Abstract
Experimental research demands the selection of appropriate models to align with study objectives and conditions. Traditional experimental models, such as in vivo animal studies and in vitro systems like organoids, present nutraceutical and pharmaceutical research limitations such as high cost, ethical concerns, long lifespan, and difficult genetic manipulation. Caenorhabditis elegans has proved to be a valuable model as a result of its genetic and physiological similarities to higher organisms, fully sequenced genome, short life cycle, and transparency. These features enable high-throughput screening, molecular pathway analysis, and lifespan and healthspan assays. C. elegans has significantly advanced the discovery of bioactive molecules with therapeutic potential, shedding light on aging, neurodegeneration, metabolic disorders, and immune responses. Its utility in pharmacokinetics and validation of nutraceuticals underscores its role in longevity and metabolic health research. Additionally, its conserved stress response, apoptosis, and pathogen recognition pathways facilitate the study of pharmacological interventions for inflammation, oxidative stress, and infections. This study evaluates the applicability of C. elegans as a model for in vivo screening, analyses its role in drug efficacy testing, and discusses relevant advancements, associated difficulties, and what to expect of C. elegans in research.
1. Introduction
The emergence of new viruses and the prevalence of chronic illnesses have resulted in an increasing demand for pharmaceutical and nutraceutical products [1]. Moreover, the limited availability of medicines and the possibility of developing resistance is a major concern for human health. As a result, research on developing novel pharmaceuticals and nutraceuticals has become crucial [2,3,4].
Nutraceuticals are bioactive substances, often obtained from food sources, that provide health benefits outside regular nutrition regimes. Pharmaceuticals are substances that have been chemically produced or derived from chemical or biological sources. They are intended to prevent, diagnose, treat, and cure diseases [3]. Pharmaceuticals have traditionally been used to treat serious conditions and prolong life, while nutritional supplements improve the quality of life and attenuate illness [3,4]. To fulfill the expanding requirement for better understanding of these substances, low-cost, relevant, high-throughput screening (HTS) technologies are required [5]. The key concern of therapeutic candidates is whether they exert the expected physiological effect at the site of action; hence, the impact of compounds must be investigated in a biological model. One such biological model is the use of in vitro cellular systems that best represent certain diseases’ physiological and biochemical processes [6]. In developing new drugs, such in vitro systems have been argued to provide more accurate predictions of drug efficacy before animal tests or clinical trials, with applications focusing on target validation, safety, and industrial potential [7]. Therefore, this approach is currently up-scaling the quality of potential drug candidate nominations and guiding drug development success potentials [8,9,10].
Nonetheless, from prior research, the challenges associated with in vitro investigations include the inability to simulate how a pharmaceutical drug would interact with all of the chemicals and cell types found within a complex organ and how to overcome the dynamism of the body’s ecosystem which has various cellular processes in constant communication [11]. To overcome these challenges, technologies based on more advanced co-culture technologies involving living cells in vitro are already emerging. There is currently a movement from two-dimensional cell cultures to three-dimensional cell cultures, a new approach that is more successful at capturing the physiological environment due to many queries about its efficacy [12]. Specifically, 2D cultures have numerous disadvantages, including disrupting the interplay that exists in the cells and outside the cell environments, coupled with changes in cell shape, polarity, and division mechanisms. These drawbacks prompted the development of models that are largely related to in vivo situations. A typical example of such an approach is three-dimensional culture [13].
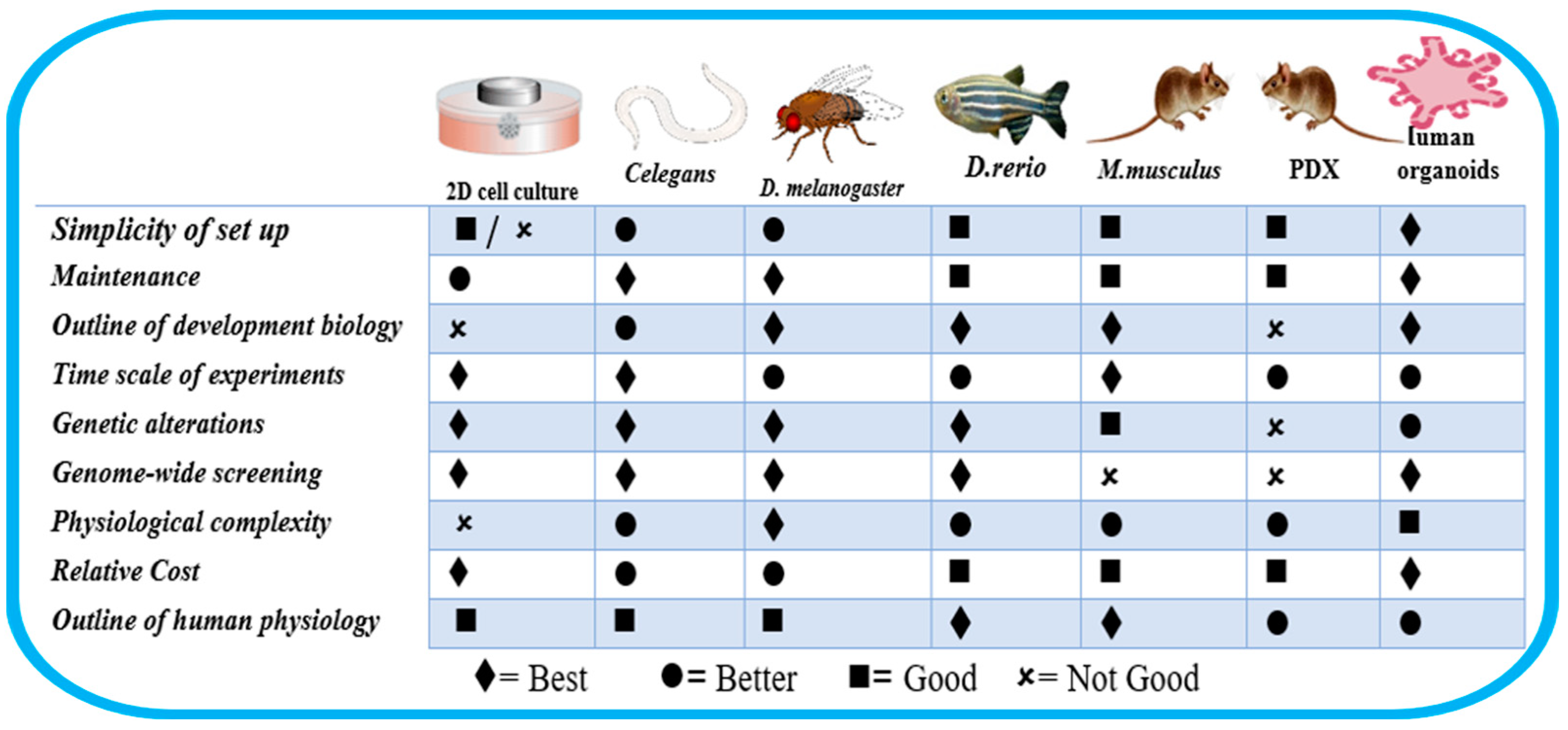
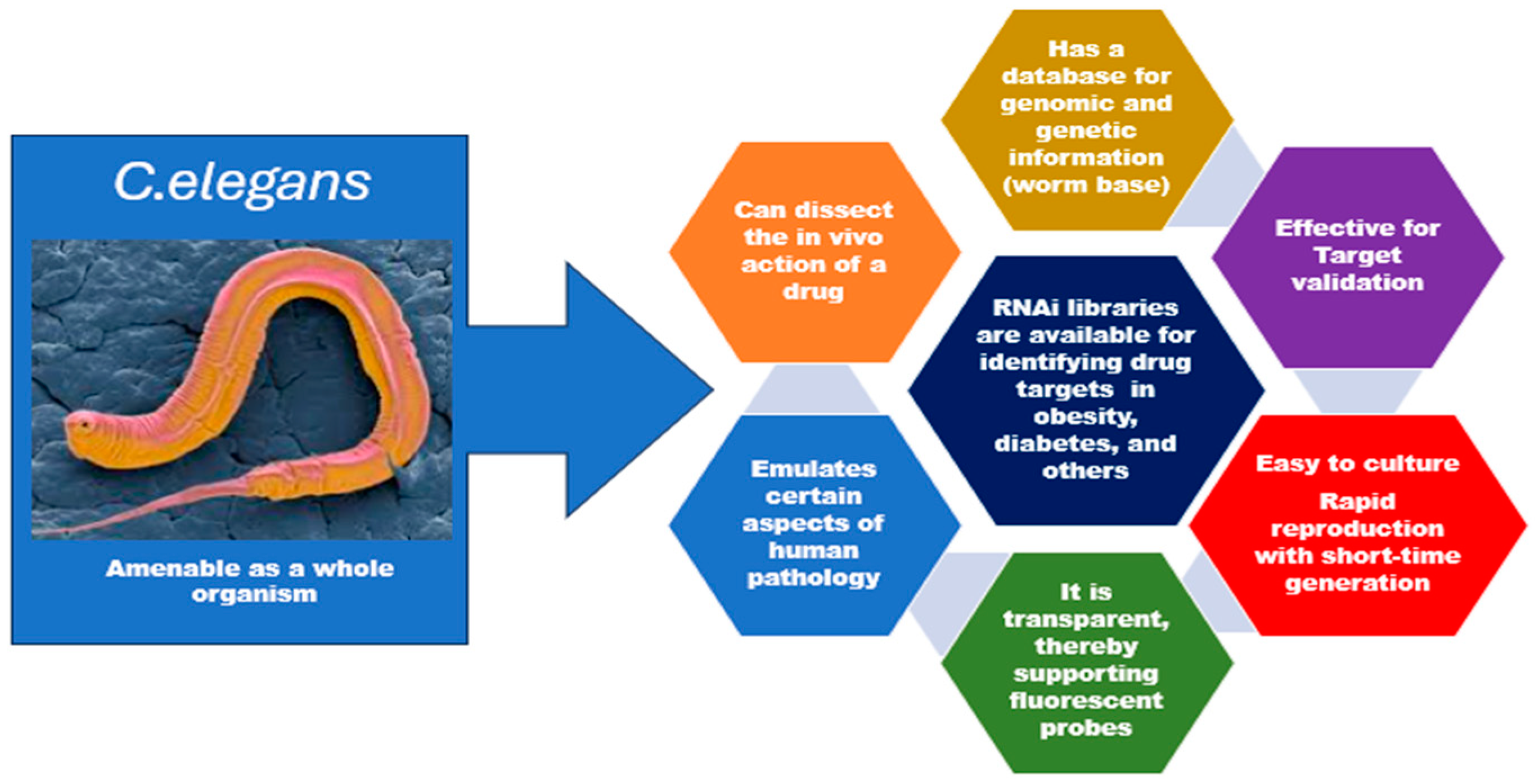
This innovative method can provide three-dimensional architecture and retain the cell heterogeneity found in vitro (Figure 1). However, there is a need to examine its peculiarities, potentials, and possible pitfalls holistically. It is important to examine closely its implications on the overall applications of in vitro systems. For instance, organoids are three-dimensional in vitro cell preparations that can better mimic disease heterogeneity, physiological function, gene specificity, and other features [14]. However, the upregulation of certain protein biomarkers in malignancies was not replicated in organoids. In addition, organoids could only be propagated for 3 weeks before the first passing, and the absence of a well-developed vascular system remains a crucial factor that has impeded the interpretation of drug tests [14,15]. Also, in studies related to congenital disorders, primary organoids may only be derived from pregnancies that have been terminated, which limits prenatal research into fetal development and congenital disorders [16]. It is crucial to remember that experimental models each have their unique set of strengths and limitations [17]. A specific study’s unique research questions and objectives determine the chosen experimental model [17,18]. The organism Caenorhabditis elegans has shown a lot of desirable characteristics such as simplicity, the possibility of genetic manipulation, evolutionary conservation, amenability, and its use in high-throughput screening (HTS) [19]. This points to the need for an increase in the popularity and usage of C. elegans as a more trusted in vivo research model in relevant research (Figure 2).
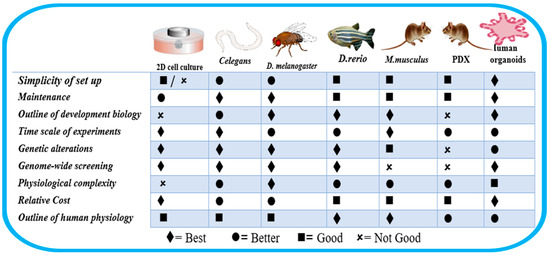
Figure 1.
Characteristics of some cell lines and animal models. Adapted from Kim et al. [20].

Figure 2.
Desirable characteristics of C. elegans for pharmaceutical and nutraceutical research.
Several reviews exist attempting to highlight the role of C. elegans in pharmaceutical development. O’Reilly et al. [19] trace the evolution of C. elegans-based drug screening, addressing the challenges associated with its use and highlighting recent technological advancements that have enhanced its potential for future drug discovery. Huang et al. [21] illustrate the advantages of C. elegans in high-throughput screening (HTS) for animal models. They further explore the mechanisms of candidate compounds, emphasizing their transformative role in translational research for drug discovery and development. Zarroug et al. [22] provide key insights into in vivo antimicrobial efficacy and the toxicity of natural products. It compares their evaluation in conventional vertebrate models. This underscores the value of C. elegans as a powerful model for identifying promising antimicrobial agents. Giunti et al. [23] emphasize the significant role of C. elegans in the discovery of diverse candidate compounds, particularly in research areas such as neurodegeneration, pathogenic infections, and metabolic disorders. Similarly, Kropp et al. [24] discuss how C. elegans contributes to the understanding of rare diseases and supports the development of potential treatments. Unlike these cited studies, our study focuses on using C. elegans in age-related decline, oxidative stress, and its critical role in longevity and healthspan research. We also provide mechanistic insights, including response pathways, beyond its use in basic drug screening, providing strong implications for its use in drug, nutraceutical, and dietary interventions.
C. elegans can be used as an animal HTS model beyond the limitations of 2D cell systems or 3D organoids [25] (Figure 1) because proteome analysis comparing C. elegans and human protein sequences has revealed that human genetic homologs are present for 83% of the C. elegans proteome [26]. Moreover, C. elegans preserves biochemical and molecular biological pathways similar to those of humans. This review will offer a deeper comprehension of the use of C. elegans in evaluating medication effects and discovering their mechanisms in relevant pathways [14].
Section 1 introduces C. elegans and its desirable characteristics over other models, while Section 2 highlights its role in early drug development and target identification. Section 3 and Section 4 explain its role in elucidating action mechanisms and pharmacokinetics. Section 5 and Section 6 delve into the various roles of C. elegans in nutraceutical development and antiaging research, highlighting gene identification, dietary restriction, specific gene mutations, cellular pathways, and the development of medications for age-related disorders. Section 7, Section 8, Section 9 and Section 10 elucidate the role of C. elegans in screening antimicrobial compounds, lipid quantification, antiobesity research, neurodegenerative diseases, and gut health permeability research. Section 10 and Section 11 looks into its limitations and associated toxicity issues, followed by our conclusion.
1.1. Molecular Basis for Using C. elegans in Biomedical Research
The small roundworm Caenorhabditis elegans is a helpful model organism in drug investigation because of its physiological similarities to humans and other higher species, its genetic tractability, and its simplicity [27,28]. In this sense, several biochemical and genetic traits support its efficacy: it possesses numerous important biological pathways and molecular mechanisms that humans and C. elegans share that have evolved throughout time. In both animals, genes related to development, aging, neurology, and illness frequently have functional similarities [23]. As a result, knowledge about these processes in C. elegans may be applied to humans [29,30]. The study of cellular signaling pathways, organ development, and medication effects at the cellular level is made easier by its straightforward architecture, which consists of well-defined cells and tissues and its transparent body [19,31]. Hence, microscopy tools can easily visualize interior structures and cellular processes. Additionally, it possesses about 302 neurons and a thoroughly mapped network of connections of a comparatively simple nervous system. The genome of C. elegans is well-annotated, and strong genetic tools are available for its modification [26]. Precise control of gene expression and functions is made possible by methods like transgenesis, RNA interference (RNAi), and genome editing, making it possible for researchers to investigate how particular genes affect drug responses and disease characteristics. Moreover, C. elegans has a reproduction time of three days and yields a vast number of progenies, approximately 300 eggs for each hermaphrodite adult [32], thus facilitating fast and affordable large-scale screening assays to find possible drug candidates or investigate drug toxicity. Due to its simplicity, the worm is a great model for research in various fields such as neurobiology, particularly in terms of how medicines affect the behavior and function of neurons [19,27,28,32].
1.2. Is C. elegans Suitable as a Reliable Model?
Although imperfect, it has been considered an ideal model for various studies [33]. In comparison to humans, C. elegans has simpler biology. This implies that results might not always apply to humans directly. C. elegans also lacks some human organs. Therefore, it may not be the best model to investigate complicated disorders or those strongly influenced by those organs, and the nutritional research results may not immediately apply to C. elegans because it has different dietary requirements from humans. Also, C. elegans does not perfectly recapitulate the complete pathophysiology of vertebrates [34]. Although this experimental model has several drawbacks, C. elegans’ ability to construct an overexpression model of any gene, in a single or combined state, and to conduct toxicology, recovery, or survival trials in short periods and at low cost is desirable. A continuous increase in its employment is expected [19,27,34].
The systematic combination of its genetic amenability, simplicity, and significance to human biology makes C. elegans a highly versatile and reputable standard model organism for several research areas, including developmental biology, neuroscience, genetics, and drug discovery [35]. It has found application in modelling various diseases such as high blood glucose, cardiovascular diseases, hyperlipidemia (obesity), and neurodegenerative diseases, it has helped to provide critical clues into the underlying systems of these diseases as well as unravel the beginning and progression of such illnesses [36]. The evolutionary conservation of C. elegans and humans has paved the way for identifying novel therapeutic targets, whereby prospective therapeutic targets are validated in vivo.
2. C. elegans for Early Drug Development and Target Identification
Drug discovery can be viewed as a three-component equation in which bioactive chemicals are generated to act on molecular targets and change disease phenotypes [37]. As a result, drug development efforts begin with different experimental starting points, such as target identification or disease models explored by bioactive molecules. The principal target of most drugs is protein, since it is a major biochemical component of biological systems [38]. C. elegans has been used to expatiate the basic elements of pivotal biology processes, such as apoptosis, aging, and gene expression control, and to discover innovative drug targets and therapy in the pharmaceutical research sector [28].
Target validation is critical to any drug development effort [39]. It is a major challenge for post-genomic drug development, and selecting promising therapeutic targets from a large candidate pool seems outright impossible without a model system capable of reliably replicating in vivo processes and symptoms. To address this challenge, C. elegans has been leveraged as a well-characterized experimental tool for genetic research of biological regulatory networks [24,39]. Using this model system, it is possible to observe the impact of mutations on normal embryonic and post-embryonic development (before and after fertilization) and signal transduction pathways [28]. To this end, C. elegans is also used to model complex illnesses such as human diseases [40]. Leveraging its relatively small size and use of fluorescent SiNPs as low-toxicity probes, it has now become possible to observe the physiological and pathophysiological processes of living organisms [41,42,43,44]. This, in turn, simplifies the screening process of new pharmaceuticals and nutraceuticals for their impact on the intricate biological mechanisms involved in human illness; thus, C. elegans offers great potential for both target identification and early drug development [36].
Using a drug to modulate the activity of a specific target protein in an organism and track the effects over time has enabled the development of genetic screens for identifying the proteins and genes that may influence the pharmacological response [14]. In previous years, the process was largely manual and lacked scalability. However, recent developments in high-throughput liquid screening, image-based equipment, and data processing have made the cellular model a viable candidate for drug screening automation [45,46,47]. Unlike single-celled organisms and in vitro experimentation, C. elegans can simulate complex human diseases and further assess ADMET parameters (absorption, metabolism, distribution, excretion, and toxicity) at the initiation of drug discovery and then through the subsequent course. Additionally, the nematode contains various phenotypes that can be measured, and its multicellular structure offers further advantages [48]. Moreover, C. elegans has existing genetic tools and genomic resources [45,48].
C. elegans also possesses easily accessible, time-tested genetic tools and genomic resources (such as the RNAi-feeding library), allowing for greater precision in identifying drug targets and their putative mechanisms of action.
High-throughput drug screening assays are primarily centered around image-based phenotyping, with a diverse extent of throughput resolution and additional requirements for specialized tools such as microfluidic devices and worm trackers [49,50,51,52]. These assays mainly focus on morphological-descriptive characteristics of C. elegans while giving limited attention to vital locomotor parameters, which are crucial for screening drugs for muscle-related disorders [48]. Significant achievements in fields such as neurodegeneration, cancer, and metabolic diseases emphasize the relevance of this approach in applied research. Ongoing progress in genetic methodologies, combined with multi-omics technology, is set to further increase the utility of C. elegans in identifying new therapeutic targets [34].
3. C. elegans for Evaluation of Drug Effects and Possible Action Mechanisms
C. elegans self-fertilizes quickly, has a brief lifespan, and a straightforward maintenance routine in the laboratory [44]. This facilitates the easy elucidation of several metabolic pathways. Using C. elegans has enabled the examination of behavioral effects (activity, feeding, and chemotaxis) at different magnitudes of pharmaceutical and nutraceutical intake and the establishing of a dose-dependent relationship of various active ingredients and bioactive substances [48]. Furthermore, C. elegans is particularly relevant for studying aging and other metabolic processes due to its distinct life stages that can be physiologically and genetically examined [53,54,55]. The highly conserved insulin/IGF-1 signaling system (daf-2/daf-16) in C. elegans has been critical in aging research, suggesting therapeutic targets for healthy aging. For example, studies in C. elegans have demonstrated that inhibiting the mTOR pathway (a cellular regulator of growth and metabolism) increases lifespan. These discoveries have influenced drug development efforts, with molecules such as rapamycin being investigated for their anti-aging and therapeutic potential in age-related disorders [56,57,58].
In cancer research, C. elegans is a biosensor for general cancer screening [59]. It has conserved genetic pathways involved in cell cycle control and apoptosis, making it a useful model for discovering potential cancer targets. In the apoptosis pathway, the identification of the fundamental apoptotic machinery in C. elegans, which includes ced-3, ced-4, and ced-9 (homologous to human caspases and BCL-2 family members), has identified targets for drugs aimed at influencing cell death in cancer [60]. Unlike in Drosophila and mice, where apoptosis occurs via proteolytic cascades of initiator and executioner caspases, CED-3 is the only major caspase required for apoptosis in C. elegans [61]. Specifically, BH3 mimetics that inhibit anti-apoptotic proteins in cancer cells were developed using insights from C. elegans research [62]. Moreover, genes such as HCP-1, HIM-10, ZW10, CENP-A, and CENP-C involved in chromosomal segregation have been examined in C. elegans for a proper understanding of their involvement in genomic stability [63]. Dysregulation of these pathways has influenced the development of cancer treatments that target mitotic checkpoints.
C. elegans has been used to study the pharmacodynamics and drug–drug interactions of anthelmintic ivermectin (IVM) and eprinomectin (EPM) [55]. It has also been used as a promising model in validating novel effective medications in treating helminth infections and elucidating the mechanism of action of anthelmintic compounds [5]. In addition, high-throughput behavioral screens of C. elegans have exposed potential drug candidates for Parkinson’s disease (PD), emphasizing the utilization of high-throughput platforms and methodology that evaluate the automation of C. elegans postures [42]. The nematode has also assisted in investigating dose, exposure time, and drug–drug interactions [64,65,66]. A C. elegans model for Amyotrophic Lateral Sclerosis (ALS) has been developed using the dnc-1 KD approach. Incorporating the Multi-Worm Tracker (MWT) with the dnc-1 KD model creates a behavior-based, automated, and quantitative method for drug screening [56,67,68]. C. elegans has also provided insights into the action mechanism of Metformin by modifying microbial folate and methionine metabolism through the lysosomal pathway [69,70]. Additionally, it has contributed to understanding dopaminergic degeneration in neuroprotection and the progression of Alzheimer’s disease [71,72,73]. Synaptic transmissions and damage, the cause of cognitive impairment in Alzheimer’s disease, have been widely studied using C. elegans. A resveratrol-loaded nanostructured lipid carrier that increased acetylcholine concentration at the synapses was employed to treat Alzheimer’s disease using C. elegans [73]. Interestingly, aldicarb, a carbamate of acetylcholine esterase inhibitors, is known to induce paralysis in C. elegans by persistently activating ionotropic acetylcholine receptors. The severity of paralysis and the rate at which it develops are dependent on the dosage. RNA-mediated interference has revealed that mobility assays apply to probes related to both genetic and drug screening. Essentially, C. elegans presents a viable choice for drug screening and the research assessment of pharmaceuticals and other genetic and RNAi-mediated research [74].
4. C. elegans in Pharmacokinetic Studies
Pharmacokinetics examines the absorption, distribution, associated metabolism, and excretion of drugs and related substances (ADME). Traditionally, it relies on mammalian and in vitro models [48]. However, C. elegans has become visible as an affordable, genetically tractable alternative [75]. Despite its simplicity, it shares key drug metabolism pathways with mammals, including CYP enzymes, ABC transporters, and detoxification mechanisms, making it valuable for high-throughput pharmacokinetic research [75,76,77]. Interestingly, utilizing C. elegans in pharmacokinetic research goes beyond typical studies, as advances in microfluidic technologies now allow for real-time monitoring of medication uptake and metabolism within individual worms. Additionally, genetically engineered strains expressing human CYP enzymes can potentially upscale the prediction accuracy of drug metabolism investigations [77]. Table 1 highlights the pharmacokinetic applications of C. elegans in drug discovery.
Researchers identified several metabolic pathways in C. elegans, including the formation of glucoside metabolites for drugs like albendazole and genistein, which appear to differ from mammalian metabolism. Amirthagunabalasingam et al. [51] developed an epifluorescent microscopy approach to monitor doxorubicin uptake into the pharynx of C. elegans, highlighting the role of organic cation transporters in this process. The accumulation of fluorescein, an organic anion, in the intestinal lumen and cells of C. elegans has demonstrated the functionality of organic anion transporters (OATs) in this organism. However, Hartman et al. [75] noted the presence of physical barriers to chemical uptake in C. elegans, emphasizing the importance of considering these factors in pharmacokinetic studies. Fluorescent tracers and high-performance liquid chromatography (HPLC-MS/MS) have enabled real-time tracking of drug absorption and distribution within the worm’s tissues [78]. Hence, various compounds, including anticancer drugs, anthelmintics, and antidepressants, have also been tested using methods such as HPLC-MS/MS and fluorescence microscopy to track drug uptake and metabolism. However, important limitations were noted, including how environmental factors (such as E. coli presence) affect drug processing and how C. elegans’ metabolism differs from that of mammals. Accordingly, Van der Most et al. [79] highlighted the influence of environmental factors, such as the presence of E. coli as a food source, on the uptake and elimination of fluoxetine in C. elegans. While C. elegans offers advantages like easy genetic manipulation and quick experiments, the distinct metabolic pathways observed suggest results may not directly translate to higher organisms, indicating that C. elegans can be valuable for initial drug metabolism research, but additional testing in more complex organisms remains necessary for drug development applications [19,75].
A major advantage of C. elegans is its ability to model drug absorption. The worm’s intestine, its primary site of drug uptake, allows researchers to study how compounds permeate biological membranes. Studies have shown that lipophilic drugs, such as rifampicin, are more readily absorbed, whereas hydrophilic drugs, such as metformin, exhibit limited uptake trends that mirror those observed in mammalian models [77]. Beyond absorption, C. elegans is also a valuable tool for studying drug metabolism. Its xenobiotic detoxification pathways include phase I modifications via CYP enzymes and phase II conjugation through UDP-glucuronosyltransferases (UGTs) and glutathione-S-transferases (GSTs) [76,77]. For instance, Shen [80] demonstrated that acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) undergo a metabolic transformation in C. elegans through homologs of human CYP1A2 and CYP3A4. These findings propose that C. elegans can be used to predict drug metabolism patterns before progressing to mammalian studies. The excretion of drugs in C. elegans further highlights its pharmacokinetic relevance. Efflux transporters, particularly P-glycoprotein (PGP-1), are crucial in drug clearance. Studies have shown that C. elegans mutants lacking PGP-1 exhibited increased doxorubicin accumulation, thereby reinforcing the importance of ABC transporters in drug efflux. This ability to study transporter-mediated drug excretion provides valuable insights into mechanisms of drug resistance and clearance [76,77].
While it does not fully replace mammalian models, it is an efficient preclinical tool for early-stage pharmacokinetic screening, helping to optimize drug candidates before transitioning to more complex systems. The progressive development of more advanced technologies, such as integrating artificial intelligence and machine learning models with C. elegans pharmacokinetic data, could further refine drug screening pipelines, reducing reliance on costly mammalian trials.

Table 1.
Examples of pharmacokinetic application of C. elegans in drug discovery.
Table 1.
Examples of pharmacokinetic application of C. elegans in drug discovery.
| S/N | Technical Approaches | Applications | Drug Class | Limitations | Ref |
|---|---|---|---|---|---|
| 1 | Fluorescence microscopy and plate reader | Quantifying organic anion uptake and accumulation | Organic anions (Fluorescein) | Limited to fluorescent compounds | [51] |
| 2 | Epifluorescent microscopy | Quantifying organic anion uptake and accumulation | Anticancer (Doxorubicin) | Limited to fluorescent compounds | [52] |
| 3 | In-silico docking and ADMET analysis | Predicting drug interactions and properties | Herbicide (Paraquat), Antiparkinson (Levodopa) | Requires experimental validation | [81,82] |
| 4 | High-Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS) | Metabolite identification and characterization | Anthelmintic (Albendazole) | Requires specialized equipment | [83] |
| 5 | Whole genome microarrays, Reverse Transcription Quantitative Polymerase Chain Reaction (RT-QPCR), HPLC-MS | Gene expression analysis and metabolite identification | Anthelmintics (Ivermectin, Albendazole) | Complex data interpretation | [84] |
| 6 | Liquid Chromatography with Diode Array Detection (LC-DAD) and LC-MS/MS | Detailed metabolite characterization | Isoflavone (Genistein) | Requires specialized equipment | [85] |
| 7 | Toxicokinetics experiments, one-compartment model | Studying drug absorption, distribution, metabolism, and excretion | Antidepressant (Fluoxetine) | Model assumptions may not fully represent biological complexity | [79,82] |
| 8 | Behavioral analysis | Assessing drug effects on nematode locomotion, and potential resistance. | Anthelmintic (Pyrantel) | Indirect measure of pharmacokinetics | [76,85] |
5. C. elegans and Nutraceutical Development
Nutraceuticals are bioactive food products that present additional health advantages beyond regular nutrition [86]. These compounds are sometimes linked to oxidative stress reduction, preventing inflammation, and promoting longevity [86,87]. This model organism functions as a valuable tool for researchers exploring the mechanistic effects of nutraceuticals [88].
A key strategy in nutraceutical development using C. elegans involves examining the impact of bioactive components on the worm’s healthspan, lifespan, and overall physiology [89]. C. elegans plays a crucial role in uncovering genetic pathways linked to various physiological processes and assessing the influence of nutraceuticals on these pathways. Notably, two well-characterized pathways regulating these processes in C. elegans are the transforming growth factor-β (TGF-β) and insulin/insulin-like growth factor-1 (IGF-1) pathways. These pathways provide critical molecular insights into the mechanisms underlying numerous pathological conditions and support the development of novel nutraceuticals [89].
Strong evidence supports the potential of nutraceuticals, such as chondroitin sulfate, in developing therapeutic agents for Alzheimer’s disease. Studies using transgenic strains of C. elegans have shown their ability to protect against oxidative stress and Aβ-induced toxicity [90,91]. The neuroprotective effects of certain nutraceuticals have also been well-documented. For instance, epigallocatechin-3-gallate, found in green tea and rapeseed pomace, has been shown to suppress neurodegeneration, while Grifola frondosa contributes to reduced fat accumulation and extended lifespan through the DAF-16/FOXO and SKN-1/NRF signaling pathways. These effects have been extensively studied in C. elegans, particularly with insulin signaling and other regulatory pathways [92]. Additionally, C. elegans serves as a valuable model for screening potential nutraceuticals by assessing their effects on specific phenotypes. Research has identified bioactive compounds with antioxidant properties that enhance mitochondrial function and promote healthy aging. These findings pave the way for developing novel anti-aging compounds [93,94].
Bioactive compounds are often assessed for bioactivity by evaluating a variety of phenotypes, including a significant reduction in growth rate, lethality, uncoordinated movement, and other morphological defects in wild-type or mutant animals. C. elegans worms show a variety of intrinsic behaviors, including chemotaxis toward attractive signals, aversion to unpleasant stimuli, and mating. Immune cells employ chemotaxis to move between the vascular and lymphatic systems and migrate from the blood to infection sites [95,96]. This appears to be a reliable strategy for studying the behavioral patterns of bioactivity in nutraceuticals. A major bottleneck worthy of note is in the administration of hydrophobic compounds to C. elegans, as highly hydrophobic nutraceuticals such as curcumin are prone to precipitation when added to aqueous media containing C. elegans growth medium, often resulting in low and varied levels of bioaccumulation in C. elegans, thereby complicating the standardization of experiments and the interpretation of the results during nutraceutical development and analysis [96].
6. C. elegans in Pharmaceutical Development in Anti-Aging Research
6.1. C. elegans in the Identification of Genes That Affect Lifespan
C. elegans is used in aging, age-related disorders, longevity, and drug screening, owing to its well-defined physiological characteristics with regard to aging [97]. Both genetic and environmental factors influence lifespan. Researchers have used C. elegans to identify genes that regulate aging, demonstrating that certain gene mutations can extend lifespan by up to 50%. Many of these genes are linked to key signaling pathways that connect aging to environmental conditions [98]. C. elegans has fundamentally shifted the understanding of aging from a passive deterioration to an actively regulated process. One of the most significant findings was identifying the IGF-1 signaling pathway as a key controller of lifespan in C. elegans. This discovery provided critical insights into aging mechanisms and established insulin signaling as the first known pathway involved in lifespan regulation [99]. Research using C. elegans has also uncovered various anti-aging strategies, including dietary restriction, specific gene mutations, cellular pathways, and natural compounds that can slow aging.
Epigenetic modifications also play a pivotal role in the response to environmental stress. C. elegans can adapt to various stressors, such as heat and oxidative stress, through epigenetic changes that help protect against age-related decline. Researchers have utilized C. elegans to investigate how these epigenetic mechanisms are affected by factors such as diet, lifestyle, and pharmaceutical compounds. Notably, compounds that target epigenetic regulators, such as histone deacetylases (HDACs), are being explored for their potential to extend lifespan and promote healthier aging [99,100].
6.2. Dietary Restriction and Specific Gene Mutation
Aging significantly increases the risk of developing chronic diseases. One effective strategy for delaying age-related illnesses is dietary restriction (DR), which involves reducing food intake without causing malnutrition. Research across various organisms has shown that DR can extend lifespan, and model organisms like C. elegans are widely used to investigate the underlying mechanisms with potential therapeutic applications [101,102]. Studies in C. elegans have demonstrated that reducing calorie intake can substantially increase longevity, sparking further research into dietary interventions for lifespan and healthspan extension. However, in laboratory settings, C. elegans is typically fed attenuated E. coli bacteria (OP50) on agarose plates [102]. To this end, Greer et al. [101] identified several distinct DR regimes in C. elegans: A genetic mutation (eat-2) that reduces pharyngeal pumping, thereby decreasing food intake; bacterial dilution in liquid cultures (bacterial DR (bDR) and liquid DR (lDR)); two chemically defined liquid media—axenic medium and chemically defined liquid medium (CDLM)—which mimic DR-like effects; peptone dilution on agarose plates (DP), which inhibits bacterial growth; complete absence of bacteria on plates, referred to as dietary deprivation (DD); and a recently developed approach which requires the serial dilution of bacteria on plates, known as solid DR (sDR).
Lowering metabolic rate and reducing IGF-1 signaling are two proposed mechanisms through which dietary restriction (DR) extends lifespan [103]. While experimental data on this topic remain limited, interaction studies suggest that DR and insulin signaling may function in parallel within similar regulatory systems [104,105]. Additionally, evidence indicates that the target of rapamycin (TOR) pathway is responsible for the impact of DR on longevity in C. elegans [106]. This suggests that DR’s impact on lifespan likely involves multiple metabolic pathways, which may vary depending on the specific DR regimen used and the organism studied [101].
Certain gene mutations in C. elegans have been found to prolong lifespan, in some instances nearly doubling it. These mutations often enhance stress resistance, improve protein quality control, or optimize metabolic efficiency [101].
6.3. Natural Chemicals and Cellular Pathways
Natural compounds that target conserved cellular pathways pave the way for the development of novel nutraceuticals and pharmaceuticals to promote longevity and healthspan [107]. Researchers have identified numerous anti-aging substances by feeding C. elegans various natural compounds, including antioxidants, sirtuin activators, and rapamycin. For instance, Ganoderma lucidum promotes oxidative stress resistance and extends lifespan in C. elegans. Its therapeutic effects may be mediated through multiple signaling pathways [108].
Additionally, administering Momordica saponin extract to both stressed and normal C. elegans increases the expression of key antioxidant enzymes [109]. Studies across various model organisms also suggest that curcumin supplementation extends lifespan by enhancing SOD activity while reducing levels of malondialdehyde (MDA) and lipofuscin, both of which are markers of oxidative damage and aging [110,111,112,113].
Curcumin also alters critical signaling pathways that regulate organism lifespan, such as the IIS, mTOR, PKA, and FOXO signaling pathways. It also induces HSP expression, enhances oxidative stress resistance, reduces ROS accumulation, and promotes autophagy [110], thus enhancing motility, healthspan and lifespan in C. elegans. Berberine, epigallocatechin gallate, and quercetin are other natural substances for which mechanistic studies have revealed their lifespan-promoting effect in C. elegans through the modulation of the IIS pathway, AMPK activation, improvement of mitochondrial function, inhibition of the TOR pathway, induction of autophagy, and activation of DAF-16. Undeniably, C. elegans research has shed light on several cellular pathways that play critical roles in aging, including the insulin/IGF-1 signaling system, the mTOR pathway, and the unfolded protein response [109,114,115,116].
Various methods of modifying nutrition and chemical substances increase longevity via separate and non-linear genetic pathways. Numerous peptides interacting with the insulin/IGF-1 receptor regulate the IIS pathway in invertebrates. The IIS pathway in C. elegans includes several proteins encoded by some genes, including daf-18 (encoding the human tumor suppressor PTEN homolog), daf-2 (mammalian insulin/IGF-1 receptor homolog), age-1 (PI3K homolog), and akt-1 (serine–threonine protein kinases) [109]. The reduced activity of the genes age-1, akt-1, and daf-2 was demonstrated to down-regulate this signaling cascade, and worms with mutations in these genes were proven to live longer. This important finding that lower insulin/IGF-1 signaling (IIS) levels can elicit lifetime extension in the worm C. elegans was published 24 years ago [109]. Alterations in the insulin/IGF-1 receptor gene daf-2 or other genes in this pathway can double longevity in this organism. On the other hand, activating the IIS pathway shortens the lifespan of C. elegans. Resveratrol notably increased the longevity of wild-type worms by a small but statistically significant amount. Resveratrol, on the other hand, did not extend the lifetime of aak-2(ok524) mutant worms, suggesting that AMPK is required for resveratrol’s favorable effects on longevity [103,114]. AMPK activity is not necessarily linked to FoxO activation, even though AMPK’s capacity to increase longevity is dependent on the presence of FoxO. Resveratrol has been shown to extend the lifespan of daf-16(mu86) mutant worms, meaning that its longevity-promoting effects occur through an AMPK-dependent but FoxO-independent pathway [103,114]. The improved stress resilience and health-promoting effects associated with Momordica saponin extract (MSE) in C. elegans may stem from its dual function as a direct free radical scavenger and a regulator of signaling pathways involved in stress adaptation. MSE-mediated stress resistance appears to include the insulin signaling (IIS) pathway [109,115].
6.4. Developing New Drugs to Improve Age-Linked Diseases
A critical part of aging research is the quest for drugs that promote lifespan and healthspan, followed by a thorough examination of their mechanisms of action. However, identifying both medications and their targets is difficult. C. elegans is an appropriate model for such studies due to its short lifetime and genetic tractability [115]. By suppressing 18S rRNA/ribosome, fatty acid amide hydrolase-4, Hsp90, and the Ca2+-activated K+ (BK) channel SLO-1, minocycline, JZL184, monorden, and paxillin have been shown to improve the lifespan of C. elegans. [116,117]. In several animal species, including C. elegans, decreasing mRNA translation and protein synthesis increases lifespan. Rapamycin, which reduces mRNA translation by blocking the mechanistic target of rapamycin (mTOR), increases lifespan and healthspan in various organisms from yeast to mammals. As a result, new chemicals that inhibit protein synthesis are attractive antiaging therapeutic prospects. In recent times, numerous antiaging compounds have been identified through the use of C. elegans models [117,118]. It has been used to study drug development that can reduce lipofuscin accumulation, a pigment thought to contribute to aging [118,119]. Because many of the processes that govern aging and longevity in C. elegans are evolutionarily conserved, these newly discovered lifespan-extending chemicals may help lead the development of antiaging drugs for human applications.
C. elegans has been utilized as an organismal model to assay for survival against a range of mitochondrial inhibitors by measuring worm longevity and aging indicators such as pharyngeal muscle contraction, pigment buildup, lipofuscin, and ATP levels [120]. C. elegans has thus helped in the development of FDA-approved medications and nutritional alternatives that not only elongate but also improve the overall quality of life [120,121,122].
Organisms constantly experience environmental fluctuations and disruptions in intracellular homeostasis, which can negatively impact their proteome and overall physiology [123]. To subdue these constraints, they have evolved specialized and highly regulated stress responses that repair cellular damage and maintain homeostasis. These protective mechanisms include the heat shock response (HSR), the unfolded protein response in the endoplasmic reticulum, the unfolded protein response in mitochondria, and the oxidative stress response [123]. Research on stress resistance provides valuable insights into how internal and external stressors influence biological processes such as cellular homeostasis. The ability of C. elegans to withstand various abiotic stressors, including oxidative stressors, hypoxia, hyperoxia, extreme temperatures, osmotic stressors, ultraviolet (UV) radiation, endoplasmic reticulum (ER) unfolded protein stress, and heavy metal toxicity, has significantly contributed to our understanding of complex disease pathways [124].
Scientists have utilized C. elegans to investigate how various stresses impact aging. For example, studies have unveiled that exposure to oxidative stress may reduce lifespan. Research into stress responses in C. elegans has uncovered a range of complex processes and disorders [124]. However, there is a lack of consensus in the heat shock/heat stress field regarding the optimal timing, temperature, and duration of exposure, leading to significant variation in experimental protocols across different laboratories. This lack of standardization makes results comparison a Herculean task across studies. Determining the best approach when developing a new protocol has also become increasingly difficult [125]. The desire to understand how cells respond to physical and chemical stressors has increased interest in stress proteins as potential toxicological endpoints. In this context, transgenic strains of C. elegans have been created, featuring a reporter enzyme controlled by a stress-inducible promoter [126]. This reporter is easily measurable in live nematodes and responds to several chemical stimuli, making transgenic C. elegans a valuable tool for developing rapid, accessible, and informative bioassays.
7. C. elegans in Screening Novel Antimicrobial Compounds
The rise of antibiotic resistance and the urgent need for novel antimicrobial agents have driven researchers to explore alternative high-throughput screening (HTS) models for drug discovery [127]. C. elegans infection models allow researchers to study host–pathogen interactions, drug efficacy, and toxicity within a living organism. Although C. elegans lacks an adaptive immune system, its innate immune responses are similar to mammalian immunity, including TLR signaling, MAPK pathways (e.g., p38 MAPK in pathogen response), and antimicrobial peptide (AMP) synthesis [128,129]. Because of its conserved pathways, it is an ideal surrogate host for testing immune regulation by antimicrobial drugs. C. elegans may be cultivated on 24, 48, 96-well plates, and more, allowing for automated HTS of chemical libraries [130]. Also, C. elegans investigations require 100–1000 times lower medication doses than mammalian models, resulting in significant cost reductions [5].
C. elegans models can help identify new antimicrobial compounds, including those that might work against antibiotic-resistant strains and persister cells [131,132]. These models appeared particularly useful in identifying compounds that work in living organisms but might be missed by traditional laboratory testing methods, possibly because they capture host and pathogen relationships. C. elegans models proved effective in identifying novel antimicrobial compounds across various studies. For instance, Escobar et al. [46] discovered N-[3,5-Bis(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide (IMD0354), a kinase inhibitor with potent activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE).
A total of 28 compounds not previously reported to have antimicrobial properties have been identified [133]. Samsudin [131] found synthetic retinoids CD437 and CD1530 effective against MRSA and persister cells. Other microbial strains used are Vancomycin-Intermediate Staphylococcus aureus (VISA), Vancomycin-Resistant Staphylococcus aureus (VRSA), and Methicillin-Susceptible Staphylococcus aureus [134,135]. Four novel anti-staphylococcal compounds with minimum inhibitory concentrations (MICs) ranging from 2 to 8 ffg/mL against MRSA have been discovered [136]. These discoveries highlight C. elegans models’ potential to identify compounds with novel mechanisms of action, the ability to modulate host defense, the capacity to target bacterial virulence factors, and effects beyond direct inhibition of bacterial growth.
The compounds identified through C. elegans screening show promising therapeutic potential (Table 2). IMD0354 demonstrated potent bacteriostatic activity against various vancomycin-resistant strains with low toxicity. Synthetic retinoids CD437 and CD1530 showed efficacy against both growing and persister MRSA cells, thus addressing a significant clinical challenge. Additionally, Brevinin-2 family peptides improved the survival of MRSA-infected worms and showed potential as new applied peptide candidates [137]. These studies report that C. elegans models identified compounds effective against antibiotic-resistant strains, which may be relevant to current clinical needs.

Table 2.
C. elegans models in identifying novel antimicrobial compounds across various studies.
8. C. elegans in Lipid Quantification and Antiobesity
8.1. Genetic Regulators of Lipid Metabolism
The importance of using model systems that compare favorably with in vivo high-throughput genetic screening to relate genome-wide sequencing (GWAS) gene candidates to several clinical disorders is on the rise [142]. Obesity and its associated metabolic syndrome are a fast-rising public health challenge. Given its high impact on people and the peculiarity of the healthcare system, there has been a surge in interest in discovering pharmaceutical targets for obesity therapy and prevention [143]. Although C. elegans is evolutionarily distant from humans, the two species share key lipid, sugar, and protein metabolic pathways [144]. In both organisms, regulators like TOR kinase and AMPK, as well as transcription factors like Sterol response element binding Protein (SREBP), Peroxisome proliferator-activated receptor gamma (PPAR), and Transcription Factor EB (TFEB), regulate genes related to metabolism and cell responses when nutrients are available. In C. elegans and human systems, the loss of function of such regulators generates comparable metabolic dysregulation, such as obesity or resistance to it [142,143,144]. Although GWAS has been employed to identify variants in human genetics that are associated with obesity, there is a need for experimental assertion, which consequently provides a springboard and direction for its prevention and treatment. As a result, hundreds of human genes related to obesity have been identified and classified using C. elegans [145].
A similar study identified 14 genes that support obesity and three genes that inhibit diet-induced obesity when silenced. The holistic effect of this prevention results in the prevention of excess fat accumulation and an overall lifespan expansion in C. elegans [146]. The use of model systems for in vivo high-throughput genetic screening is becoming increasingly important for linking GWAS gene candidates to various clinical disorders [142].
8.2. Experimental Approaches and Applications of C. elegans in Lipid Antiobesity Studies
The model organism C. elegans has been extensively used to investigate lipid metabolism through a variety of techniques, such as imaging lipid droplets, analyzing total fatty acids, and utilizing lipidomics approaches to study complex lipids [146]. Several transcription factors have been identified as key regulators of lipid metabolism in C. elegans. For instance, the loss of mdt-15, a homolog of peroxisome proliferator-activated receptor-gamma coactivator 1 α, results in reduced fat storage in nematodes [147,148,149]. Additionally, cebp-2 is important in regulating fatty acid mitochondrial β-oxidation and desaturation [148,149]. Increased expression of nhr-49 and ech-1.1 enhances mitochondrial β-oxidation of fatty acids. Furthermore, delta-9 desaturases, including fat-5, fat-6, and fat-7, regulate overall body fat by modulating fatty acid production [148,149,150,151].
In a study investigating the potential antiobesity effects of a natural product on C. elegans, Shen et al. [147] analyzed 18 lipid and glucose metabolism genes using an RT-qPCR assay. Their findings revealed that the citrus extract under consideration significantly reduced the expression levels of genes such as mdt-15, tub-1, cebp-2, nhr-80, fat-7, and sbp-1, to mention a few. These results suggest that the extract may inhibit lipid accumulation (fat-5, fat-6, fat-7, fat-4, fat-2, sbp-1), modulate transcriptional regulators involved in lipid metabolism, such as those linked to β-oxidation and fatty acid desaturation (mdt-15 and cebp-2), and influence lipid signaling (nhr-80 and tub-1). However, expression of atgl-1, acs-2, aak-2, hosl-1, elo-2, ech-1.1, and nhr-49 did not show significant changes. Furthermore, C. elegans can synthesize various lipids, and lipid metabolic genes present in the worm have been identified by comparative genomics. In particular, 71 of these conserved genes are linked to human metabolic disorders. The various lipid classes identified in C. elegans have been reviewed by Witting et al. [146], as well as their biosynthetic processes and chosen biological and therapeutic functions.
Fatty acids are utilized as building blocks for complex lipids. They also function as signaling molecules, and fatty amides are crucial molecules for metabolic signaling, such as relating diet to lifespan extension [148]. In C. elegans, PUFAs are essential for proper neurotransmission, and the C. elegans mutant fat-3 causes functional but not developmental nervous system abnormalities and inadequate neurotransmitter release [148,149]. Comprehensively, the central nervous system plays a crucial role in regulating energy balance. Studies aimed at understanding the physiological mechanisms of body weight management identified dopamine as a key regulator of energy balance [150,151,152]. Knockout mice with impaired dopamine function consume significantly less food and succumb to malnutrition within a few weeks after birth. Additionally, hormones such as leptin, insulin, and ghrelin, which relay the energy state of the peripheral nervous system to the CNS, act on dopaminergic neurons [153,154,155]. Genetic studies of fat storage in C. elegans exposed to varying dopamine concentrations have identified potential molecular targets involved in fat deposition and provided insights into the effects of dopamine signaling on fat accumulation [154]. Notably, the exposure of C. elegans to exogenous dopamine resulted in a dose-dependent decrease in fluorescent signals, a response that was reversible upon termination of the dopamine treatment [149].
Like in other animals, fat regulation in C. elegans reflects the consequence of behavioral, physiological, and metabolic processes. Its amenability to testing has led to its use in unraveling the complicated homeostatic systems that underpin energy balance in whole animals. This, coupled with its optical advantages, has allowed for the investigation of cell biological mechanisms of fat absorption, transport, storage, and usage [149,155]. Homogenates from C. elegans have been used to access phospholipid and triglyceride levels by diverse chromatographic methods. Various methodologies for de novo fat synthesis, fatty acid uptake, and fatty acid breakdown can now be adequately investigated using different C. elegans strains, thereby expanding the scope of fatty acid and obesity research [149].
8.3. Applications in Obesity Research
Obesity has become a global epidemic, affecting approximately one-third of the United States population. It is currently a public health challenge in terms of its prevalence and the escalating economic burden it imposes on healthcare systems [154]. The C. elegans model is a reliable tool in advancing our understanding of obesity and its underlying molecular mechanisms. C. elegans has been extensively employed to explore critical pathways in obesity research, particularly in insulin signaling, dietary glucose response, and the role of serotonin in regulating satiety, eating behaviors, and hypoxia-related metabolic disorders [104]. Its use in studying how dietary factors influence metabolic responses has provided significant insights into the complex interplay between genetics, environment, and obesity. Furthermore, the nematode model has been instrumental in evaluating potential therapies for obesity, helping to identify new compounds and treatments aimed at modulating fat storage and energy metabolism [145]. Studying C. elegans is critical for improving our understanding of obesity and its intricacies, paving the way for new approaches to combating this rising public health concern.
9. C. elegans in Neurodegenerative Diseases
Neurodegenerative disorders (NDs) refer to a group of age-related diseases characterized by the breakdown of cellular proteostasis and the misfolding of various proteins, leading to the formation of toxic neuronal aggregates [156,157]. In C. elegans, ND models are commonly created by overexpressing pathogenic proteins in specific cells (such as neurons and body wall cells) using different promoters [28]. As a result, various transgenic models of NDs have been developed in C. elegans. The response of the IGF-1 signaling pathway to both biotic and abiotic stresses presents valuable insight into the connections between different neurodegenerative diseases. The Alzheimer model of C. elegans, which expresses Alpha/Beta-amyloid protein aggregates in the body wall muscle, is paralyzed and reaches smaller sizes than normal. IGF-1, in turn, through the activation of DAF 16/FOXO and HSF-1, reduces these symptoms and induces autophagic clearance of protein aggregates [157]. Interestingly, when the activity of Sir 2.1 is increased, inflammation is reduced, followed by a significant lifespan expansion.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that gradually impairs memory, cognitive abilities, and the capacity to carry out even the easiest tasks [158]. It is characterized by two key neuropathological features: the presence of intraneuronal neurofibrillary tangles, which are associated with neuronal destruction and death in brain regions such as the cerebral cortex and hippocampus, and the accumulation of extracellular amyloid plaques, also known as senile plaques [159,160]. The accumulation of amyloid precursor protein (APP) cleavage products called amyloid plaques results from the insoluble buildup of beta-amyloid peptide (Aβ). In mammals, the APP protein family includes three members: APP, APLP1, and APLP2, each containing a single transmembrane domain and playing a crucial role in brain development [161,162]. In C. elegans, there is a single APP-related gene, APL-1, which is exhibited in various organs and is essential for survival [117,146,158].
Notably, C. elegans does not produce beta-amyloid peptides naturally because its APL-1 gene lacks an Aβ sequence and the β-secretase enzyme (BACE). To mimic the characteristics of Alzheimer’s disease (AD), including Aβ peptide toxicity, several transgenic C. elegans strains that express human pathological proteins in muscle cells or neurons have been developed [145,157,161]. Additionally, transgenic C. elegans lines replicate the clinical profile of APOE polymorphism-associated neurodegeneration, which provides a valuable model for uncovering novel molecular insights into AD [28]. Several acetylcholinesterase (AChE) inhibitors, such as rivastigmine, donepezil, and galantamine, have been developed to restore normal cholinergic function and support synaptic transmission in the CNS of AD patients. However, the effectiveness of these treatments is limited, with various adverse effects, including gastrointestinal issues [162].
The tau protein homolog in C. elegans, called Protein with Tau-like Repeats-1 (ptl-1), plays a key role in neuronal health. A loss-of-function mutant of ptl-1 showed normal development but produced fewer offspring and exhibited reduced touch sensitivity. Additionally, PTL-1/tau impacts neuronal integrity and influences the lifespan of C. elegans [140,157]. These findings have enabled the development of transgenic C. elegans models that accurately reflect tau-related changes seen in Alzheimer’s disease (AD). In Parkinson’s disease (PD), the brain gradually degenerates, leading to the dysfunction of dopaminergic neurons in the substantia nigra pars compacta (nigrostriatal pathway). This region of the basal ganglia is crucial for voluntary motor control. PD is typically marked by symptoms such as bradykinesia (slowness of movement), muscle stiffness, postural instability, and resting tremors [163].
Various C. elegans models have shown great promise in uncovering the factors that could prevent, halt, or even reverse Parkinson’s disease (PD) symptoms. These models have also helped identify numerous genetic and pharmacological agents that could prevent dopaminergic neurodegeneration. Among the most notable C. elegans models for PD are those that focus on alpha-synuclein (α-syn), a protein prone to misfolding and a key pathogenic factor in PD. This has become a major target for scientific research [164,165,166,167,168]. Transgenic C. elegans worms expressing either single or multiple copies of normal or disease-associated proteins under neuronal-specific promoters allow for targeted expression in specific neuronal subtypes. The worms’ anatomical transparency makes it possible to co-express fluorescent proteins, enabling the tracking of neurodegeneration as the worms age [167]. Additionally, the fully mapped connectome of C. elegans provides detailed insights into how neurodegeneration affects overall organismal health. It also enables the accurate correlation of cell death with behavioral dysfunction or phenotypic changes in vivo [28].
Since C. elegans lacks a homolog of PARK1, which encodes α-synuclein, transgenic C. elegans are created by overexpressing either wild-type or mutant versions of human synuclein in dopaminergic neurons [166]. In addition, several C. elegans genes serve as orthologs to human genes linked to PD, such as PARK2 and PAKR5-9, which encode Parkin [125]. Other key C. elegans orthologs associated with PD onset and progression include pdr-1 (an ortholog of the human Parkin gene), lrk-1 (an ortholog of the human LRRK2 gene), and dj-1 (an ortholog of the human DJ-1 gene, which encodes glyoxalase mutations). Additionally, pinl-1, an ortholog of the human PINK1 protein kinase, has been linked to autosomal recessive, early-onset PD [159,167]. The versatility of C. elegans, combined with continuous discoveries from transgenic models such as those for α-synuclein in PD, demonstrates its potential as a research tool. These findings suggest that C. elegans can help divulge new conserved modifiers of genetics. Ultimately, these insights may be translatable to human treatments [160,169].
Huntington’s disease results from an increasing trinucleotide CAG (cytosine-adenine-guanine) repeat in the huntingtin gene. This leads to the production of an enlarged polyglutamine (polyQ) tract in the N-terminal region of the huntingtin protein (Htt) [170,171]. Although it lacks a homolog of the huntingtin gene, researchers have created various transgenic C. elegans models to study Htt aggregation and toxicity for HD research. While the cholinergic hypothesis is widely accepted in Alzheimer’s disease research, there is currently no known drug for this condition. Table 3 lists various chemical compounds that have been shown to alleviate certain neurological conditions in C. elegans.

Table 3.
Compounds with therapeutic effects on C. elegans models in neurodegenerative diseases.
10. C. elegans in the Study of Gut Health Permeability
The human gastrointestinal system and that of C. elegans share a similar developmental pattern, with the C. elegans GI often resembling that of humans [177]. The C. elegans gut has microvilli. When observed through an electron microscope, it appears similar to the crypt and villus structures present in mammalian tissue. Its GI system performs essential functions such as nutrient absorption, content transport, and waste elimination. Digestion starts with the pharyngeal grinder, which physically breaks down bacteria, the primary food source for the worm [178,179]. A network of intestinal transporters regulates the balance between nutrients and pathogens. Finally, a rhythmic defecation cycle, driven by calcium ion fluctuations, ensures proper waste elimination [180].
These characteristics make it an excellent representation for studying the gut microbiome. The worm hosts a unique and diverse microbiome, consisting of 12 bacterial species from nine different families, which form the core microbiome based on intestinal colonization studies [181,182]. In the wild, C. elegans recruits its gut microbiome from the surrounding environment [183]. Given its bacterial diet, C. elegans has adequately represented host–microbiota interactions in mammals [184].
Interactions between gut bacteria and their hosts have been associated with the onset of various human diseases, though the precise mechanisms remain largely unknown. The nematode C. elegans, which is genetically tractable, serves as a valuable model for studying gut health interactions, as harmful bacteria such as the human gut commensal Enterococcus faecalis can be introduced through feeding. Sim and Hibberd [181] conducted a study demonstrating that infection with E. faecalis (along with other potentially pathogenic bacteria) reduced the lifespan of C. elegans. An RNAi screen of 17 candidate genes identified the transcription factor nhr-49, a crucial regulator of fat metabolism, as responsible for this effect when knocked down. Notably, the similarity between nhr-49 and mammalian peroxisome proliferator-activated receptors (PPARs) suggests that fatty acid metabolism and innate immunity are involved in this process. C. elegans is a bacterivore susceptible to infection through feeding; it represents an excellent system for exploring gut bacteria interactions and pathogenic host–microbiome relationships [183,184].
Graphene oxide (GO) has garnered significant attention for its applicability in biological systems. However, its toxicity is a concern in both biomedical uses and non-biomedical products exposed to the environment. Therefore, understanding its action mechanisms is crucial to identifying potential therapeutic targets in appropriate animal models. A study by Ren et al. [184] suggests that GO toxicity in C. elegans may result from a combination of oxidative stress in the intestinal barrier, increased permeability of the biological barrier, and reduced defecation behavior.
A gap in the intestinal junction barrier is referred to as a leaky gut. When this essential barrier is breached, it causes increased permeability in the gut, allowing big molecules that would usually be unable to cross the gut barrier to seep through and enter the circulation [185]. Models such as pigs, horses, zebrafish, mice, and birds are used for leaky gut assays. However, some of these models are expensive and difficult to maintain. Chickens are susceptible to pathogenic invasion, which could alter research outcomes. Moreover, some biomarkers related to inflammation in mice have been changed due to the absence of specificity in some ELISA isoforms, hence the need for transgenic mouse models, which comes at an extra cost [186,187].
The possession of a worm cell–cell adhesion complex (the apical junction), which has a single electron-dense area, LET-413 that does not colocalize with DLG-1, and the tubular organ membranes’ proteins, which include AR-3, PAR-6, PKC-3, and CRB-3, are some of the several ways in which C. elegans differs from mammals, vertebrates, and drosophila. However, investigations have discovered that C. elegans epithelial cells tend to follow similar pathways as human cells, making them excellent for simulating microbial dysbiosis, gastrointestinal inflammation, and disease pathogenesis in the intestine [188]. Indeed, studies have shown that successful treatment techniques for lowering intestinal inflammation in C. elegans may be translated into mouse models [189]. This suggests that, despite their simplified anatomy, therapies obtained from C. elegans (Table 4) investigations may be used in more complicated mammalian models. Nevertheless, the absence of well-developed organs and systems is a major challenge and could be overcome by combining C. elegans studies with other more sophisticated models.

Table 4.
Compounds with therapeutic effects on C. elegans models in gut health and intestinal permeability related diseases.
11. Limitations of Using C. elegans Models in Pharmaceutical Research
Despite the apparent benefits of utilizing C. elegans for drug screening, several factors need consideration. C. elegans has a thick cuticle, which often interferes with drug absorption. According to some research, the internal concentration of the tested substance might be less than half of the external concentration, meaning that certain medications cannot cross the cuticle barrier and are thereby dismissed [194,195]. Using E. coli as the primary food source has added complexity to the issue. Due to the chemical metabolism or degradation by the bacteria, the use of live bacteria may reduce the effective dosage delivered to the worms. Therefore, higher initial chemical concentrations ranging from (25–100 M) are often employed in C. elegans-based pharmacological screening for these reasons [45,195]. Even so, a newly published prospective solution is the use of E. coli killed with paraformaldehyde, on which C. elegans grows normally and, interestingly, has a normal (non-extended) lifetime despite infection abrogation [196]. Another significant issue is that E. coli may biotransform the medication before it reaches the worm. However, these effects could offer a valuable model for studying how the microbiome influences drug action [196].
Recent research has explored the complex relationships between medications and the bacterial food source of nematodes, as well as the challenge of drugs not being effectively absorbed into nematode tissues. Liposome-mediated delivery has been suggested as a potential solution to address some of the limitations associated with using C. elegans as a model for discovering and evaluating anti-aging medicines [195]. The study found that liposome encapsulation positively impacted lifespan extension, reduced the number of compounds needed for analysis, and enhanced the uptake of several dyes into the intestine. However, Texas Red did not penetrate nematode tissues, indicating that liposomes cannot guarantee the pickup of substances [195]. Liposome encapsulation should help minimize drug effects on E. coli and drug bio-transformation, often caused by E. coli. [196].
Drugs need to reach a specific concentration at their site of action to be effective, and some compounds require much higher levels to have a biological effect. To address this challenge, mutant C. elegans strains with wounded cuticles upscale drug absorption [195]. Additionally, the simple anatomy of C. elegans, which lacks human organs such as the lungs, liver, kidneys, and blood transport systems, limits its use as a model for studying human disorders linked to these organs. Furthermore, its miniature size can make experimental manipulation difficult for inexperienced researchers [84,94].
While C. elegans is an excellent model for studying fundamental biological processes, it is not well-suited for investigating burn wounds or most infections that involve complex tissue repair, immune responses, and systemic interactions [197]. However, recent research on the structure and physiological roles of the C. elegans cuticle has provided new insights, highlighting its potential as a valuable model for skin-related studies. This is due to the unique arrangement and organization of collagen proteins in its cuticle [198,199,200].
C. elegans may facilitate the understanding of associated immune response, host–pathogen interactions, and genetic susceptibility. However, factors including limited infection models involving skin or internal organs, lack of complex host–microbiome interaction, and different pathogen responses are common limitations to its use. For such studies, mammalian models are preferred due to their advanced tissue structures [200].
Model Variation and Reproducibility
Validation entails ensuring that experimental findings in C. elegans adequately reflect the biological processes they want to describe. This procedure frequently involves cross-species comparisons and the use of computational tools. Research has shown that toxicity ratings in C. elegans correlate well with those in mouse models. For example, studies examining the effects of various metal salts discovered that toxicity orders based on C. elegans adult mortality coincided with rat and mouse oral LD50 rankings [201]. Interestingly, developing software frameworks like open worm stimulation stack enables the rigorous, data-driven evaluation of computational models in C. elegans. These technologies enable researchers to continuously compare model behavior with experimental data, thereby improving simulation accuracy [202].
Validation of C. elegans screening models varies across studies, with some providing strong statistical measures while others offer limited validation details. For example, Moy et al. [134] reported a Z’-factor of 0.61 and a high correlation (R2 = 0.98) with manual scoring, while Rajamuthiah et al. [139] achieved a Z’-factor of 0.77 and validated their results against an agar-based assay. In contrast, other studies provided less comprehensive validation. Kong et al. [137] compared liquid-based and agar-based methods but lacked specific validation metrics, and Moy et al. [138] reported false-positive and false-negative rates without detailed reproducibility data.
Reproducibility is a key component of scientific research, ensuring that results are consistent and dependable. In C. elegans investigations, various factors influence reproducibility: A study of over 5000 wild-type C. elegans found high inherent variability in lifespan measures, even under controlled settings [203]. This variability shows that finding modest but meaningful effects necessitates bigger sample sizes than are often employed. Also, minor changes in environmental variables, such as temperature, nutrition availability, or salt concentration, can cause adaptive responses in C. elegans, drastically affecting experimental outcomes. As a result, stringent adherence to defined cultural practices and handling methods is required to reduce variability [203,204]. Differences in experimental techniques between laboratories may also produce conflicting results. Advocating for consistent reporting and methodological transparency can help to reduce inter-lab variability and increase reproducibility in C. elegans research [205].
12. C. elegans and Toxicity Issues: A Comparison with Mammals
Drugs are particularly harmful products, and as a result, they must undergo a stringent examination procedure before they can be made commercially available. A major case study of a previous episode is the case of Thalidomide [204]. Consequently, all drugs must be safe, or at least, the benefits must always outweigh the risks [205]. Unlike other products, drug safety cannot be characterized as the complete elimination of risk, as achieving this objective is generally unfeasible within this context. Instead, a drug is deemed fit for use when its potential dangers are manageable, based on an evaluation of its anticipated benefits and available treatment options [204]. Preclinical animal studies are done to elucidate the way they act, describe the proof of concept, and determine how safe new medications are.
Most research, particularly safety assessments, is conducted following worldwide regulatory guidelines. However, animal models used to justify the start of clinical studies have limitations [206], coupled with several regulatory limits associated with their usage that stem from ethical and environmental issues. Since there are no regulatory restrictions on C. elegans research, unlike other animal models, developmental, reproductive, and acute toxicity can be assessed in a single experimental design. Additionally, chronic toxicity can be observed as the worms age. The toxicity ranking of various substances in C. elegans closely aligns with results from mammals, even though the exact quantities may not be directly comparable [205,206,207,208]. Thus, C. elegans trials can offer early indications of potential safety risks associated with drugs and nutraceuticals. More interestingly, acute reactions are sensitive probes for evaluating the biological connectivity of the worms to pharmacological treatment, and these connectivities can help to adequately elucidate the activation of previously unknown metabolic pathways both in drugs and in nutraceutical development.
The literature on ethical principles and regulatory frameworks that regulate C. elegans in preclinical drug screening processes was quite scarce. However, van der Voet et al. [208] provided 30 reporting criteria for Developmental and Reproductive Toxicology (DART) testing in C. elegans. These standards are consistent with the 3Rs principles (Reduction, Refinement, and Replacement) of animal testing and seek to standardize non-vertebrate organism testing for regulatory hazard assessment. The proposed criteria prioritize the documentation of experimental details such as compound purity, lot/batch number, and container type. The study’s authors developed these criteria to address reproducibility in C. elegans investigations. Their analysis discovered insufficient documentation of ethical principles and regulatory frameworks related to C. elegans use in preclinical drug screening. suggesting that current research techniques differ, and existing regulatory frameworks may not adequately cover C. elegans models. Additional studies may assist in defining standards for using C. elegans in preclinical drug-screening methods.
13. Conclusions
Despite widespread use of various C. elegans mutant worms to unravel various disease conditions and drug development processes, it is still difficult to genetically manipulate worms that can accurately mimic pathological conditions of diseases, especially their onset and progression. Also, there is a clear difference between metabolic and nutrient requirements between humans and C. elegans, thereby reducing the accuracy of what has been shown in the worms. However, C. elegans continues to provide basic and mechanistic insights into biological processes related to the development of pharmaceuticals and nutraceuticals; this is a springboard for further clinical investigations and applications, and a correct appraisal of its knowledge base through standardized and quantitative assays has great potential to speed up the progress of pharmaceutical and nutraceutical research.
Author Contributions
Conceptualization, O.O.D.-O., T.O.E., S.A.A. and M.J.; writing, original draft preparation, O.O.D.-O. and T.O.E.; writing—review and editing, O.O.D.-O., T.O.E., S.A.A. and M.J.; supervision, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Venkatesan, P. Re-emergence of infectious diseases associated with the past. Lancet Microbe 2021, 2, e140. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A comprehensive review on nutraceuticals: Therapy support and formulation challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Esther, B.; Rapport, L.; Lockwood, B. 1. What is a nutraceutical. Stroke 2018, 13, 57. [Google Scholar]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 550909. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Crespo, D.; Reguera, R.M.; Rojo-Vázquez, F.; Balaña-Fouce, R.; Martínez-Valladares, M. Drug discovery technologies: Caenorhabditis elegans as a model for anthelmintic therapeutics. Med. Res. Rev. 2020, 40, 1715–1753. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Z.; Tang, Z.; Chen, Y.; Huang, M.; Liu, H.; Huang, W.; Ye, Q.; Jia, B. Research progress, challenges, and breakthroughs of organoids as disease models. Front. Cell Dev. Biol. 2021, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.; Solis, G.M.; Petrascheck, M. C. elegans as a model for drug discovery. Curr. Top. Med. Chem. 2017, 17, 2067–2076. [Google Scholar] [CrossRef]
- Morgan, P.; Brown, D.G.; Lennard, S.; Anderton, M.J.; Barrett, J.C.; Eriksson, U.; Fidock, M.; Hamrén, B.; Johnson, A.; March, R.E.; et al. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat. Rev. Drug Discov. 2018, 17, 167–181. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kaneko, M.; Narukawa, M. Approval success rates of drug candidates based on target, action, modality, application, and their combinations. Clin. Transl. Sci. 2021, 14, 1113–1122. [Google Scholar] [CrossRef]
- Grimstein, M.; Yang, Y.; Zhang, X.; Grillo, J.; Huang, S.-M.; Zineh, I.; Wang, Y. Physiologically based pharmacokinetic modeling in regulatory science: An update from the US Food and Drug Administration’s Office of Clinical Pharmacology. J. Pharm. Sci. 2019, 108, 21–25. [Google Scholar] [CrossRef]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Galindo, F.J.; Ai, R.; Fang, E.F.; Nilsen, H.L.; SenGupta, T. C. elegans as an animal model to study the intersection of DNA repair, aging and neurodegeneration. Front. Aging 2022, 3, 916118. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, G.R.; Wilson, C.V.; Raviendran, S.; Xiao, Q. Recent Advances and Future Perspectives in Vascular Organoids and Vessel-on-Chip. Organoids 2024, 3, 203–246. [Google Scholar] [CrossRef]
- Gerli, M.F.M.; Calà, G.; Beesley, M.A.; Sina, B.; Tullie, L.; Sun, K.Y.; Panariello, F.; Michielin, F.; Davidson, J.R.; Russo, F.M.; et al. Single-cell guided prenatal derivation of primary fetal epithelial organoids from human amniotic and tracheal fluids. Nat. Med. 2024, 30, 875–887. [Google Scholar] [CrossRef]
- Singh, R.; Gholipourmalekabadi, M.; Shafikhani, S.H. Animal models for type 1 and type 2 diabetes: Advantages and limitations. Front. Endocrinol. 2024, 15, 1359685. [Google Scholar] [CrossRef]
- Simon, F.; Oberhuber, A.; Schelzig, H. Advantages and disadvantages of different animal models for studying ischemia/reperfusion injury of the spinal cord. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 744. [Google Scholar] [CrossRef]
- O’Reilly, L.P.; Luke, C.J.; Perlmutter, D.H.; Silverman, G.A.; Pak, S.C. C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 247–253. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ma, L.; Mishra, A.; Turnbull, J.E.; Tu, H. Editorial: C. elegans as an emerging model of pharmacological innovation. Front. Pharmacol. 2022, 13, 1029752. [Google Scholar] [CrossRef] [PubMed]
- Zarroug, S.H.O.; Bajaman, J.S.; Hamza, F.N.; Saleem, R.A.; Abdalla, H.K. Caenorhabditis elegans as an in vivo model for the discovery and development of natural plant-based antimicrobial compounds. Pharmaceuticals 2023, 16, 1070. [Google Scholar] [CrossRef]
- Giunti, S.; Andersen, N.; Rayes, D.; De Rosa, M.J. Drug discovery: Insights from the invertebrate Caenorhabditis elegans. Pharmacol. Res. Perspect. 2021, 9, e00721. [Google Scholar] [CrossRef] [PubMed]
- Kropp, P.A.; Bauer, R.; Zafra, I.; Graham, C.; Golden, A. Caenorhabditis elegans for rare disease modeling and drug discovery: Strategies and strengths. Dis. Model. Mech. 2021, 14, dmm049010. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Hwang, J.; Kwack, K. Comparative genomic analysis of the human and nematode Caenorhabditis elegans uncovers potential reproductive genes and disease associations in humans. Physiol. Genom. 2018, 50, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, B.; Oshiro-Rapley, N.; Li, M.; Paulo, J.A.; Webster, C.M.; Mou, F.; Kacergis, M.C.; Talkowski, M.E.; Carr, C.E.; et al. An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell 2016, 167, 1705–1718.e13. [Google Scholar] [CrossRef]
- Paschinger, K.; Yan, S.; Wilson, I.B.H. N-glycomic Complexity in Anatomical Simplicity: Caenorhabditis elegans as a Non-model Nematode? Front. Mol. Biosci. 2019, 6, 9. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Zhou, T.; Wang, G.; Li, Z. Caenorhabditis elegans as a useful model for studying aging mutations. Front. Endocrinol. 2020, 11, 554994. [Google Scholar] [CrossRef]
- Roussos, A.; Kitopoulou, K.; Borbolis, F.; Palikaras, K. Caenorhabditis elegans as a model system to study human neurodegenerative disorders. Biomolecules 2023, 13, 478. [Google Scholar] [CrossRef]
- Athar, F.; Templeman, N.M. C. elegans as a model organism to study female reproductive health. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 266, 111152. [Google Scholar] [CrossRef]
- Higgins, D.P.; Weisman, C.M.; Lui, D.S.; A D’agostino, F.; Walker, A.K. Defining characteristics and conservation of poorly annotated genes in Caenorhabditis elegans using WormCat 2.0. Genetics 2022, 221, iyac085. [Google Scholar] [CrossRef] [PubMed]
- A Calarco, J.; Taylor, S.R.; Miller, D.M. Detecting gene expression in Caenorhabditis elegans. Genetics 2024, 229, iyae167. [Google Scholar] [CrossRef]
- Li, G.; Gong, J.; Liu, J.; Liu, J.; Li, H.; Hsu, A.-L.; Liu, J.; Xu, X.S. Genetic and pharmacological interventions in the aging motor nervous system slow motor aging and extend life span in C. elegans. Sci. Adv. 2019, 5, eaau5041. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Alvarez-Illera, P.; Santo-Domingo, J.; Fonteriz, R.I.; Montero, M. Modeling Alzheimer’s Disease in Caenorhabditis elegans. Biomedicines 2022, 10, 288. [Google Scholar] [CrossRef]
- Romussi, S.; Giunti, S.; Andersen, N.; De Rosa, M.J. C. elegans: A prominent platform for modeling and drug screening in neurological disorders. Expert Opin. Drug Discov. 2024, 19, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.K.; Priya, A.; Malik, Z.; Thanaraj, T.A.; Singh, A.K.; Mago, P.; Ghosh, C.; Shalimar; Tandon, R.; Chaturvedi, R. A bioinformatics approach to elucidate conserved genes and pathways in C. elegans as an animal model for cardiovascular research. Sci. Rep. 2024, 14, 7471. [Google Scholar] [CrossRef]
- Xia, X. Bioinformatics and Drug Discovery. Curr. Top. Med. Chem. 2017, 17, 1709–1726. [Google Scholar] [CrossRef]
- Meissner, F.; Geddes-McAlister, J.; Mann, M.; Bantscheff, M. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat. Rev. Drug Discov. 2022, 21, 637–654. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Caldwell, K.A.; Willicott, C.W.; Caldwell, G.A. Modeling neurodegeneration in Caenorhabditis elegans. Dis. Model. Mech. 2020, 13, dmm046110. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, Y.; Song, B.; Fu, R.; Zhou, Y. The In vivo toxicity assessments of water-dispersed fluorescent silicon nanoparticles in Caenorhabditis elegans. Int. J. Environ. Res. Public Health 2022, 19, 4101. [Google Scholar] [CrossRef] [PubMed]
- Sofela, S.; Sahloul, S.; Song, Y.-A. Biophysical analysis of drug efficacy on C. elegans models for neurodegenerative and neuromuscular diseases. PLoS ONE 2021, 16, e0246496. [Google Scholar] [CrossRef]
- Meneely, P.M.; Dahlberg, C.L.; Rose, J.K. Working with worms: Caenorhabditis elegans as a model organism. Curr. Protoc. Essent. Lab. Tech. 2019, 19, e35. [Google Scholar] [CrossRef]
- Hashmi, S.; Wang, Y.; Parhar, R.S.; Collison, K.S.; Conca, W.; Al-Mohanna, F.; Gaugler, R. A C. elegans model to study human metabolic regulation. Nutr. Metab. 2013, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- E Escobar, I.; White, A.; Kim, W.; Mylonakis, E. New antimicrobial bioactivity against multidrug-resistant gram-positive bacteria of kinase inhibitor IMD0354. Antibiotics 2020, 9, 665. [Google Scholar] [CrossRef]
- Jim, V.; Slauter, R. Overview of ADME Science. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development; Academic Press: Cambridge, MA, USA, 2024; pp. 49–82. [Google Scholar]
- van der Most, M.A.; Estruch, I.M.; Brink, N.W.v.D. Contrasting dose response relationships of neuroactive antidepressants on the behavior of C. elegans. Ecotoxicol. Environ. Saf. 2023, 250, 114493. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y.; Bao, W.; Li, G.; Wang, Q.; et al. Oxidation and antioxidation of natural products in the model organism Caenorhabditis elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Harold, A.L.; Woock, A.E.; Bridges, A.A.; Cecile, J.P. Organic Anion Transport in Caenorhabditis elegans. FASEB J. 2013, 27, 732.1. [Google Scholar] [CrossRef]
- Amirthagunabalasingam, S.; Papaluca, A.; Harihar, T.; Ramotar, D. Imaging the Pharynx to Measure the Uptake of Doxorubicin in Caenorhabditis elegans. Bio-Protocol 2017, 7, e2291. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Jin, S.; Lu, Y.; Peng, Y.; Zhao, L.; Wang, X. Cannabidiol protects against Alzheimer’s disease in C. elegans via ROS scavenging activity of its phenolic hydroxyl groups. Eur. J. Pharmacol. 2022, 919, 174829. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Sewell, A.K.; Wu, Z.; Han, M. TOR signaling in Caenorhabditis elegans development, metabolism, and aging. Genetics 2019, 213, 329–360. [Google Scholar] [CrossRef]
- Suárez, G.; Alcántara, I.; Salinas, G. Caenorhabditis elegans as a valuable model for the study of anthelmintic pharmacodynamics and drug-drug interactions: The case of ivermectin and eprinomectin. Front. Pharmacol. 2022, 13, 984905. [Google Scholar] [CrossRef]
- Sohrabi, S.; Mor, D.E.; Kaletsky, R.; Keyes, W.; Murphy, C.T. High-throughput behavioral screen in C. elegans reveals Parkinson’s disease drug candidates. Commun. Biol. 2021, 4, 203. [Google Scholar] [CrossRef] [PubMed]
- Chrienova, Z.; Rysanek, D.; Oleksak, P.; Stary, D.; Bajda, M.; Reinis, M.; Mikyskova, R.; Novotny, O.; Andrys, R.; Skarka, A.; et al. Discovery of small molecule mechanistic target of rapamycin inhibitors as anti-aging and anti-cancer therapeutics. Front. Aging Neurosci. 2022, 14, 1048260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Ma, X.; Liu, J.-L.; Liu, A.-L. Exploring the potential use of Caenorhabditis elegans as an animal model for evaluating chemical-induced intestinal dysfunction. Toxicol. Appl. Pharmacol. 2024, 493, 117140. [Google Scholar] [CrossRef] [PubMed]
- Eric, D.L.; Morishita, M.; Hirotsu, T. C. elegans as a Powerful Tool for Cancer Screening. Biomedicines 2022, 10, 2371. [Google Scholar] [CrossRef]
- Hussar, P. Apoptosis regulators bcl-2 and caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Horowitz, L.B.; Shaham, S. Apoptotic and Nonapoptotic Cell Death in Caenorhabditis elegans Development. Annu. Rev. Genet. 2024, 58, 113–134. [Google Scholar] [CrossRef]
- La Marca, J.E.; Kelly, G.L.; Strasser, A.; Diepstraten, S.T. Don’t fear the reaper: The role of regulated cell death in tumorigenesis and BH3-mimetics for cancer therapy. Dev. Cell 2024, 59, 2532–2548. [Google Scholar] [CrossRef]
- Kovalchuk, I. Genome stability in Caenorhabditis elegans. In Genome Stability, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 177–200. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Hazbun, T.R.; Mayhoub, A.S.; Seleem, M.N. Ospemifene displays broad-spectrum synergistic interactions with itraconazole through potent interference with fungal efflux activities. Sci. Rep. 2020, 10, 6089. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.; Gcilitshana, O.; Pohl, C.H. Inhibitory effect of polyunsaturated fatty acids alone or in combination with fluconazole on Candida krusei biofilms in vitro and in Caenorhabditis elegans. Med. Mycol. 2021, 59, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Y.; Liu, M.; Huang, Y.; Wang, S.; An, J.; Wang, Y.; Shang, Y. The joint effects of nanoplastics and TBBPA on neurodevelopmental toxicity in Caenorhabditis elegans. Toxicol. Res. 2023, 12, 76–85. [Google Scholar] [CrossRef]
- Ikenaka, K.; Tsukada, Y.; Giles, A.C.; Arai, T.; Nakadera, Y.; Nakano, S.; Kawai, K.; Mochizuki, H.; Katsuno, M.; Sobue, G.; et al. A behavior-based drug screening system using a Caenorhabditis elegans model of motor neuron disease. Sci. Rep. 2019, 9, 10104. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Quan, J.I.; Li, L.; Kinghorn, K.J.; Ivanov, D.K.; Tain, L.S.; Slack, C.; Kerr, F.; Nespital, T.; Thornton, J.; Hardy, J.; et al. Lithium Promotes Longevity through GSK3/NRF2-Dependent Hormesis. Cell Rep. 2016, 15, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 22839. [Google Scholar] [CrossRef]
- Chen, J.; Ou, Y.; Li, Y.; Hu, S.; Shao, L.-W.; Liu, Y. Metformin extends C. elegans lifespan through lysosomal pathway. eLife 2017, 6, e31268. [Google Scholar] [CrossRef]
- Ahmad, W.; Ebert, P.R. Metformin Attenuates Aβ Pathology Mediated Through Levamisole Sensitive Nicotinic Acetylcholine Receptors in a C. elegans Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 5427–5439. [Google Scholar] [CrossRef]
- Saewanee, N.; Praputpittaya, T.; Malaiwong, N.; Chalorak, P.; Meemon, K. Neuroprotective effect of metformin on dopaminergic neurodegeneration and α-synuclein aggregation in C. elegans model of Parkinson’s disease. Neurosci. Res. 2021, 162, 13–21. [Google Scholar] [CrossRef]
- Udono, H.; Nishida, M. Metformin-ROS-Nrf2 connection in the host defense mechanism against oxidative stress, apoptosis, cancers, and ageing. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130171. [Google Scholar] [CrossRef]
- Markaki, M.; Tavernarakis, N. Caenorhabditis elegans as a model system for human diseases. Curr. Opin. Biotechnol. 2020, 63, 118–125. [Google Scholar] [CrossRef]
- Göethel, G.; Augsten, L.V.; das Neves, G.M.; Gonçalves, I.L.; de Souza, J.P.S.; Garcia, S.C.; Eifler-Lima, V.L. The role of alternative toxicological trials in drug discovery programs. The case of Caenorhabditis elegans and other methods. Curr. Med. Chem. 2022, 29, 5270–5288. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.H.; Widmayer, S.J.; Bergemann, C.M.; King, D.E.; Morton, K.S.; Romersi, R.F.; Jameson, L.E.; Leung, M.C.K.; Andersen, E.C.; Taubert, S.; et al. Xenobiotic metabolism and transport in Caenorhabditis elegans. J. Toxicol. Environ. Health Part B 2021, 24, 51–94. [Google Scholar] [CrossRef]
- Sharma, N.; Au, V.; Martin, K.; Edgley, M.L.; Moerman, D.; Mains, P.E.; Gilleard, J.S. Multiple UDP glycosyl-transferases modulate benzimidazole drug sensitivity in the nematode Caenorhabditis elegans in an additive manner. Int. J. Parasitol. 2024, 54, 535–549. [Google Scholar] [CrossRef]
- Raza, A.; Williams, A.R.; Abeer, M.M. Importance of ABC Transporters in the Survival of Parasitic Nematodes and the Prospect for the Development of Novel Control Strategies. Pathogens 2023, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.T.; Ivens, A.; Butler, V.; Ravikumar, S.P.; Laing, R.; Woods, D.J.; Gilleard, J.S. The transcriptional response of Caenorhabditis elegans to ivermectin exposure identifies novel genes involved in the response to reduced food intake. PLoS ONE 2012, 7, e31367. [Google Scholar] [CrossRef]
- van der Most, M.A.; Bakker, W.; Wesseling, S.; Brink, N.W.v.D. Toxicokinetics of the Antidepressant Fluoxetine and Its Active Metabolite Norfluoxetine in Caenorhabditis elegans and Their Comparative Potency. Environ. Sci. Technol. 2024, 58, 3129–3140. [Google Scholar] [CrossRef]
- Shen, M. Pain Chemogenomics Knowledgebase (PAIN-CKB) for Systems Pharmacology Target Mapping and PBPK Modeling Investigation of Opioid Drug-Drug Interactions. Doctoral Dissertation, University of Pittsburgh, Pittsburgh, PA, USA, 2020. [Google Scholar]
- Jatti, T.; Maniyal, N.; Mouli, S.; Shenoy, R.; Bhat, S. Molecular Docking and ADMET of Levodopa against Leucine-Rich Repeat Kinases, and In-Vitro Mobility Analysis in C. elegans for Parkinson’s Disease. In Proceedings of the 2023 IEEE 23rd International Conference on Bioinformatics and Bioengineering (BIBE), Dayton, OH, USA, 4–6 December 2023. [Google Scholar]
- Stasiuk, S.J.; MacNevin, G.; Workentine, M.L.; Gray, D.; Redman, E.; Bartley, D.; Morrison, A.; Sharma, N.; Colwell, D.; Ro, D.K.; et al. Similarities and differences in the biotransformation and transcriptomic responses of Caenorhabditis elegans and Haemonchus contortus to five different benzimidazole drugs. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 13–29. [Google Scholar] [CrossRef]
- Kim, D.K.; Park, J.Y.; Kang, Y.J.; Khang, D. Drug repositioning of metformin encapsulated in PLGA combined with photothermal therapy ameliorates rheumatoid arthritis. Int. J. Nanomed. 2023, 18, 7267–7285. [Google Scholar] [CrossRef]
- Soukup, S.T.; Spanier, B.; Grünz, G.; Bunzel, D.; Daniel, H.; Kulling, S.E. Formation of Phosphoglycosides in Caenorhabditis elegans: A Novel Biotransformation Pathway. PLoS ONE 2012, 7, e46914. [Google Scholar] [CrossRef]
- Sheeja, M.D.; Prasanth, M.I.; Brimson, J.M.; Verma, K.; Prasansuklab, A.; Tencomnao, T. Hibiscus sabdariffa extract protects HT-22 cells from glutamate-induced neurodegeneration by upregulating glutamate transporters and exerts lifespan extension in C. elegans via DAF-16 mediated pathway. Nutr. Healthy Aging 2021, 6, 229–247. [Google Scholar] [CrossRef]
- Ha, N.M.; Tran, S.H.; Shim, Y.-H.; Kang, K. Caenorhabditis elegans as a powerful tool in natural product bioactivity research. Appl. Biol. Chem. 2022, 65, 18. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Okpala, C.O.R. Natural nutraceuticals, especially functional foods, their major bioactive components, formulation, and health benefits for disease prevention: An overview. J. Food Bioact. 2022, 19, 97–123. [Google Scholar] [CrossRef]
- Loo, J.; Bana, M.A.F.S.; Tan, J.K.; Goon, J.A. Effect of dietary restriction on health span in Caenorhabditis elegans: A systematic review. Exp. Gerontol. 2023, 182, 112294. [Google Scholar] [CrossRef]
- Long, N.P.; Kang, J.S.; Kim, H.M. Caenorhabditis elegans: A model organism in the toxicity assessment of environmental pollutants. Environ. Sci. Pollut. Res. 2023, 30, 39273–39287. [Google Scholar] [CrossRef]
- Nitin, V.; Sharma, S.; Thakur, N.; Kaur, N.; Dua, K. Nanotherapeutics for Alz-heimer’s disease using metal nanocomposites. Met. Nanocompos. Nanother. Oxidative Stress-Induc. Metab. Disord. 2024, 1, 372–391. [Google Scholar]
- Aranaz, P.; Peña, A.; Vettorazzi, A.; Fabra, M.J.; Martínez-Abad, A.; López-Rubio, A.; Pera, J.; Parladé, J.; Castellari, M.; Milagro, F.I.; et al. Grifola frondosa (Maitake) Extract Reduces Fat Accumulation and Improves Health Span in C. elegans through the DAF-16/FOXO and SKN-1/NRF2 Signalling Pathways. Nutrients 2021, 13, 3968. [Google Scholar] [CrossRef]
- Lippi, L.; Uberti, F.; Folli, A.; Turco, A.; Curci, C.; d’Abrosca, F.; de Sire, A.; Invernizzi, M. Impact of nutraceuticals and dietary supplements on mitochondria modifications in healthy aging: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 2659–2674. [Google Scholar] [CrossRef]
- Deledda, A.; Giordano, E.; Velluzzi, F.; Flore, G.; Franceschelli, S.; Speranza, L.; Ripari, P. Mitochondrial aging and senolytic natural products with protective potential. Int. J. Mol. Sci. 2022, 23, 16219. [Google Scholar] [CrossRef]
- Iwanir, S.; Ruach, R.; Itskovits, E.; Pritz, C.O.; Bokman, E.; Zaslaver, A. Irrational behavior in C. elegans arises from asymmetric modulatory effects within single sensory neurons. Nat. Commun. 2019, 10, 3202. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, R.; McClements, D.J.; Park, Y. Nanoemulsion-based delivery systems for testing nutraceutical efficacy using Caenorhabditis elegans: Demonstration of curcumin bioaccumulation and body-fat reduction. Food Res. Int. 2019, 120, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. Npj Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Pedro, F.J.; Pitt, B.; Zannad, F. Histone deacetylase inhibitors for cardiovascular conditions and healthy longevity. Lancet Healthy Longev. 2021, 2, e371–e379. [Google Scholar] [CrossRef] [PubMed]
- Revtovich, A.V.; Lee, R.; Kirienko, N.V. Interplay between mitochondria and diet mediates pathogen and stress resistance in Caenorhabditis elegans. PLoS Genet. 2019, 15, e1008011. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 2009, 8, 113–127. [Google Scholar] [CrossRef]
- Ewald, C.Y.; Castillo-Quan, J.I.; Blackwell, T.K. Untangling longevity, dauer, and healthspan in Caenorhabditis elegans insulin/IGF-1-signalling. Gerontology 2017, 64, 96–104. [Google Scholar] [CrossRef]
- Hahm, J.-H.; Seo, H.-D.; Jung, C.H.; Ahn, J. Longevity through diet restriction and immunity. BMB Rep. 2023, 56, 537–544. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2021, 23, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Cuong, V.T.; Chen, W.; Shi, J.; Zhang, M.; Yang, H.; Wang, N.; Yang, S.; Li, J.; Yang, P.; Fei, J. The anti-oxidation and anti-aging effects of Ganoderma lucidum in Caenorhabditis elegans. Exp. Gerontol. 2019, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chen, Y.; Lin, Y.; Wang, X.; Hu, L.; Cao, Y.; Chen, Y. Antistress and anti-aging activities of Caenorhabditis elegans were enhanced by Momordica saponin extract. Eur. J. Nutr. 2020, 60, 1819–1832. [Google Scholar] [CrossRef]
- Bahrami, A.; Montecucco, F.; Carbone, F.; Sahebkar, A. Effects of curcumin on aging: Molecular mechanisms and experimental evidence. BioMed. Res. Int. 2021, 2021, 8972074. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, Y.; Xiao, J.; Lan, Y.; Cao, Y.; Chen, Y. Effect of Momordica saponin and Cyclocarya paliurus pol-ysaccharide-enriched beverages on oxidative stress and fat accumulation in Caenorhabditis elegans. J. Sci. Food Agric. 2021, 101, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Razavi, S.M.; Arab, Z.N.; Hosseini, Y.; Niknejad, A.; Momtaz, S.; Abdolghaffari, A.H.; Sathyapalan, T.; Jamialahmadi, T.; Kesharwani, P.; et al. Superoxide dismutase: A key target for the neuroprotective effects of curcumin. Mol. Cell. Biochem. 2023, 479, 693–705. [Google Scholar] [CrossRef]
- Turer, B.Y.; Sanlier, N. Relationship of Curcumin with Aging and Alzheimer and Parkinson Disease, the Most Prevalent Age-Related Neurodegenerative Diseases: A Narrative Review. Nutr. Rev. 2024, 83, e1243–e1258. [Google Scholar] [CrossRef]
- Park, H.-E.H.; Hwang, W.; Ham, S.; Kim, E.; Altintas, O.; Park, S.; Son, H.G.; Lee, Y.; Lee, D.; Heo, W.D.; et al. A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling. Nat. Commun. 2021, 12, 5631. [Google Scholar] [CrossRef]
- Yoon, D.S.; Cha, D.S.; Choi, Y.; Lee, J.W.; Lee, M. MPK-1/ERK is required for the full activity of resveratrol in extended lifespan and reproduction. Aging Cell 2018, 18, e12867. [Google Scholar] [CrossRef]
- Miller, B.C.; Mathai, M.; Yadav, H.; Jain, S. Geroprotective potential of microbiome modulators in the Caenorhabditis elegans model. GeroScience 2023, 46, 129–151. [Google Scholar] [CrossRef]
- Solis, G.M.; Kardakaris, R.; Valentine, E.R.; Bar-Peled, L.; Chen, A.L.; Blewett, M.M.; A McCormick, M.; Williamson, J.R.; Kennedy, B.; Cravatt, B.F.; et al. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. eLife 2018, 7, e40314. [Google Scholar] [CrossRef]
- Janssens, G.E.; Lin, X.-X.; Millan-Ariño, L.; Kavšek, A.; Sen, I.; Seinstra, R.I.; Stroustrup, N.; Nollen, E.A.; Riedel, C.G. Transcriptomics-based screening identifies pharmacological inhibition of Hsp90 as a means to defer aging. Cell Rep. 2019, 27, 467–480.e6. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, X.; Kong, X.-M.; Liu, T.-W.; Feng, X.-Q.; Chen, F.-E.; Zhuang, Z.-H. Anti-aging effect of methylurolithin A and its amide derivatives on nematode Caenorhabditis elegans. Tetrahedron Lett. 2023, 118, 154389. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Brooks, D.R.; Shepherd, S.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria extend lifespan in C. elegans. Aging 2023, 15, 11764–11781. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Lin, D.; Guo, W.; Xu, Z.; Wang, L.; Guan, S. Ginsenoside prolongs the lifespan of C. elegans via lipid metabolism and activating the stress response signaling pathway. Int. J. Mol. Sci. 2021, 22, 9668. [Google Scholar] [CrossRef]
- Snell, T.W.; Johnston, R.K.; Matthews, A.B.; Zhou, H.; Gao, M.; Skolnick, J. Repurposed FDA-approved drugs targeting genes influencing aging can extend lifespan and healthspan in rotifers. Biogerontology 2018, 19, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ziv, R.; Frakes, A.E.; Higuchi-Sanabria, R.; Bolas, T.; Frankino, P.A.; Gildea, H.K.; Metcalf, M.G.; Dil-lin, A. Measurements of Physiological Stress Responses in C. elegans. J. Vis. Exp. 2020, 21, e61001. [Google Scholar]
- Dues, D.J.; Andrews, E.K.; Schaar, C.E.; Bergsma, A.L.; Senchuk, M.M.; Van Raamsdonk, J.M. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress response pathways. Aging 2016, 8, 777–795. [Google Scholar] [CrossRef]
- Kyriakou, E.; Taouktsi, E.; Syntichaki, P. The thermal stress coping network of the nematode Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 14907. [Google Scholar] [CrossRef]
- Szigeti, B.; Gleeson, P.; Vella, M.; Khayrulin, S.; Palyanov, A.; Hokanson, J.; Currie, M.; Cantarelli, M.; Idili, G.; Larson, S. OpenWorm: An open-science approach to modeling Caenorhabditis elegans. Front. Comput. Neurosci. 2014, 8, 137. [Google Scholar] [CrossRef]
- Norris, D.D. Design and Application of Alternative Transgenic Reporter Systems in Caenorhabditis elegans. Master’s Thesis, Michigan Technological University, Houghton, MI, USA, 2024. [Google Scholar]
- Nance, J.; Frøkjær-Jensen, C. The Caenorhabditis elegans transgenic toolbox. Genetics 2019, 212, 959–990. [Google Scholar] [CrossRef] [PubMed]
- Ayon, N.J. High-throughput screening of natural product and synthetic molecule libraries for antibacterial drug discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef]
- Foster, K.J.; Cheesman, H.K.; Liu, P.; Peterson, N.D.; Anderson, S.M.; Pukkila-Worley, R. Innate immunity in the C. elegans intestine is programmed by a neuronal regulator of AWC olfactory neuron development. Cell Rep. 2020, 31, 107478. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, R.; Ilangovan, P.; Nongbri, D.; Suchiang, K. Probiotics interactions and the modulation of major signalling pathways in host model organism Caenorhabditis elegans. Indian J. Microbiol. 2021, 61, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Midkiff, D.; San-Miguel, A. Microfluidic technologies for high throughput screening through sorting and on-chip culture of C. elegans. Molecules 2019, 24, 4292. [Google Scholar] [CrossRef]
- Samsudin, S.; Al-Talib, H.; Zain, Z.M.; Murugaiah, C. Panton-Valentine Leukocidin-Positive Staphylococcus aureus: A successful infectious enemy? Int. Med. J. 2020, 27, 540–544. [Google Scholar]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial persisters: Molecular mechanisms and therapeutic development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Moy, T.I.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; Mazitschek, R.; Casadei, G.; Lewis, K.; Carpenter, A.E.; Ausubel, F.M. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009, 4, 527–533. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Rajmuthiah, R.; Kim, W.; Fuchs, B.B.; Jeyamani, E.; Kelso, M.J.; Mylonakis, E. Antibacterial properties of four novel hit compounds from a methicillin-resistant Staphylococcus aureus–Caenorhabditis elegans high-throughput screen. Microb. Drug Resist. 2018, 24, 666–674. [Google Scholar] [CrossRef]
- Xie, H.; Zhan, Y.; Chen, X.; Zeng, Q.; Chen, D.; Liang, J. Brevinin-2 drug family—New applied peptide candidates against methicillin-resistant Staphylococcus aureus and their effects on Lys-7 expression of innate immune pathway DAF-2/DAF-16 in Caenorhabditis elegans. Appl. Sci. 2018, 8, 2627. [Google Scholar] [CrossRef]
- Iatsenko, J.; Yim, J.; Schroeder, F.; Iatsenko, R.S. B. subtilis GS67 protects C. elegans from Gram-positive pathogens via fengycin-mediated microbial antagonism. Curr. Biol. 2014, 24, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; A Yehye, W.; Rahman, N.A.; Tan, M.-W.; Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement. Altern. Med. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Moy, T.I.; Ball, A.R.; Anklesaria, Z.; Casadei, G.; Lewis, K.; Ausubel, F.M. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl. Acad. Sci. USA 2006, 103, 10414–10419. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Fuchs, B.B.; Jayamani, E.; Kim, Y.; Larkins-Ford, J.; Conery, A.; Ausubel, F.M.; Mylonakis, E. Whole animal automated platform for drug discovery against multi-drug-resistant Staphylococcus aureus. PLoS ONE 2014, 9, e89189. [Google Scholar] [CrossRef]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef]
- Wu, K.; E Simor, A.; Vearncombe, M.; McClure, J.-A.; Zhang, K. A Caenorhabditis elegans host model correlates with invasive disease caused by Staphylococcus aureus recovered during an outbreak in neonatal intensive care. Can. J. Infect. Dis. Med. Microbiol. 2012, 23, 130–134. [Google Scholar] [CrossRef]
- Spreafico, R.; Soriaga, L.B.; Grosse, J.; Virgin, H.W.; Telenti, A. Advances in genomics for drug development. Genes 2020, 11, 942. [Google Scholar] [CrossRef]
- Ke, W.; Reed, J.N.; Yang, C.; Higgason, N.; Rayyan, L.; Wählby, C.; Carpenter, A.E.; Civelek, M.; O’rourke, E.J. Genes in human obesity loci are causal obesity genes in C. elegans. PLoS Genet. 2021, 17, e1009736. [Google Scholar] [CrossRef]
- Subhadra, M.; Mir, D.A.; Ankita, K.; Sindunathy, M.; Kishore, H.D.; Ravichandiran, V.; Balamurugan, K. Exploring diabesity pathophysiology through proteomic analysis using Caenorhabditis elegans. Front. Endocrinol. 2024, 15, 1383520. [Google Scholar] [CrossRef]
- Goh, G.Y.S.; Winter, J.J.; Bhanshali, F.; Doering, K.R.S.; Lai, R.; Lee, K.; Veal, E.A.; Taubert, S. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell 2018, 17, e12743. [Google Scholar] [CrossRef]
- Witting, M.; Schmitt-Kopplin, P. The Caenorhabditis elegans lipidome: A primer for lipid analysis in Caenorhabditis elegans. Arch. Biochem. Biophys. 2016, 589, 27–37. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.C.; Dunbar, T.L.; Nargund, A.M.; Haynes, C.M.; Troemel, E.R. The C. elegans CCAAT-enhancer-binding protein gamma is required for surveillance immunity. Cell Rep. 2016, 14, 1581–1589. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Hu, J.-P.; Wu, M.-M.; Wang, L.-S.; Fang, N.-Y. CCAAT/enhancer-binding protein CEBP-2 controls fat consumption and fatty acid desaturation in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2015, 468, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Mitra, S.; Basmayor, A.M.; Lu, B.; Taylor, B.E.; Bult-Ito, A. Genetic silencing of fatty acid desaturases modulates α-synuclein toxicity and neuronal loss in Parkinson-like models of C. elegans. Front. Aging Neurosci. 2019, 11, 207. [Google Scholar] [CrossRef]
- An, L.; Fu, X.; Chen, J.; Ma, J. Application of Caenorhabditis elegans in lipid metabolism research. Int. J. Mol. Sci. 2023, 24, 1173. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.G.d.A.; Bridi, J.C.; de Souza, B.R.; Júnior, C.d.C.; Torres, K.C.d.L.; Malard, L.; Jorio, A.; de Miranda, D.M.; Ashrafi, K.; Romano-Silva, M.A. Dopamine signaling regulates fat content through β-oxidation in Caenorhabditis elegans. PLoS ONE 2014, 9, e85874. [Google Scholar] [CrossRef]
- Berthoud, H.; Seeley, R.J.; Roberts, S.B. Physiology of energy intake in the weight-reduced state. Obesity 2021, 29, S25–S30. [Google Scholar] [CrossRef]
- Ekraminasab, S.; Dolatshahi, M.; Sabahi, M.; Mardani, M.; Rashedi, S. The interactions between adipose tissue secretions and Parkinson’s disease: The role of leptin. Eur. J. Neurosci. 2022, 55, 873–891. [Google Scholar] [CrossRef]
- Soares, M.V.; Viçozzi, G.P.; Kuhn, E.C.; Weishaupt, A.-K.; Kubens, L.; Bornhorst, J.; Avila, D.S. Neurotoxicology of organic environmental toxicants using Caenorhabditis elegans as a model. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2023; Volume 9, pp. 149–180. [Google Scholar] [CrossRef]
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef]
- Liang, J.J.H.; McKinnon, I.A.; Rankin, C.H. The contribution of C. elegans neurogenetics to understanding neurodegenerative diseases. In Nature’s Gift to Neuroscience; CRC Press: Boca Raton, FL, USA, 2022; pp. 317–338. [Google Scholar]
- Rani, N.; Alam, M.M.; Jamal, A.; Bin Ghaffar, U.; Parvez, S. Caenorhabditis elegans: A transgenic model for studying age-associated neurodegenerative diseases. Ageing Res. Rev. 2023, 91, 102036. [Google Scholar] [CrossRef]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of advanced prostate cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef]
- Ahmad, W. Glucose enrichment impair neurotransmission and induce Aβ oligomerization that cannot be reversed by manipulating O-β-GlcNAcylation in the C. elegans model of Alzheimer’s disease. J. Nutr. Biochem. 2022, 108, 109100. [Google Scholar] [CrossRef]
- Chau, D.D.-L.; Ng, L.L.-H.; Zhai, Y.; Lau, K.-F. Amyloid precursor protein and its interacting proteins in neurodevelopment. Biochem. Soc. Trans. 2023, 51, 1647–1659. [Google Scholar] [CrossRef]
- Jing, Y. Regulation of Caenorhabditis elegans model in Alzheimer’s Disease. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 185, p. 03043. [Google Scholar] [CrossRef]
- He, C.-L.; Tang, Y.; Wu, J.-M.; Long, T.; Yu, L.; Teng, J.-F.; Qiu, W.-Q.; Pan, R.; Yu, C.-L.; Qin, D.-L.; et al. Chlorogenic acid delays the progression of Parkinson’s disease via autophagy induction in Caenorhabditis elegans. Nutr. Neurosci. 2021, 26, 11–24. [Google Scholar] [CrossRef]
- Abusrair, A.H.; Elsekaily, W.; Bohlega, S. Tremor in Parkinson’s Disease: From Pathophysiology to Advanced Therapies. Tremor. Other Hyperkinetic Mov. 2022, 12, 29. [Google Scholar] [CrossRef]
- Poewe, W.; Volc, D.; Seppi, K.; Medori, R.; Lührs, P.; Kutzelnigg, A.; Djamshidian, A.; Thun-Hohenstein, C.; Meissner, W.G.; Rascol, O.; et al. Safety and tolerability of active immunotherapy targeting α-synuclein with PD03A in patients with early Parkinson’s disease: A randomized, placebo-controlled, phase 1 study. J. Park. Dis. 2021, 11, 1079–1089. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Y.; Chen, Y.; Cheng, B.; Peng, A.; Huang, K. Caenorhabditis elegans as a model system for target identification and drug screening against neurodegenerative diseases. Eur. J. Pharmacol. 2018, 819, 169–180. [Google Scholar] [CrossRef]
- Gaeta, A.L.; Caldwell, K.A.; Caldwell, G.A. Found in translation: The utility of C. elegans alpha-synuclein models of Parkinson’s disease. Brain Sci. 2019, 9, 73. [Google Scholar] [CrossRef]
- Fan, H.-C.; Ho, L.-I.; Chi, C.-S.; Chen, S.-J.; Peng, G.-S.; Chan, T.-M.; Lin, S.-Z.; Harn, H.-J. Polyglutamine (PolyQ) Diseases: Genetics to Treatments. Cell Transplant. 2014, 23, 441–458. [Google Scholar] [CrossRef]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite Nanoplatelets and Caenorhabditis elegans: Insights from an in Vivo Model. Nano Lett. 2012, 12, 2740–2744. [Google Scholar] [CrossRef]
- Golegaonkar, S.; Tabrez, S.S.; Pandit, A.; Sethurathinam, S.; Jagadeeshaprasad, M.G.; Bansode, S.; Sampathkumar, S.; Kulkarni, M.J.; Mukhopadhyay, A. Rifampicin reduces advanced glycation end products and activates DAF-16 to increase lifespan in Caenorhabditis elegans. Aging Cell 2015, 14, 463–473. [Google Scholar] [CrossRef]
- Abu-Elfotuh, K.; Al-Najjar, A.H.; Mohammed, A.A.; Aboutaleb, A.S.; Badawi, G.A. Fluoxetine ameliorates Alzheimer’s disease progression and prevents the exacerbation of cardiovascular dysfunction of socially isolated depressed rats through activation of Nrf2/HO-1 and hindering TLR4/NLRP3 inflammasome signaling pathway. Int. Immunopharmacol. 2022, 104, 108488. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Link, P. Natural Products Against Neurodegenerative Diseases: Effects in the Model Organism Caenorhabditis elegans. Ph.D. Thesis, Ruperto-Carola University of Heidelberg, Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Sadananda, G.; Velmurugan, J.D.; Subramaniam, J.R. DMSO Delays Alzheimer Disease Causing Aβ-induced Paralysis in C. elegans Through Modulation of Glutamate/Acetylcholine Neurotransmission. Ann. Neurosci. 2021, 28, 55–64. [Google Scholar] [CrossRef]
- Pandey, T.; Smita, S.S.; Mishra, A.; Sammi, S.R.; Pandey, R. Swertiamarin, a secoiridoid glycoside modulates nAChR and AChE activity. Exp. Gerontol. 2020, 138, 111010. [Google Scholar] [CrossRef]
- Dirksen, P.; Assié, A.; Zimmermann, J.; Zhang, F.; Tietje, A.-M.; Marsh, S.A.; Félix, M.-A.; Shapira, M.; Kaleta, C.; Schulenburg, H.; et al. CeMbio-The Caenorhabditis elegans microbiome resource. G3 Genes Genomes Genet. 2020, 10, 3025–3039. [Google Scholar] [CrossRef]
- Cheddadi, R.; Yeramilli, V.; Gamra, I.; Davies, J.; Tanner, S.; Martin, C. Intestinal Development and Gut Disease: Contributions From the Caenorhabditis elegans Model. J. Surg. Res. 2024. [Google Scholar] [CrossRef]
- Dirksen, P.; Marsh, S.A.; Braker, I.; Heitland, N.; Wagner, S.; Nakad, R.; Mader, S.; Petersen, C.; Kowallik, V.; Rosenstiel, P.; et al. The native microbiome of the nematode Caenorhabditis elegans: Gateway to a new host-microbiome model. BMC Biol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Zhang, J.; Holdorf, A.D.; Walhout, A.J. C. elegans and its bacterial diet as a model for systems-level understanding of host–microbiota interactions. Curr. Opin. Biotechnol. 2017, 46, 74–80. [Google Scholar] [CrossRef]
- Singh, A.; Luallen, R.J. Understanding the factors regulating host–microbiome interactions using Caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230059. [Google Scholar] [CrossRef]
- Sim, S.; Hibberd, M.L. Caenorhabditis elegans susceptibility to gut Enterococcus faecalis infection is associated with fat metabolism and epithelial junction integrity. BMC Microbiol. 2016, 16, 6. [Google Scholar] [CrossRef]
- Kumar, A.; Baruah, A.; Tomioka, M.; Iino, Y.; Kalita, M.C.; Khan, M. Caenorhabditis elegans: A model to understand host–microbe interactions. Cell. Mol. Life Sci. 2019, 77, 1229–1249. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Bäumler, A.J. Gastrointestinal host-pathogen interaction in the age of microbiome research. Curr. Opin. Microbiol. 2020, 53, 78–89. [Google Scholar] [CrossRef]
- Ren, M.; Zhao, L.; Ding, X.; Krasteva, N.; Rui, Q.; Wang, D. Developmental basis for an intestinal barrier against the toxicity of graphene oxide. Part. Fibre Toxicol. 2018, 15, 26. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Cheng, C.K.; Huang, Y. The gut-cardiovascular connection: New era for cardiovascular therapy. Med. Rev. 2021, 1, 23–46. [Google Scholar] [CrossRef]
- Massier, L.; Chakaroun, R.; Kovacs, P.; Heiker, J.T. Blurring the picture in leaky gut research: How shortcomings of zonulin as a biomarker mislead the field of intestinal permeability. Gut 2020, 70, 1801–1802. [Google Scholar] [CrossRef]
- Kim, M.R.; Cho, S.-Y.; Lee, H.J.; Kim, J.Y.; Nguyen, U.T.T.; Ha, N.M.; Choi, K.Y.; Cha, K.H.; Kim, J.-H.; Kim, W.K.; et al. Schisandrin C improves leaky gut conditions in intestinal cell monolayer, organoid, and nematode models by increasing tight junction protein expression. Phytomedicine 2022, 103, 154209. [Google Scholar] [CrossRef]
- Rutter, J.W.; Ozdemir, T.; Galimov, E.R.; Quintaneiro, L.M.; Rosa, L.; Thomas, G.M.; Cabreiro, F.; Barnes, C.P. Detecting Changes in the Caenorhabditis elegans Intestinal Environment Using an Engineered Bacterial Biosensor. ACS Synth. Biol. 2019, 8, 2620–2628. [Google Scholar] [CrossRef]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. GeroScience 2019, 42, 333–352. [Google Scholar] [CrossRef]
- Kim, J.; Moon, Y. Worm-based alternate assessment of probiotic intervention against gut barrier infection. Nutrients 2019, 11, 2146. [Google Scholar] [CrossRef]
- Raposo, V.L. Safe Drugs Versus Innovative Drugs (Can We Have Both?). Adv. Pharm. Bull. 2019, 10, 334–337. [Google Scholar] [CrossRef]
- Anupama, S.; Kaduskar, B.; Reddy, K.C.; Kumsta, C.; Bier, E.; Hansen, M.; Chalasani, S.H. Conserved neuropeptidergic regulation of intestinal integrity in invertebrate models of aging. bioRxiv 2022. [Google Scholar] [CrossRef]
- Xiong, H.; Pears, C.; Woollard, A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017, 7, 9839. [Google Scholar] [CrossRef]
- Calahorro, F.; Holden-Dye, L.; O’connor, V. Impact of drug solvents on C. elegans pharyngeal pumping. Toxicol. Rep. 2021, 8, 1240–1247. [Google Scholar] [CrossRef]
- Zhang, A.; Hsiung, K.C.; Kern, C.C.; Wang, Y.; Girtle, A.L.; Xu, N.; Gems, D. Unraveling effects of anti-aging drugs on C. elegans using liposomes. GeroScience 2023, 45, 1583–1603. [Google Scholar] [CrossRef]
- Flavel, M.R.; Mechler, A.; Shahmiri, M.; Mathews, E.R.; E Franks, A.; Chen, W.; Zanker, D.; Xian, B.; Gao, S.; Luo, J.; et al. Growth of Caenorhabditis elegans in defined media is dependent on presence of particulate matter. G3 Genes|Genomes|Genet. 2018, 8, 567–575. [Google Scholar] [CrossRef]
- Ramage, G.; Borghi, E.; Rodrigues, C.F.; Kean, R.; Williams, C.; Lopez-Ribot, J. Our current clinical under-standing of Candida biofilms: Where are we two decades on? APMIS 2023, 131, 636–653. [Google Scholar] [CrossRef]
- Wang, E.; Jiang, Y.; Zhao, C. Structural and physiological functions of Caenorhabditis elegans epidermis. Heliyon 2024, 10, e38680. [Google Scholar] [CrossRef]
- Sundaram, M.V.; Pujol, N. The Caenorhabditis elegans cuticle and precuticle: A model for studying dynamic apical extracellular matrices in vivo. Genetics 2024, 227, iyae072. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2016, 37, 50–59. [Google Scholar] [CrossRef]
- Sarma, G.P.; Lee, C.W.; Portegys, T.; Ghayoomie, V.; Jacobs, T.; Alicea, B.; Cantarelli, M.; Currie, M.; Gerkin, R.C.; Gingell, S.; et al. OpenWorm: Overview and recent advances in integrative biological simulation of Caenorhabditis elegans. Philos. Trans. R. Soc. B 2018, 373, 20170382. [Google Scholar] [CrossRef]
- Petrascheck, M.; Miller, D.L. Computational Analysis of Lifespan Experiment Reproducibility. Front. Genet. 2017, 8, 92. [Google Scholar] [CrossRef]
- FDA. The C. elegans Model in Toxicity Testing. Toxicology Research. 2022. Available online: https://www.fda.gov/food/toxicology-research/c-elegans-model-toxicity-testing#:~:text=elegans%20tests%20is%20comparable%20to,%2C%20sensory%2C%20and%20neuromuscular%20systems (accessed on 15 March 2025).
- Urban, N.D.; Cavataio, J.P.; Berry, Y.; Vang, B.; Maddali, A.; Sukpraphrute, R.J.; Schnell, S.; Truttmann, M.C. Explaining inter-lab variance in C. elegans N2 lifespan: Making a case for standardized reporting to enhance reproducibility. Exp. Gerontol. 2021, 156, 111622. [Google Scholar] [CrossRef]
- van der Zanden, T.M.; Mooij, M.G.; Vet, N.J.; Neubert, A.; Rascher, W.; Lagler, F.B.; Male, C.; Grytli, H.; Halvorsen, T.; de Hoog, M.; et al. Benefit-Risk Assessment of Off-Label Drug Use in Children: The Bravo Framework. Clin. Pharmacol. Ther. 2021, 110, 952–965. [Google Scholar] [CrossRef]
- van Meer, P.J.; Graham, M.L.; Schuurman, H.-J. The safety, efficacy and regulatory triangle in drug development: Impact for animal models and the use of animals. Eur. J. Pharmacol. 2015, 759, 3–13. [Google Scholar] [CrossRef]
- van der Voet, M.; Teunis, M.; de Haar, J.L.-V.; Stigter, N.; Bhalla, D.; Rooseboom, M.; Wever, K.E.; Krul, C.; Pieters, R.; Wildwater, M.; et al. Towards a reporting guideline for developmental and reproductive toxicology testing in C. elegans and other nematodes. Toxicol. Res. 2021, 10, 1202–1210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).