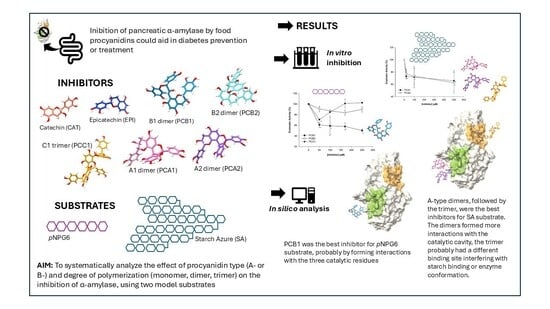
The Inhibition of Pancreatic α-Amylase by Monomeric, Dimeric and Trimeric Procyanidins Is Dependent upon the Structural Characteristics of Inhibitors and Substrates
Abstract
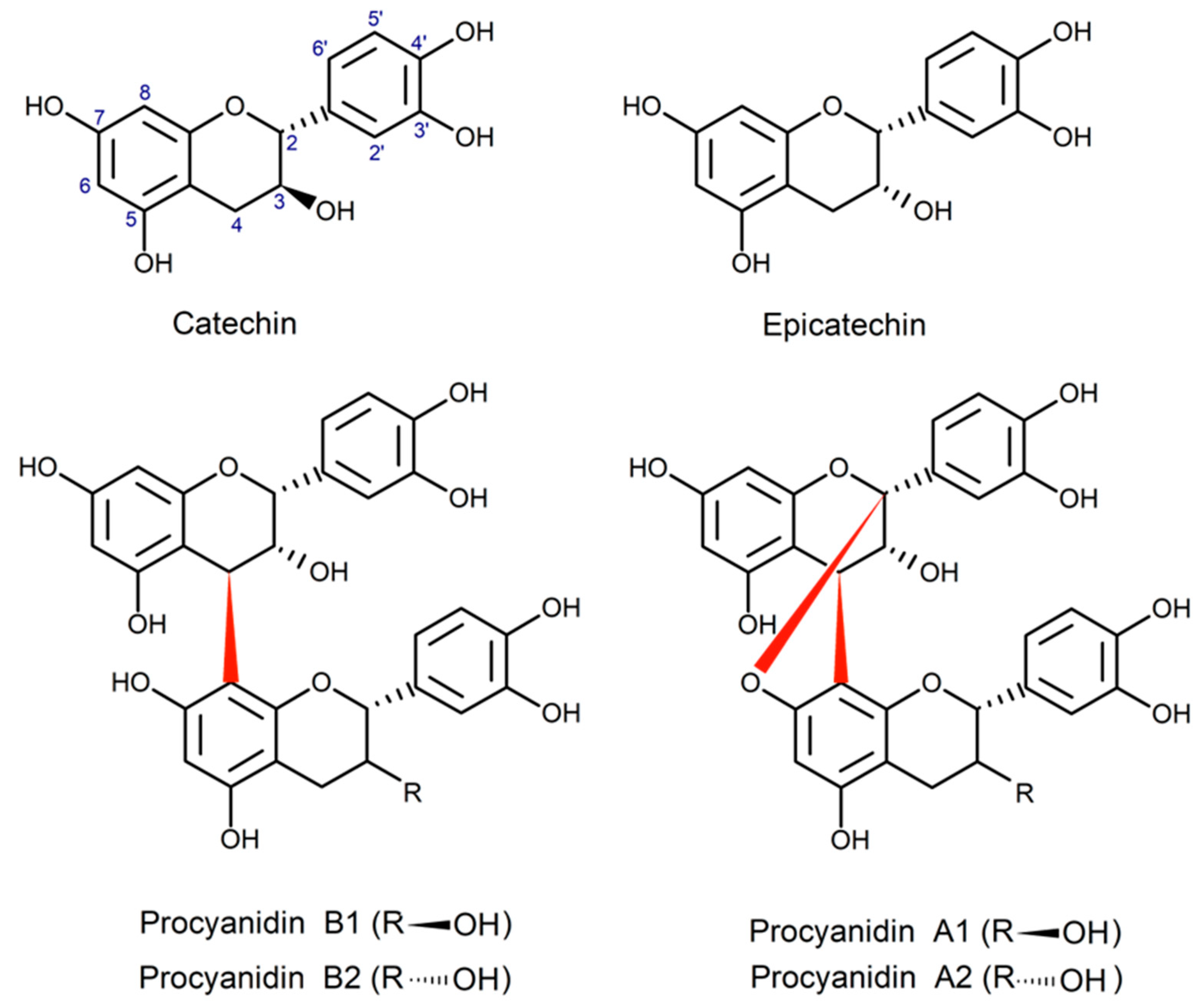
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzyme Activity of Pancreatic α-Amylase
2.2.1. p-NPG6 as Substrate
2.2.2. Starch Azure as Substrate
2.3. Inhibition of Pancreatic α-Amylase Enzyme Activity
2.3.1. Determination of the Percent Inhibition with p-NPG6 as Substrate
2.3.2. Determination of the Percent Inhibition with Starch Azure as Substrate
2.4. Molecular Docking Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Enzyme Activity of α-Amylase Towards Two Model Substrates
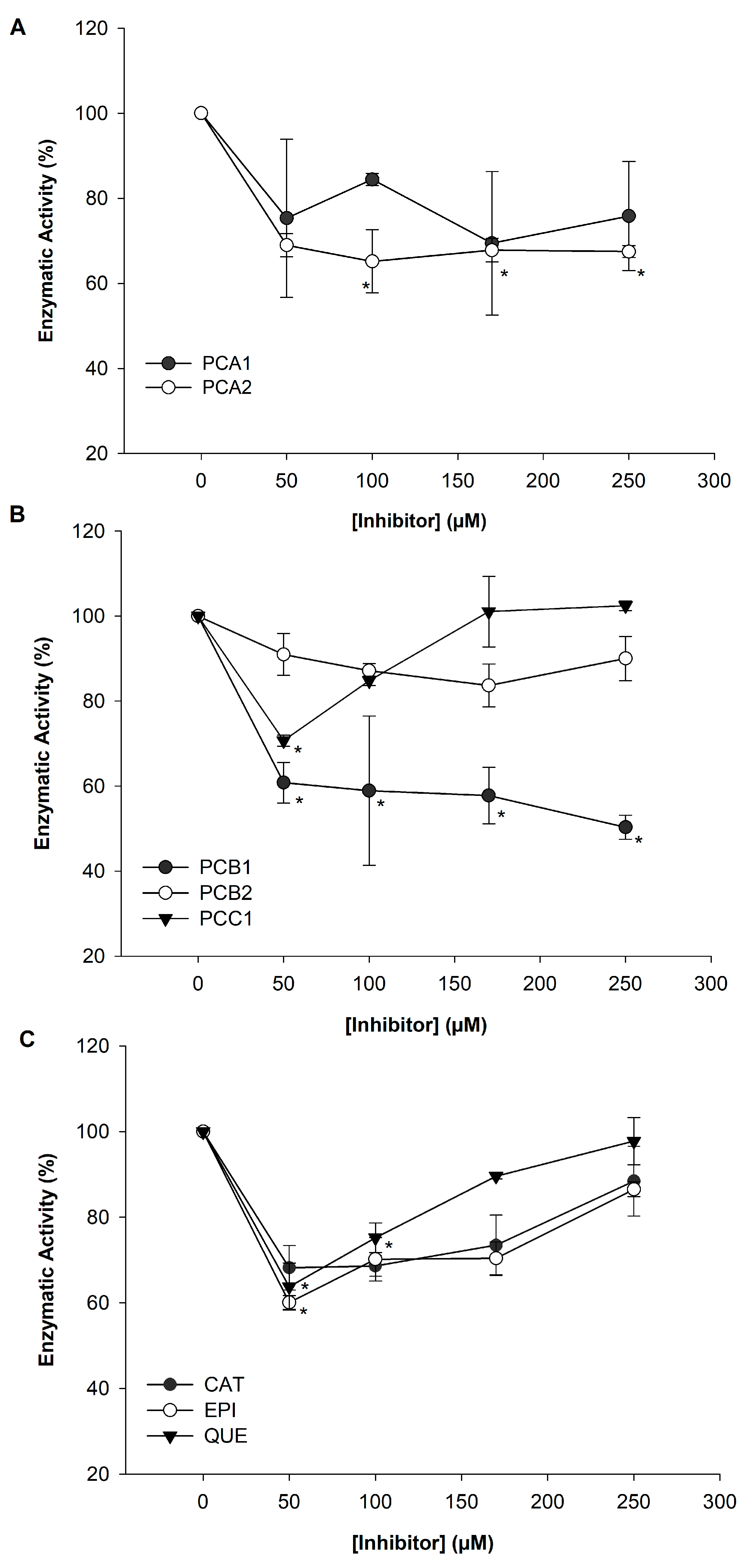
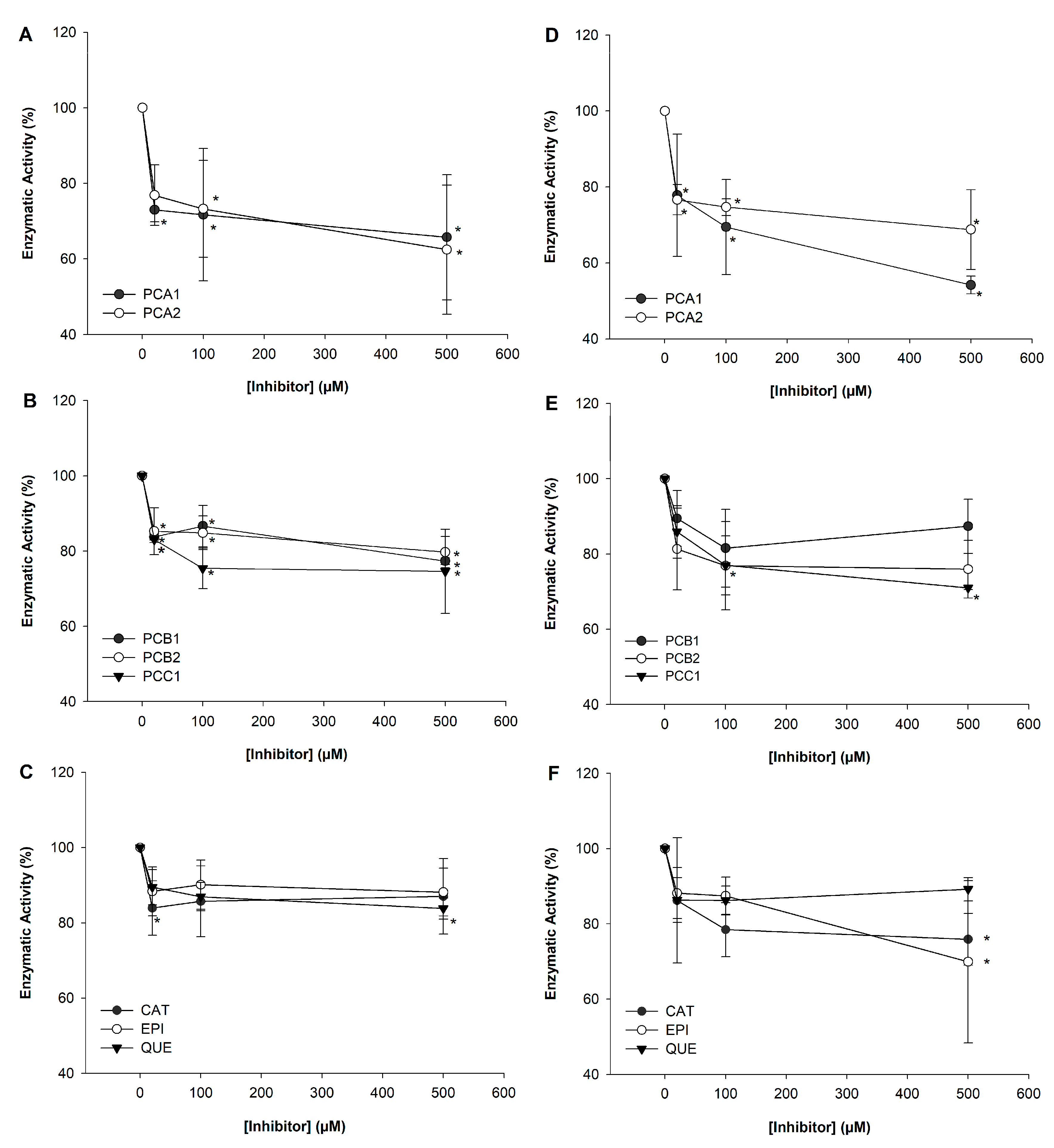
3.2. Inhibition of α-Amylase Using p-NPG6 as Substrate
3.3. Inhibition of α-Amylase Using Starch Azure as Substrate
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| p-NPG6 | p-nitrophenyl-α-D-maltohexaoside |
| CAT | Catechin |
| EPI | Epicatechin |
| QUE | Quercetin |
| PCA1 | Procyanidin A1 |
| PCA2 | Procyanidin A2 |
| PCB1 | Procyanidin B1 |
| PCB2 | Procyanidin B2 |
| PCC1 | Procyanidin C1 |
References
- Kanter, J.E.; Bornfeldt, K.E. Impact of Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1049–1053. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-Amylase by Flavonoids: Structure Activity Relationship (SAR). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins. Front. Nutr. 2017, 3, 57. [Google Scholar] [CrossRef]
- Lam, T.-P.; Tran, N.-V.N.; Pham, L.-H.D.; Lai, N.V.-T.; Dang, B.-T.N.; Truong, N.-L.N.; Nguyen-Vo, S.-K.; Hoang, T.-L.; Mai, T.T.; Tran, T.-D. Flavonoids as Dual-Target Inhibitors against α-Glucosidase and α-Amylase: A Systematic Review of in Vitro Studies. Nat. Prod. Bioprospect 2024, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Houghton, M.J.; Barber, E.; Williamson, G. Structure-Function Relationships in (Poly)Phenol-Enzyme Binding: Direct Inhibition of Human Salivary and Pancreatic α-Amylases. Food Res. Int. 2024, 188, 114504. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Flores, A.A.; Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; González-Aguilar, G.A.; Aguilar, C.N. Proanthocyanidins with a Low Degree of Polymerization Are Good Inhibitors of Digestive Enzymes Because of Their Ability to Form Specific Interactions: A Hypothesis. J. Food Sci. 2018, 83, 2895–2902. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Effect of Tannin Structure. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 515–521. [Google Scholar]
- Jiang, C.; Chen, Y.; Ye, X.; Wang, L.; Shao, J.; Jing, H.; Jiang, C.; Wang, H.; Ma, C. Three Flavanols Delay Starch Digestion by Inhibiting α-Amylase and Binding with Starch. Int. J. Biol. Macromol. 2021, 172, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.; Kan, J.; Wen, X.; Jin, C. Synthesis, Characterization and in Vitro Anti-Diabetic Activity of Catechin Grafted Inulin. Int. J. Biol. Macromol. 2014, 64, 76–83. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.-J.; Song, H.-C. α-Glucosidase and α-Amylase Inhibitory Activities of Guava Leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Tao, W.; Pan, H.; Jiang, H.; Wang, M.; Ye, X.; Chen, S. Extraction and Identification of Proanthocyanidins from the Leaves of Persimmon and Loquat. Food Chem. 2022, 372, 130780. [Google Scholar] [CrossRef]
- Darnis, S.; Juge, N.; Guo, X.-J.; Marchis-Mouren, G.; Puigserver, A.; Chaix, J.-C. Molecular Cloning and Primary Structure Analysis of Porcine Pancreatic α-Amylase. BBA-Protein Struct. Mol. Enzymol. 1999, 1430, 281–289. [Google Scholar] [CrossRef]
- Baks, T.; Janssen, A.E.M.; Boom, R.M. A Kinetic Model to Explain the Maximum in A-amylase Activity Measurements in the Presence of Small Carbohydrates. Biotechnol. Bioeng. 2006, 94, 431–440. [Google Scholar] [CrossRef]
- Gaquere-Parker, A.; Taylor, T.; Hutson, R.; Rizzo, A.; Folds, A.; Crittenden, S.; Zahoor, N.; Hussein, B.; Arruda, A. Low Frequency Ultrasonic-Assisted Hydrolysis of Starch in the Presence of α-Amylase. Ultrason. Sonochem 2018, 41, 404–409. [Google Scholar] [CrossRef]
- Rinderknecht, H.; Wilding, P.; Haverback, B.J. A New Method for the Determination of α-Amylase. Experientia 1967, 23, 805. [Google Scholar] [CrossRef] [PubMed]
- Abeliovich, H. On Hill Coefficients and Subunit Interaction Energies. J. Math. Biol. 2016, 73, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N. The Hill Equation Revisited: Uses and Misuses. FASEB J. 1997, 11, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, M.; Balestri, F.; Moschini, R.; Del-Corso, A.; Mura, U. Apparent Cooperativity and Apparent Hyperbolic Behavior of Enzyme Mixtures Acting on the Same Substrate. J. Enzyme Inhib. Med. Chem. 2016, 31, 1556–1559. [Google Scholar] [CrossRef]
- Ishikura, K.; Nitta, Y.; Watanabe, T. Hydrolysis of Phenyl β-Maltoside Catalyzed by Saccharifying α-Amylase from Bacillus subtilis. J. Biochem. 1977, 81, 1187–1192. [Google Scholar] [PubMed]
- Yoshida, H.; Hiromi, K.; Ono, S. Kinetics and Mechanism of Hydrolysis of Phenyl α-Maltoside by Saccharifying α-Amylase of Bacillus subtilis. J. Biochem. 1969, 65, 741–750. [Google Scholar] [CrossRef]
- Nielsen, J.W.; Kramhøft, B.; Bozonnet, S.; Abou Hachem, M.; Stipp, S.L.S.; Svensson, B.; Willemoës, M. Degradation of the Starch Components Amylopectin and Amylose by Barley α-Amylase 1: Role of Surface Binding Site 2. Arch. Biochem. Biophys. 2012, 528, 1–6. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Seo, E.-S.; Bozonnet, S.; Aghajari, N.; Robert, X.; Haser, R.; Svensson, B. Multi-site Substrate Binding and Interplay in Barley A-amylase 1. FEBS Lett. 2008, 582, 2567–2571. [Google Scholar] [CrossRef]
- Apar, D.K.; Özbek, B. Estamation of Kinetic Parameters for Rice Starch Hydrolysis Inhibited by Added Materials. Chem. Eng. Commun. 2007, 194, 334–344. [Google Scholar] [CrossRef]
- Yoshino, M.; Murakami, K. Analysis of the Substrate Inhibition of Complete and Partial Types. Springerplus 2015, 4, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Wang, G.; Cao, J.; Yang, X.; Liu, X.; Sun, L. α-Amylase Inhibition of a Certain Dietary Polyphenol Is Predominantly Affected by the Concentration of α-1, 4-Glucosidic Bonds in Starchy and Artificial Substrates. Food Res. Int. 2022, 157, 111210. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Lu, J.; Li, W.; Wolynes, P.G.; Wang, W. Frustration and the Kinetic Repartitioning Mechanism of Substrate Inhibition in Enzyme Catalysis. J. Phys. Chem. B 2022, 126, 6792–6801. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.-W.; Kim, J.-I.; Kim, M.-J.; Kim, J.C. Porcine Pancreatic α-Amylase Hydrolysis of Native Starch Granules as a Function of Granule Surface Area. Biotechnol. Prog. 2008, 19, 1162–1166. [Google Scholar] [CrossRef]
- Lehoczki, G.; Kandra, L.; Gyémánt, G. The Use of Starch Azure for Measurement of Alpha-Amylase Activity. Carbohydr. Polym. 2018, 183, 263–266. [Google Scholar] [CrossRef]
- Guerra, N.P.; Pastrana Castro, L. Modelling the Effects of Ageing Time of Starch on the Enzymatic Activity of Three Amylolytic Enzymes. Sci. World J. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, L.; Lu, Q.; Liu, R. Interaction Mechanism between α-Glucosidase and A-Type Trimer Procyanidin Revealed by Integrated Spectroscopic Analysis Techniques. Int. J. Biol. Macromol. 2020, 143, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as Human Intestinal α-Glucosidase Inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Ao, J.; Hou, Y.; Xi, M.; Cai, Y.; Li, M.; Luo, A. Structure-Activity Relationships and the Underlying Mechanism of α-Amylase Inhibition by Hyperoside and Quercetin: Multi-Spectroscopy and Molecular Docking Analyses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121797. [Google Scholar] [CrossRef]
- Peng, Q.; Ma, Y.; Wang, Z.; Wang, J. Inhibition Mechanism of Different Structural Polyphenols against α-Amylase Studied by Solid-State NMR and Molecular Docking. Int. J. Biol. Macromol. 2024, 275, 133757. [Google Scholar] [CrossRef]
- Deogratias, G.; Shadrack, D.M.; Munissi, J.J.E.; Kinunda, G.A.; Jacob, F.R.; Mtei, R.P.; Masalu, R.J.; Mwakyula, I.; Kiruri, L.W.; Nyandoro, S.S. Hydrophobic π-π Stacking Interactions and Hydrogen Bonds Drive Self-Aggregation of Luteolin in Water. J. Mol. Graph. Model. 2022, 116, 108243. [Google Scholar] [CrossRef]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing Starch Digestion and Inhibiting Digestive Enzyme Activity Using Plant Flavanols/Tannins—A Review of Efficacy and Mechanisms. LWT Food Sci. Technol. 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A Comprehensive Review Encompassing Structure Elucidation via Mass Spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Spiegler, V. Anthelmintic A-Type Procyanidins and Further Characterization of the Phenolic Composition of a Root Extract from Paullinia pinnata. Molecules 2020, 25, 2287. [Google Scholar] [CrossRef] [PubMed]
- Prigent, S.V.E.; Voragen, A.G.J.; van Koningsveld, G.A.; Baron, A.; Renard, C.M.G.C.; Gruppen, H. Interactions between Globular Proteins and Procyanidins of Different Degrees of Polymerization. J. Dairy. Sci. 2009, 92, 5843–5853. [Google Scholar] [CrossRef]
- Li, B.; Fu, R.; Tan, H.; Zhang, Y.; Teng, W.; Li, Z.; Tian, J. Characteristics of the Interaction Mechanisms of Procyanidin B1 and Procyanidin B2 with Protein Tyrosine Phosphatase-1B: Analysis by Kinetics, Spectroscopy Methods and Molecular Docking. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 259, 119910. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Structural Features of Procyanidin Interactions with Salivary Proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, T.; Joshi, T.; Chandra, S.; Tamta, S. Molecular Dynamics Simulation for Screening Phytochemicals as α-Amylase Inhibitors from Medicinal Plants. J. Biomol. Struct. Dyn. 2020, 39, 6524–6538. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Interactions of Flavonoids with α-Amylase and Starch Slowing down Its Digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on in Vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Bozonnet, S.; Jensen, M.T.; Nielsen, M.M.; Aghajari, N.; Jensen, M.H.; Kramhøft, B.; Willemoës, M.; Tranier, S.; Haser, R.; Svensson, B. The ‘Pair of Sugar Tongs’ Site on the Non-catalytic Domain C of Barley A-amylase Participates in Substrate Binding and Activity. FEBS J. 2007, 274, 5055–5067. [Google Scholar] [CrossRef]
- Agu, K.C.; Eluehike, N.; Ofeimun, R.O.; Abile, D.; Ideho, G.; Ogedengbe, M.O.; Onose, P.O.; Elekofehinti, O.O. Possible Anti-Diabetic Potentials of Annona muricata (Soursop): Inhibition of α-Amylase and α-Glucosidase Activities. Clin. Phytosci. 2019, 5, 21. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Nada, A.A.; Metwally, A.M.; Asaad, A.M.; Celik, I.; Ibrahim, R.S.; Eldin, S.M.S. Synergistic Effect of Potential Alpha-Amylase Inhibitors from Egyptian Propolis with Acarbose Using in Silico and in Vitro Combination Analysis. BMC Complement. Med. Ther. 2024, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Chen, J.; McClements, D.J.; Li, T.; Liu, C. Investigation the Interaction between Procyanidin Dimer and α-Glucosidase: Spectroscopic Analyses and Molecular Docking Simulation. Int. J. Biol. Macromol. 2019, 130, 315–322. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tomé, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araújo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a Flavonoids Library for Inhibition of Pancreatic α-Amylase towards a Structure–Activity Relationship. J. Enzyme Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Possible Effects of Dietary Polyphenols on Sugar Absorption and Digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
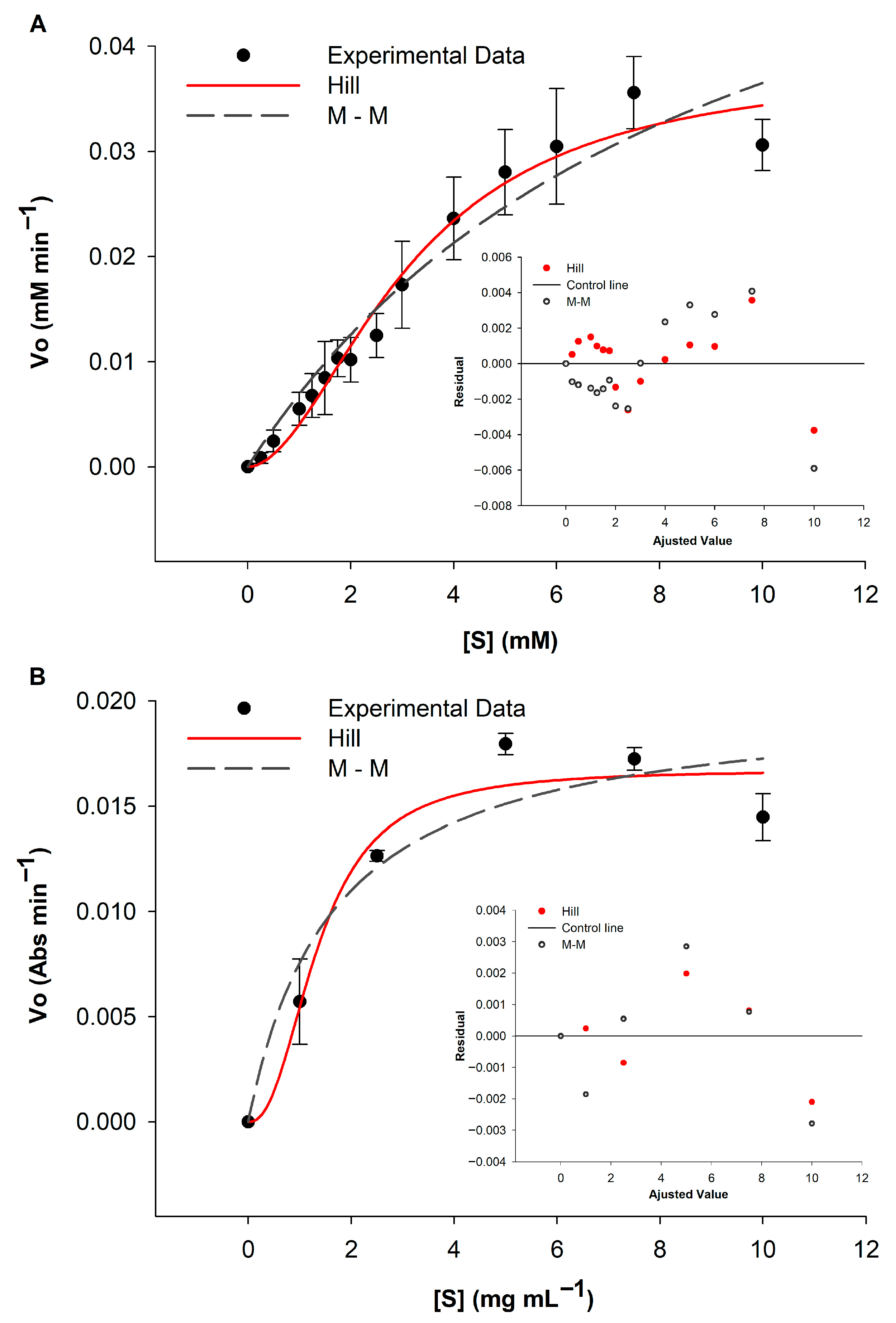

| Substrate | Fit | Vmax | KM | R2 | h |
|---|---|---|---|---|---|
| p-NPG6 | Hill | 0.0383 ± 0.0038 mM min−1 | 3.1447 ± 0.4526 mM | 0.9745 | 1.871 ± 0.2965 |
| Michaelis–Menten | 0.0698 ± 0.0144 mM min−1 | 9.1077 ± 2.9978 mM | 0.9444 | NA | |
| Starch azure | Hill | 0.0167 ± 0.0018 AU min−1 | 1.3601 ± 0.345 mg mL−1 | 0.8983 | 2.3454 ± 1.3201 |
| Michaelis–Menten | 0.0201 ± 0.0036 AU min−1 | 1.6591 ± 1.0779 mg mL−1 | 0.7901 | NA |
| Ligand | ΔG (Kcal mol−1) | Molecular Structure | H-Bonds | π-π Stacking | ||
|---|---|---|---|---|---|---|
| Residues | d (Å) | Residues | d (Å) | |||
| PCA1 | −8.88 | 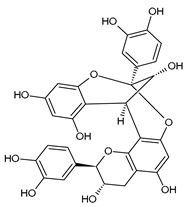 | Glu352 | 1.88–2.94 | Trp59 | 4.47–4.63 |
| Gln63 | 3.27–3.29 | |||||
| Asp197 | 1.81–2.54 | |||||
| Asp300 | 3.81 | |||||
| PCA2 | −8.42 |  | Arg195 | 2.71 | Trp59 | 3.91–4.41 |
| Asp197 | 2.64 | Tyr62 | 3.66–4.67 | |||
| Asp300 | 1.70 | |||||
| Hse305 | 2.77 | |||||
| Gln63 | 2.98 | |||||
| PCB1 | −8.85 | 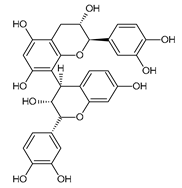 | Asp300 | 1.77 | Trp59 | 3.85–4.95 |
| Glu233 | 2.06 | |||||
| Asp197 | 1.94 | |||||
| Tyr151 | 3.51 | |||||
| Lys200 | 2.38 | |||||
| PCB2 | −8.68 |  | Hse305 | 2.34–2.69 | Tyr62 | 3.67–4.50 |
| Asp356 | 2.36 | Trp59 | 3.94–4.26 | |||
| Asp300 | 1.94 | |||||
| Asp197 | 2.69 | |||||
| PCC1 | −9.41 |  | Asp290 | 3.65 | Phe406 | 4.59–4.59 |
| Glu282 | 2.12–2.53 | Tyr2 | 4.60–4.84 | |||
| Asp402 | 2.78 | |||||
| Gly403 | 2.14 | |||||
| Arg421 | 1.97 | |||||
| CAT | −7.58 | 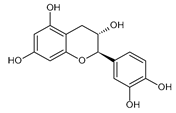 | Asp197 | 1.93–2.11 | Tyr62 | 3.79 |
| Hse101 | 2.37 | Trp59 | 3.97–4.20 | |||
| Asp300 | 1.84 | |||||
| EPI | −7.56 | 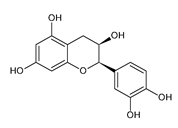 | Glu233 | 1.98 | Tyr62 | 4.35 |
| Tyr151 | 2.53 | |||||
| Asp197 | 2.77 | |||||
| Gln63 | 2.41 | |||||
| QUE | −7.74 |  | Hse305 | 2.59 | Trp58 | 3.50–4.29 |
| Asp356 | 3.61 | Tyr62 | 3.72–4.21 | |||
| Gln63 | 2.78 | |||||
| Asp197 | 2.24–2.37 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-López, J.V.; Arras-Gardea, A.V.; Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; de la Rosa, L.A. The Inhibition of Pancreatic α-Amylase by Monomeric, Dimeric and Trimeric Procyanidins Is Dependent upon the Structural Characteristics of Inhibitors and Substrates. Appl. Biosci. 2025, 4, 49. https://doi.org/10.3390/applbiosci4040049
Aguilar-López JV, Arras-Gardea AV, Martinez-Gonzalez AI, Alvarez-Parrilla E, de la Rosa LA. The Inhibition of Pancreatic α-Amylase by Monomeric, Dimeric and Trimeric Procyanidins Is Dependent upon the Structural Characteristics of Inhibitors and Substrates. Applied Biosciences. 2025; 4(4):49. https://doi.org/10.3390/applbiosci4040049
Chicago/Turabian StyleAguilar-López, Jocelin Violeta, Ana V. Arras-Gardea, Alejandra I. Martinez-Gonzalez, Emilio Alvarez-Parrilla, and Laura A. de la Rosa. 2025. "The Inhibition of Pancreatic α-Amylase by Monomeric, Dimeric and Trimeric Procyanidins Is Dependent upon the Structural Characteristics of Inhibitors and Substrates" Applied Biosciences 4, no. 4: 49. https://doi.org/10.3390/applbiosci4040049
APA StyleAguilar-López, J. V., Arras-Gardea, A. V., Martinez-Gonzalez, A. I., Alvarez-Parrilla, E., & de la Rosa, L. A. (2025). The Inhibition of Pancreatic α-Amylase by Monomeric, Dimeric and Trimeric Procyanidins Is Dependent upon the Structural Characteristics of Inhibitors and Substrates. Applied Biosciences, 4(4), 49. https://doi.org/10.3390/applbiosci4040049