1. Introduction
Ethanol levels in blood are greater than zero even in some subjects who are completely teetotal. As an example, a study from the United Arab Emirates found that in 1557 participants of different nationalities, ages, and sexes, the median endogenous ethanol level was 0.04 mg/dl (IQR 0.01–0.09 mg/dl) [
1]. Similarly, another study found that residents from different nationalities in Saudi Arabia had a mean endogenous blood alcohol level of 0.14 mg/dl (range: 0–1.53 mg/dl) [
2].
Regarding this endogenous ethanol production, very rarely this phenomenon leads to significant levels of blood ethanol and/or human diseases, since under physiological conditions the small quantities of endogenous ethanol formed in the body, mainly in the colon and in the bladder, are rapidly eliminated during sugar metabolism [
3] by intestinal and hepatic dehydrogenases, catalases, and the microsomal ethanol oxidation system [
3]. When ethanol concentration exceeds the internal metabolic capacity, ethanol accumulates and can lead to complications and even the onset of certain diseases [
4]. This situation is due to an imbalance in the intestinal microbiota, favoring the proliferation of microbes that produce ethanol from sugars or other substrates [
5]. In this infrequent condition, some individuals might suffer from these consequences without consuming any alcohol, a condition named in different ways, such as gut fermentation syndrome, endogenous alcohol fermentation syndrome, gut fermentation syndrome, or auto-brewery syndrome (ABS) [
5]. For the sake of clarity, in this article we will refer solely to the latter.
Since the first description of this rare condition in 1894 by Bouchard [
6] in a French subject, the literature has grown consistently and it has been reviewed recently by Mbaye et al. [
7], who examined 5937 articles and found that a vast number of microorganisms species are able to produce different amounts of ethanol, the majority of which are bacteria (
N = 61) and the rest fungi (
N = 24). The interested reader is referred to this excellent systematic review [
7].
The aim of the present paper is: (1) to describe the most frequent microorganisms involved in endogenous ethanol production; (2) to recall the related metabolic pathways; (3) to address the various pathologies involved; and (4) to present some therapeutic strategies directed at minimizing endogenous ethanol production.
2. Ethanol-Producing Microorganisms in the Human Gut
The phenomenon of ethanol production by the gut microbiota has been acknowledged for over a century [
6]. It took, however, many decades to understand that this is, within certain quantitative boundaries, a physiological phenomenon occurring in the intestine of most human beings (1–2, 3–4), with blood ethanol levels ranging from 0 mg/dl to 3 mg/dl [
2,
3,
4,
8]. The description of types of endogenous ethanol-producing microorganisms and related levels of ethanol produced is, at present, incomplete, in the setting of ABS patients, since comprehensive studies on these patients are lacking, albeit eagerly awaited [
9].
The already quoted recent paper by Mbaye et al. [
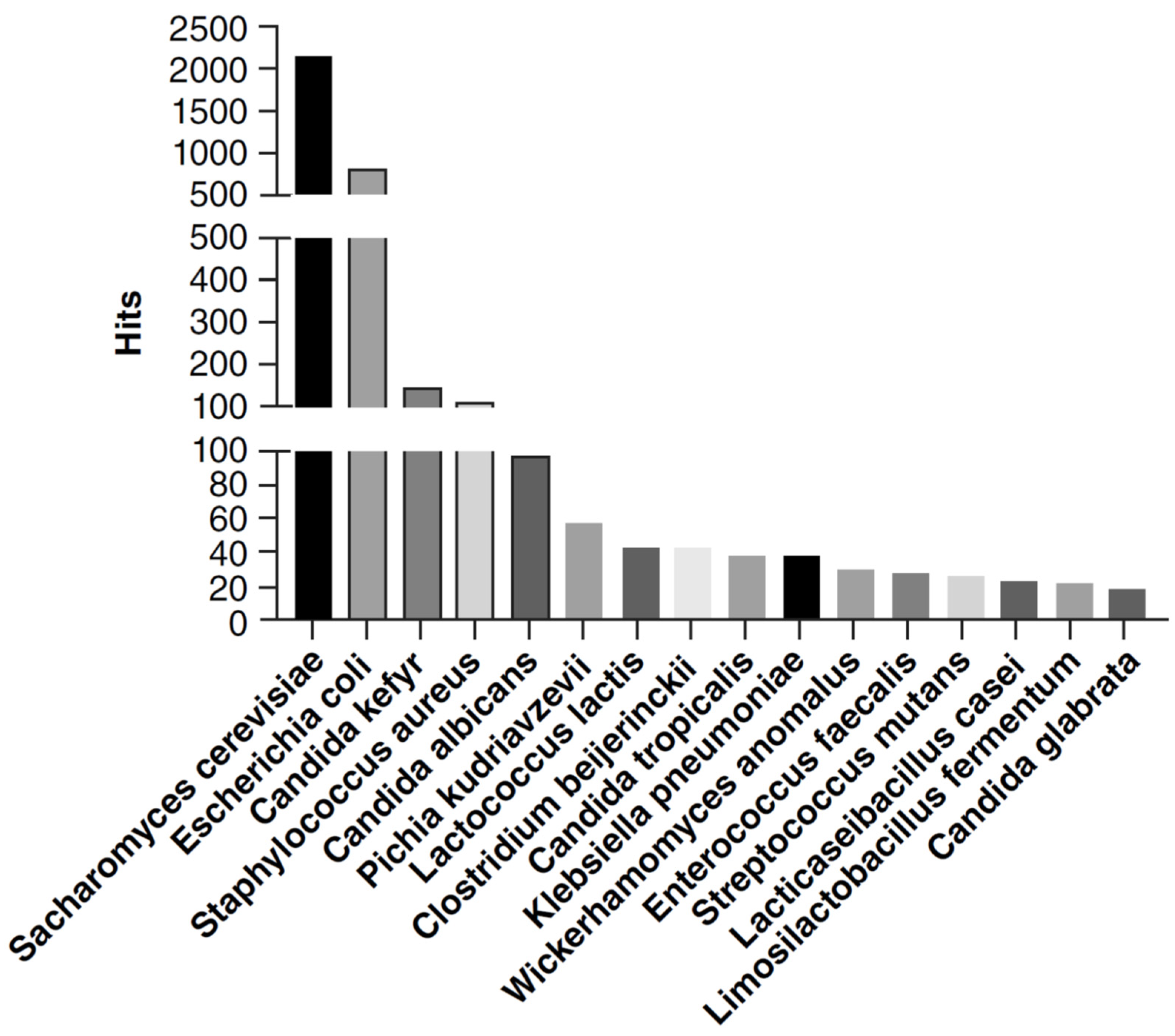
7] has screened 5397 articles in the literature dealing with ethanol-producing microorganisms and has identified a total of 340 ethanol-producer microbes, including 52.6% (179/340) fungi, 45.8% (156/340) bacteria, and 1.6% (5/340) archaea. Ultimately, 85 ethanol-producing microbes in humans (61 bacteria and 24 fungi) were identified.
Saccharomyces cerevisiae,
Candida, and
Pichia were the most represented fungi. Enterobacteriaceae was the most represented bacterial family with
Escherichia coli and
Klebsiella pneumoniae as the most prevalent species. Species of the Lachnospiraceae and Clostridiaceae family, of the Lactobacillales order, and of the Bifidobacterium genus were also identified. No Archaea found in humans were reported as being able to produce ethanol in vitro [
7].
Concerning the published articles dealing with ethanol-producing microorganisms of the human gut, the authors found that in the literature, the most frequently quoted phylum was
Pseudomonadota followed by
Bacillota, while at the family level, Enterobacteriaceae and Lactobacillaceae were the most prevalent quotations found. Finally, the three most reported genera were
Klebsiella,
Clostridium, and
Lactococcus. For fungi,
Ascomycota was the most represented phylum, while among families it was Saccharomycetaceae. Finally, 15 ethanol-producing fungal genera were reported, with the most represented belonging to
Candida. For further details, see
Figure 1.
It is of note that the catalog of microbes found in the human gut and capable of producing ethanol in vitro is not equivalent to a list of pathogen microbes, due to the great variability in the amount of ethanol production. Also, within the specific microbes there is a heavy strain-specific effect in alcohol production, a phenomenon already known [
10], while the maximum ethanol production level is much greater for yeasts (up to 6 g/L) than for bacteria (up to 1.2 g/L) [
2]. Finally, as the authors state, since microbiota is a complex system where microbe–microbe interactions modify the behavior of each microbe, the detection of an ethanol-producing microbe is not synonymous with a risk of disease linked with endogenous ethanol production, and interactions between gut microorganisms in vivo may both decrease or increase (or even abolish) the amount of ethanol production by single species [
7].
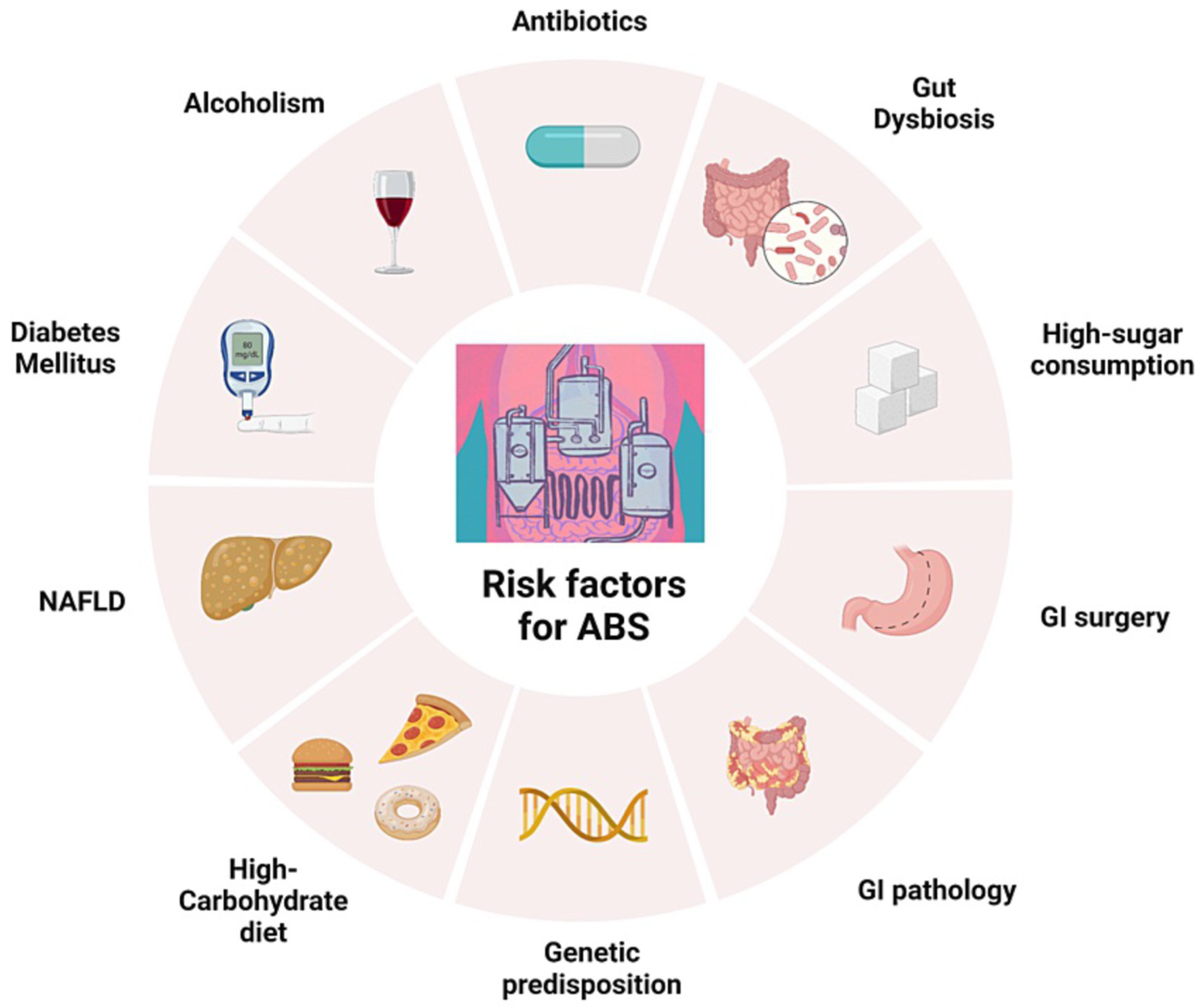
Independently from the types of microorganisms involved in endogenous ethanol production in the gut (or in the bladder), many risk factors have been postulated for the ABS [
11], which are listed in
Figure 2. Diet is very important, because a high-carbohydrate diet may increase the availability of sugar substrate to be converted into ethanol by gut microorganisms. Antibiotics use may induce dysbiosis, and a previous intake of these drugs is reported [
5]. Previous GI surgery, in particular jejunal bypass for morbid obesity or for Crohn’s disease strictures or fistulas, have been linked with ABS (see section on diseases associated with ABS). NAFLD, diabetes mellitus, and alcoholism, by directly or indirectly inducing hepatic metabolic changes, may be associated with the onset of more severe features of ABS.
3. Pathways of Microbial Ethanol Production
Ethanol is a metabolite not produced by Homo sapiens sapiens. As recalled above, under physiological conditions, small quantities of endogenous ethanol are formed in the body, particularly in the colon, and to a lesser extent also in the bladder, during sugar metabolism. Understanding the microbial fermentation pathways that produce ethanol in the human gut is useful for different reasons. Firstly, identifying the pathway(s) involved might contribute to better understanding the pathophysiology of the condition linked to excessive ethanol production. Secondly, it could help in defining potential therapeutic targets, offering antimicrobial or dietary therapeutic strategies to mitigate endogenous ethanol production.
Various microbial fermentation pathways have been identified that can produce ethanol [
5], such as the hexitol pathway, the Embden–Meyerhof–Parnas pathway (or glycolysis), the 6-Phosphogluconate pathway and hexitol pathway, the acetone–butanol pathway, the 3-butanediol fermentation pathway, the ethanol fermentation pathway, the mixed acid fermentation pathway, and the ethanolamine utilization pathway [
5]. For a detailed description of these pathways, the reader is referred to a comprehensive description in [
9]. Among the various pathways, the mixed acid fermentation and the 2,3-butanediol fermentation pathways are the predominant ones [
12]. Most frequently, microorganisms can produce ethanol via fermentation of monosaccharides such as glucose or hexitols (mannitol or sorbitol). Along the respective pathways, they can process these molecules to sugar alcohols and then ethanol and other metabolites. Most fermentation pathways primarily involve the conversion of glycolysis-produced acetyl-CoA into ethanol, involving the enzyme alcohol dehydrogenase that converts acetaldehyde into ethanol [
13,
14] (see
Figure 3). Glucose fermentation starts with glucose entry in the epithelial gut cell by active transportation by the hexose transporter and subsequently phosphorylated by hexokinase to glucose-6-phosphate by phosphohexose-isomerase. Fructose-6-phosphate is phosphorylated by phosphofructokinase-2 to fructose 1,6-bisphosphate, which subsequently converts to glyceraldehyde-3-phosphate by aldolase. The latter is converted to 1,3-biphosphoglycerate by glyceraldehyde-3-phosphate dehydrogenase. This 1,3-biphosphoglycerate will be converted to pyruvate in multiple steps. Pyruvate is converted to acetaldehyde by pyruvate decarboxylase, which is subsequently converted to the final product ethanol by alcohol dehydrogenase (see
Figure 3). As mentioned above, different taxa or strains have different ethanol production pathways and capacity [
9]. The complexity of endogenous ethanol production has two consequences: first, it involves a chain of interplay between various microbial strains rather than being attributable to a single microbial strain; secondly, since different strains use different pathways, the therapeutic approach in case of excessive and/or clinically relevant ethanol production needs to be multifaceted, and, for example, directed against bacteria and fungi at the same time. Despite the complexity of pathways and the redundance of ethanol-producing microbial strains in the human gut, in most cases, no harm is caused unless dysbiosis develops, due to exogenous factors such as diet, medication use, and intervening diseases (see next section).
4. Disease Related to Endogenous Ethanol Production
In a recent review, it has pointed out that antibiotic treatment might be a common factor in many ABS patients [
5], either before or at onset of symptoms [
15,
16,
17,
18,
19]. The use of antibiotics
per se affects the gut microbiota and is a permissive factor for colonization of alcohol-producing species. Compositional alterations translate into functional changes in gut microbiota, which, in turn, may affect many metabolic and organ functions, including the immune system function, the breakdown of nutritional components, the metabolism of xenobiotics, and other functions. An example of the pathological consequences of gut dysbiosis is its connection with metabolic diseases such as obesity [
20], metabolic syndrome [
21], and nonalcoholic steatohepatitis (NASH) and its complication, namely, hepatic cirrhosis [
22]. It has been shown [
23] that an increased abundance of Enterobacteriaceae, especially Escherichia, which is a known alcohol-producing bacterium, is present in NASH patients compared to healthy controls and that patients exhibit a higher serum alcohol concentration compared to controls [
23]. As a recent review has pointed out, the endogenous alcohol generated by gut microbiota may affect the progress of NAFLD in some patients [
24]. However, because of the variability between individuals with NAFLD and different metabolic pathways involved, it is unclear if changes in the gut microbiota are causal for NAFLD development. Hence, it is of particular interest to identify any bacteria that might account for the development of NAFLD and to elucidate the molecular mechanisms involved in the pathogenesis of this disease [
22,
25].
As an example, a recent study performed in China [
10] has shown that a high alcohol-producing
Klebsiella pneumoniae (HiAlc Kpn) was present in up to 60% of individuals with NAFLD in a Chinese cohort. Interestingly, transfer of clinical isolates of HiAlc Kpn by oral gavage into mice was able to induce NAFLD in the rodents. Likewise, fecal microbiota transplant (FMT) into mice using a HiAlc-Kpn-strain-containing microbiota isolated from an individual with NASH induced NAFLD. On the contrary, selective elimination of the HiAlc Kpn strain before FMT prevented NAFLD in the recipient mice. These results suggest that at least in some cases of NAFLD, an alteration in the gut microbiome drives the condition due to excess endogenous alcohol production.
In a case-control study conducted in five Chinese patients with ABS [
12], the characteristics and metabolites of the intestinal flora of patients were analyzed during different stages of disease and compared to a group of healthy controls. An in vitro culture system of relevant samples was used for screening drug sensitivity and ABS-inducing factors. Rabbit intestinal and murine models were established to verify if the isolated strains could induce ABS in vivo [
12]. Authors found intestinal dysbiosis, with decreased abundance of Firmicutes, and increased Proteobacteria, in the group of patients with ABS compared with healthy controls. After an in-depth investigation of patients by means of abdominal computed tomography (CT), chest CT, esophagogastroduodenoscopy, and colonoscopy, as well as neurological and psychiatric assessments, all the results were negative except for the finding of NAFLD. In addition, the alcohol dehydrogenase and aldehyde dehydrogenase levels of the five patients were all within normal ranges. In three patients, a cycle of antifungal drugs had been used for one month or more prior to sampling. Three different species of alcohol-producing
Klebsiella were isolated from these ABS patients, based on tests conducted in mice. Monosaccharide content was identified as a food-related inducing factor for alcohol production. Treatment with antibiotics, together with probiotic administration and a low-carbohydrate diet were prescribed, which cured or mitigated the ABS-related symptoms.
In the literature, ABS has been associated with various other diseases, such as, for example, surgical jejunoileal bypass for morbid obesity, resulting in short bowel syndrome, up in a third of patients in a report by Mezey et al. [
26]; or with jejunal atresia and necrotizing enterocolitis, described in a child who received neonatal extensive small bowel resection, again leading to a short bowel syndrome [
27]; or in patients who were immunocompromised or had strictures in Crohn’s disease with bacterial overgrowth [
15]. Finally, ABS has been postulated to be the cause of death in a case of sudden infant death [
28] or in a pediatric patient with explosive rupture of the stomach [
29]. A list of diseases linked with increased endogenous ethanol production is presented in
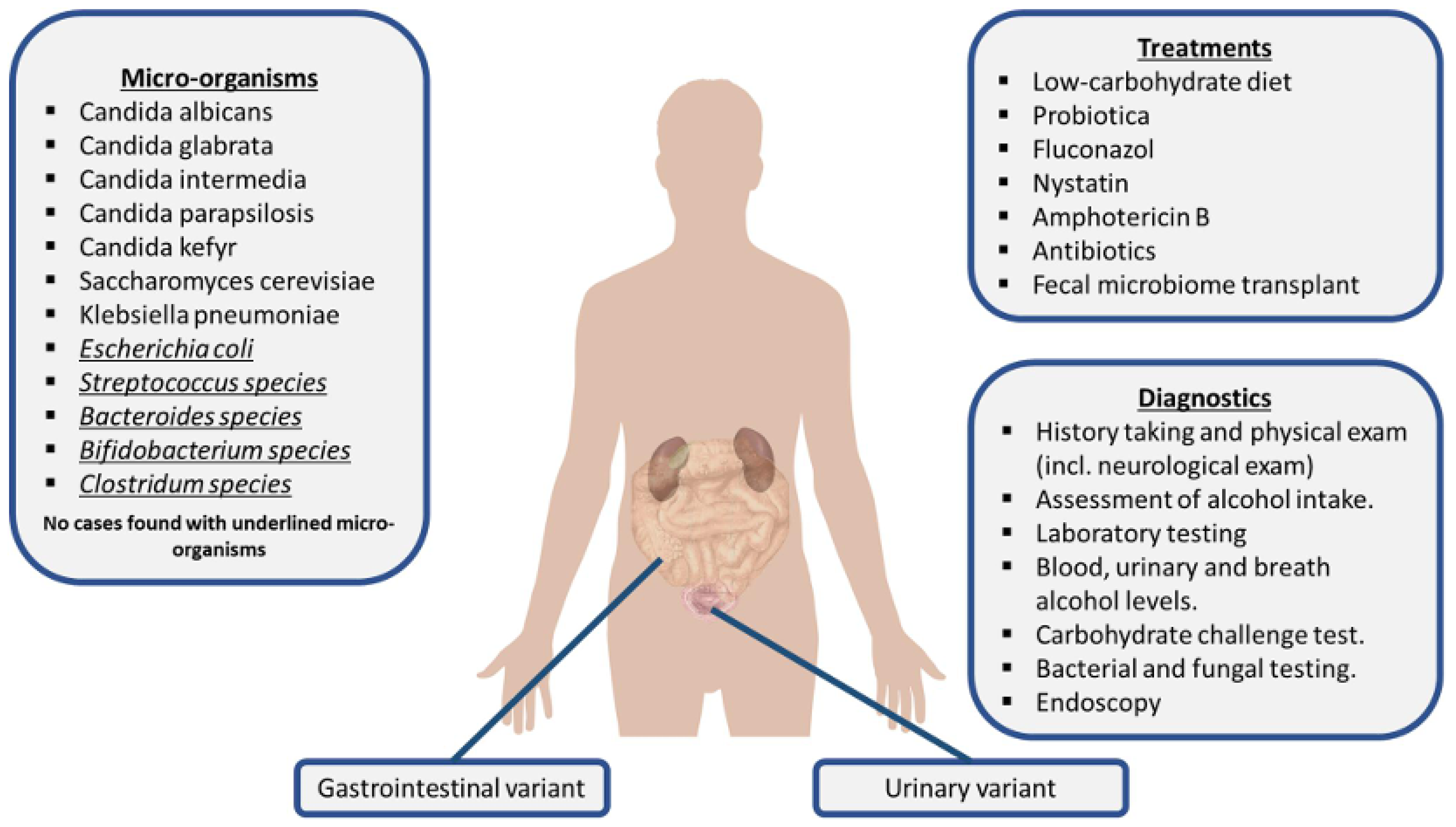
Table 1.
5. Diagnosis and Therapeutic Strategies Directed at Minimizing the Endogenous Ethanol Production
Due to its rarity and the overlap of symptoms with other conditions, ABS requires a detailed diagnosis by a team of specialists working together, including a gastroenterologist, a nutritionist, and a microbiologist to make the diagnosis, to treat the acute situation, and to prevent relapses. Currently, there are no established criteria to confirm an ABS diagnosis or to treat this condition. A detailed patient history, including dietary habits, alcohol consumption, and gastrointestinal symptoms, is, therefore, crucial. A comprehensive physical examination is essential to detect unexplained ethanol intoxication. A recently proposed diagnostic protocol starts with a standardized carbohydrate challenge test, with the patient receiving 200 g of glucose by mouth after an overnight fast with blood drawn at timed intervals of 0, ½, 1, 2, 4, 8, 16, and 24 h for glucose and blood alcohol levels [
15]. It is very important to exclude patients with suspected ABS and elevated blood or breath alcohol levels that are not surreptitiously drinking. Confirmation of ABS requires that ethanol levels are elevated during any phase of the carbohydrate challenge test. Diagnostic approach includes complete blood counts and comprehensive metabolic panels, and basic diagnostic tests to rule out other differential diagnoses that can present with similar symptoms and signs. In addition, stool testing can assist in the screening process, although small amounts of fungal colonization in the lower gastrointestinal tract can be considered as normal.
Recent research on ABS [
11] highlights the need for lifestyle modifications to manage this rare condition. Low-carbohydrate and sugar-restricted diets are essential for reducing fermentable substrates that promote endogenous ethanol production. To reduce alcohol production in the gastrointestinal and genitourinary tracts, ABS patients should adhere to a high-protein, low-carbohydrate diet [
11]. It is possible to tolerate carbohydrates found in fruits and vegetables in small amounts, but nutritionists should be involved in the management of ABS to help patients in adhering to a strict low-carb diet, starting with an initial period of six weeks [
38]. Pharmaceutical treatments for ABS primarily target underlying fungal overgrowth with antifungal medication. In several case reports, fluconazole 100 mg/day for 3 weeks and/or a low-carbohydrate diet was sufficient to treat ABS [
17,
19,
27,
37]. However, in some patients, switching to other antimicrobial medications was necessary due to fluconazole failure, and nystatin, amphotericin, micafungin, itraconazole, voriconazole, metronidazole, or combinations thereof were used. Fluconazole is often used empirically, but it would be more appropriate to treat patients based on antifungal and antibiotic sensitivity testing. As shown in the case by Kruckenberg et al. [
39], undertreated diabetes may lead to difficult-to-treat ABS. There has already been published the anecdotical use of fecal microbiota transplant (FMT) in a case where all other therapies have failed [
16].
For most patients, an integrated and coordinated treatment program is needed, which should include patient input for compliance.
Painter and coworkers have suggested the following therapeutic timeline for ABS treatment [
30]:
Immediate care: The patient with an extremely high blood alcohol level should be treated for acute alcohol poisoning and stabilized.
Drug therapy: A drug therapy should be started, based on culture and sensitivity results for the identified yeast or bacteria. It must be emphasized that the use of a week-long regimen of broad-spectrum antibiotics including metronidazole, ciprofloxacin, and clindamycin recently described in [
36] in patients with MASH may, in fact, further disrupt gut homeostasis and, therefore, may not be suggested. Instead, the experience of several case reports suggests the successful use of a narrow-spectrum antibiotic regimen for treating excessive endogenous ethanol production.
Diet therapy: An essential treatment of ABS is diet modification requiring high protein and low carbohydrates until symptoms subside. A low-carbohydrate diet, therefore, forms the cornerstone of managing endogenous ethanol production, even if it is important to note that microbial strains can vary greatly in their substrate preferences
Supplements: Multi-strain probiotic supplements help balance bacteria in the gastrointestinal tract and have been used in the treatment of ABS, but have yet to be investigated thoroughly.
The risk of relapses of ABS is lessened by avoiding carbohydrates and a nutritionist should be involved for long-term treatment and management of the disease. Anything that causes an imbalance between harmful and beneficial bacteria can potentially induce dysbiosis and increase fermentation in the gut. Antibiotics should be avoided if possible. If an antibiotic course is required, a plan should be in place to again test for fermenting pathogens and to treat them if necessary. In single and various combinations, dietary carbohydrate control, antifungal or antibiotic therapy, general antibiotic avoidance, and probiotics have all been reported as successful treatments. However, patients with long-term, chronic relapses may require fecal microbiota transplants. This has been successfully conducted in a recent case report [
16] describing a 47 yr-old patient with a history of Roux-en-Y gastric bypass surgery [
13] and with intermittent episodes of feeling drunk during the previous 2 months. These symptoms started 1 month after two consecutive courses of amoxicillin–clavulanic acid and moxifloxacin for a respiratory tract infection. A fecal culture identified
Candida glabrata, which has been reported in other cases of gut fermentation syndrome. The patient was given a 100 g oral dose of glucose, and the patient’s blood ethanol levels rose from zero to a maximum value of 8.9 mmol/L 4 h later, which was considered sufficient to establish the diagnosis of ABS. The patient was then treated with a low-carbohydrate diet combined with fluconazole, 100 mg/d orally for 3 weeks, followed by nystatin, 500 000 IU orally four times a day for another 4 weeks. Despite this treatment, the patient still had a random blood ethanol level of 23.6 mmol/L despite completely abstaining from alcohol use. Next therapy was a total of 4 weeks of amphotericin B (oral suspension, 100 mg four times a day for 2 weeks, then 100 mg/d for another 2 weeks), which revealed to be unsuccessful, leading to the withdrawal of the patient’s driving license at a random police check. At this point, a fecal microbiota transplantation was proposed and following the informed consent and appropriate donor screening, the feces of the 22-year-old daughter of the patient were used for jejunum transplant. After 34 months, the patient remained free of symptoms, with repeatedly normal ethanol levels (<2 mmol/L) and normal liver function test results.
For an overview of ABS clinical picture, including involved microorganisms, diagnostic testing, and treatment, see
Figure 4.
6. Conclusions
Endogenous ethanol production is a rare and probably neglected entity, which in some instances may become clinically relevant, and must be suspected in patients in whom ethanol consumption is excluded, either due to the patient age (e.g., in children), or for religion commitments, e.g., followers of the Muslim faith. Nevertheless, due to the growing prevalence of NAFLD (now renamed as MAFLD) worldwide, an ethanol-producing microorganism responsible for endogenous ethanol production such as
Klebsiella pneumoniae or
Saccharomices cerevisiae is increasingly sought. The so-called auto-brewery syndrome is probably the best-known disease linked to excessive endogenous ethanol production, but is certainly not the only one, with metabolic diseases such as diabetes mellitus, obesity, and NAFLD being among the more prevalent conditions. The list of microorganisms that are potentially ethanol-producers is very long and is continuously updated. However, it is important to understand that the detection of an ethanol-producing microbe is not synonymous with the risk of disease associated with endogenous ethanol production, since the gut microbiota is a complex system where microbe-microbe interactions modify the behavior of each microorganism, and also because under physiological conditions, the small quantities of endogenous ethanol formed in the body during sugar metabolism, mainly in the colon and in the bladder, are rapidly eliminated by intestinal and hepatic enzymatic systems [
3]. The clinician should be aware of the existence of multiple fermentation pathways, since this might better help to clarify the underlying pathophysiology of the condition linked to excessive ethanol production, and because it could assist in defining potential therapeutic targets and strategies to mitigate endogenous ethanol production, for example the use of drugs directed against specific microorganisms or specific dietary recommendations.
As far as the latter are concerned, even in the present lack of clinical guidelines, some recommendations may be given, pointing to an integrated and coordinated treatment program, directed by a specific team that will include a gastroenterologist, a nutritionist, a microbiologist, and possible other specialists working together to treat the acute situation and to prevent relapses.
Hopefully, scientific societies will fill the present gap and collaborate in producing international agreed recommendations for ABS and other conditions linked to excessive endogenous ethanol production.