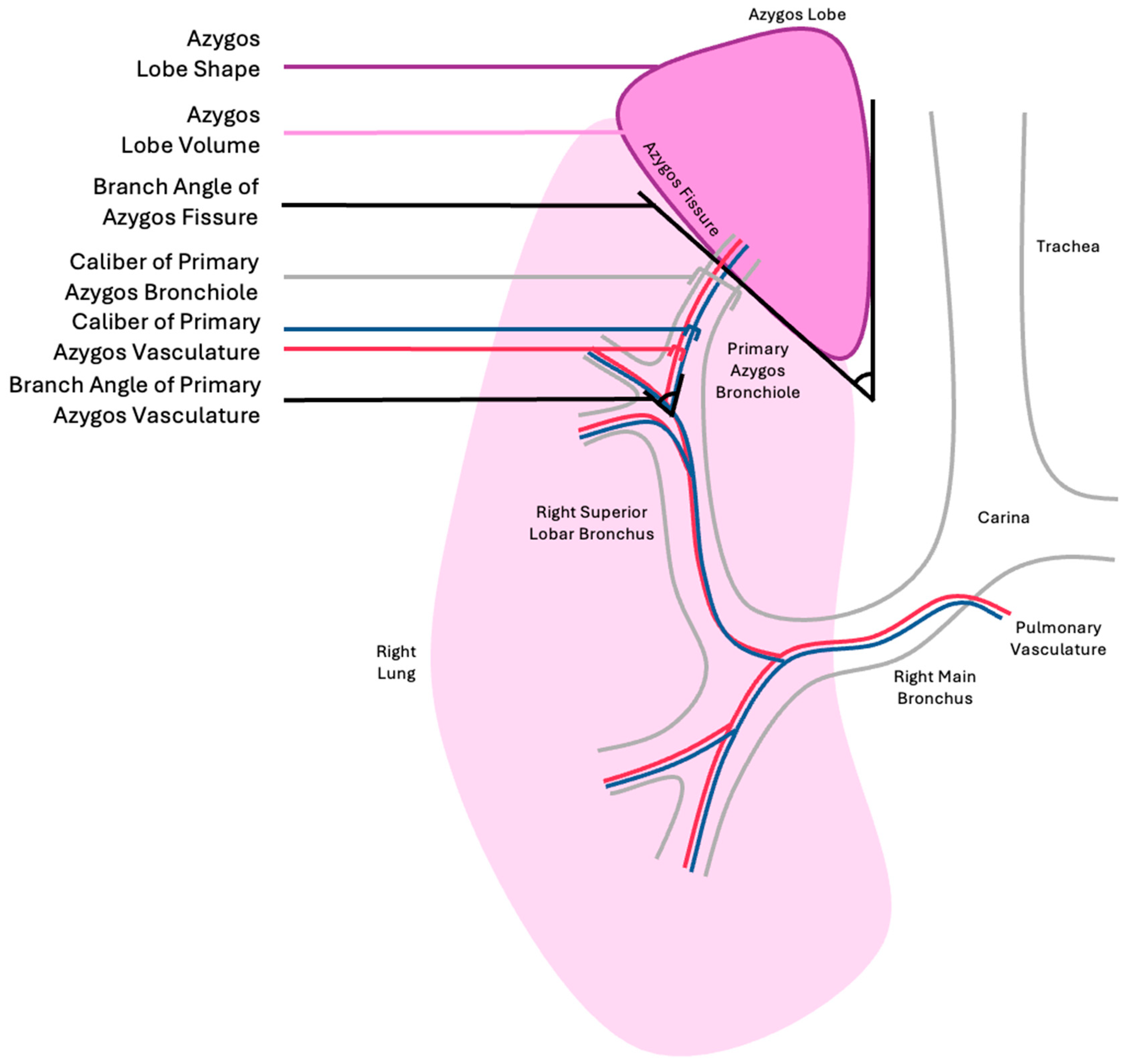
| Azygos Fissure Classification, Historical | |
|---|---|
| Type A | More or less horizontal and cutting the outer (lateral) surface of the lung at some point between the apex and a point two inches below the apex. |
| Type B | More nearly vertical, and dividing the apex of the lung into lateral halves. |
| Type C | Vertical, and cutting off a small tongue-shaped lobe from the inner (mediastinal) surface, the pedicle being attached to the upper margin of the root of the lung. |
| Azygos Fissure Classification Criteria, Proposed |
|---|
| Azygos lobe volume |
| Azygos lobe shape |
| Branch angle of azygos fissure |
| Branch angle of primary azygos pulmonary vasculature |
| Branch angle of primary azygos bronchiole |
| Caliber of primary azygos pulmonary vasculature |
| Caliber of primary azygos bronchiole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, D.; Wind, G.; Leighton, M.X.; Lashley, K.; Valenzuela-Fuenzalida, J.J.; Dimitrakoff, J.; Roth, Y.; Lenert, J.; Granite, G. Azygos Lobe in a 38-Year-Old Male Donor Diagnosed with Ogilvie’s Syndrome. Anatomia 2025, 4, 13. https://doi.org/10.3390/anatomia4030013
Johnson D, Wind G, Leighton MX, Lashley K, Valenzuela-Fuenzalida JJ, Dimitrakoff J, Roth Y, Lenert J, Granite G. Azygos Lobe in a 38-Year-Old Male Donor Diagnosed with Ogilvie’s Syndrome. Anatomia. 2025; 4(3):13. https://doi.org/10.3390/anatomia4030013
Chicago/Turabian StyleJohnson, David, Gary Wind, Maria Ximena Leighton, Kerrie Lashley, Juan Jose Valenzuela-Fuenzalida, Jordan Dimitrakoff, Yolanda Roth, Joanne Lenert, and Guinevere Granite. 2025. "Azygos Lobe in a 38-Year-Old Male Donor Diagnosed with Ogilvie’s Syndrome" Anatomia 4, no. 3: 13. https://doi.org/10.3390/anatomia4030013
APA StyleJohnson, D., Wind, G., Leighton, M. X., Lashley, K., Valenzuela-Fuenzalida, J. J., Dimitrakoff, J., Roth, Y., Lenert, J., & Granite, G. (2025). Azygos Lobe in a 38-Year-Old Male Donor Diagnosed with Ogilvie’s Syndrome. Anatomia, 4(3), 13. https://doi.org/10.3390/anatomia4030013







