Effect of Doxapram, a K2p Channel Blocker, and pH on Heart Rate: Larval Drosophila Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Fly Lines and Culturing Conditions
2.2. Dissection and Solutions
2.3. Measures of Membrane Potential and Frequency of Spontaneous Quantal Responses in Body Wall Muscles
2.4. Statistical Analysis
3. Results
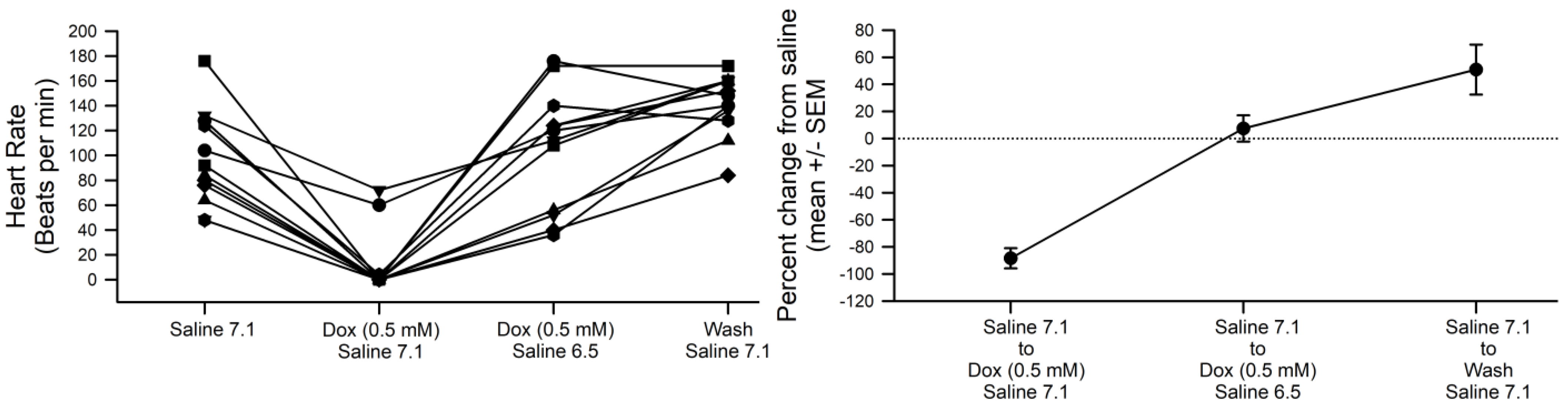
3.1. The Effect of Doxapram on Heart Rate
3.2. Effect of pH on HR
3.3. Effect of Doxapram on LPS Action
3.4. Sensitivity to pH in Hearts and Overexpressing ORK1 (a K2p Channel)
3.5. Resting Membrane Potential and Quantal Occurrences in Skeletal Muscle
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.D.; Kidd, J.F.; Law, R.J.; Franks, C.J.; Sattelle, D.B. Structure and function of two-pore-domain K+ channels: Contributions from genetic model organisms. Trends Pharmacol. Sci. 2005, 26, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Plant, L.D.; Goldstein, S.A.N. Two-Pore Domain Potassium Channels. In Handbook of Ion Channels, 1st ed.; Zheng, J., Trudeau, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429193965. [Google Scholar]
- Lee, L.M.; Müntefering, T.; Budde, T.; Meuth, S.G.; Ruck, T. Pathophysiological role of K2P channels in human diseases. Cell Physiol. Biochem. 2021, 55, 65–86. [Google Scholar] [PubMed]
- Wiedmann, F.; Frey, N.; Schmidt, C. Two-Pore-Domain Potassium (K2P−) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes. Cells 2021, 10, 2914. [Google Scholar]
- Rotstein, B.; Paululat, A. On the Morphology of the Drosophila Heart. J. Cardiovasc. Dev. Dis. 2016, 3, 15. [Google Scholar] [CrossRef]
- Badre, N.H.; Martin, M.E.; Cooper, R.L. The physiological and behavioral effects of carbon dioxide on Drosophila melanogaster larvae. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2005, 140, 363–376. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Stacy, A.; Cooper, R.L. Pharmacological identification of serotonin receptor subtypes on the Dro-sophila larval heart. J. Comp. Physiol. B 2014, 184, 205–219. [Google Scholar] [CrossRef]
- Bartos, D.C.; Grandi, E.; Ripplinger, C.M. Ion Channels in the Heart. Compr. Physiol. 2015, 5, 1423–1464. [Google Scholar]
- Severi, S.; Cavalcanti, S.; Mancini, E.; Santoro, A. Effect of electrolyte and pH changes on the sinus node pacemaking in humans. J. Electrocardiol. 2002, 35, 115–124. [Google Scholar] [CrossRef]
- Achike, F.I.; Dai, S. Effects of blood gas/pH abnormalities on the cardiovascular actions of verapamil in rats. Clin. Exp. Pharmacol. Physiol. 1990, 17, 653–663. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Price, L.A.; Rosenthal, D.N.; Pausch, M.H. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1996, 93, 13256–13261. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Wang, K.W.; Ilan, N.; Pausch, M.H. Sequence and function of the two P domain potassium channels: Implications of an emerging superfamily. J. Mol. Med. 1998, 76, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cotten, J.F.; Keshavaprasad, B.; Laster, M.J.; Eger, E.I., II; Yost, C.S. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth. Analg. 2006, 102, 779–785. [Google Scholar] [CrossRef]
- Vacassenno, R.M.; Haddad, C.N.; Cooper, R.L. The effects on resting membrane potential and synaptic transmission by Doxapram (blocker of K2P channels) at the Drosophila neuromuscular junction. Comp. Biochem. Physiol. C 2023, 263, 109497. [Google Scholar] [CrossRef]
- Vacassenno, R.M.; Haddad, C.N.; Cooper, R.L. Bacterial lipopolysaccharide hyperpolarizes the membrane potential and is antagonized by the K2p channel blocker doxapram. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2023, 266, 109571. [Google Scholar] [CrossRef]
- Lalevée, N.; Monier, B.; Sénatore, S.; Perrin, L.; Sémériva, M. Control of cardiac rhythm by ORK1, a Drosophila two-pore domain potassium channel. Curr. Biol. 2006, 16, 1502–1508. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.; Hoskins, R.A.; Galle, R.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Littleton, J.T.; Ganetzky, B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron 2000, 26, 35–43. [Google Scholar] [CrossRef]
- Patel, A.J.; Honore, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef]
- Cooper, R.L.; Krall, R.M. Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P). Int. J. Mol. Sci. 2022, 23, 15787. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.E.; Elliott, E.R.; Abul-Khoudoud, M.O.; Cooper, R.L. The effects of Gram-positive and Gram-negative bacterial toxins (LTA & LPS) on cardiac function in Drosophila melanogaster larvae. J. Insect Physiol. 2023, 147, 104518. [Google Scholar] [PubMed]
- Pleuvry, B.J. A study of the enhanced toxicity of doxapram in rodents treated with narcotic analgesics. Br. J. Anaesth. 1978, 50, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Devel. 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.S.; Rymond, K.E.; Ward, M.A.; Bocook, E.L.; Cooper, R.L. Monitoring heart function in larval Drosophila melanogaster for physiological studies. J. Vis. Exp. 2009, 33, 1596. [Google Scholar] [CrossRef]
- De Castro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A 2014, 200, 83–92. [Google Scholar] [CrossRef]
- Fast, I.; Rosenkranz, D. 2018. Temperature-dependent small RNA expression in Drosophila melanogaster. RNA Biol. 2018, 15, 308–313. [Google Scholar] [CrossRef]
- Haltenhof, T.; Kotte, A.; De Bortoli, F.; Schiefer, S.; Meinke, S.; Emmerichs, A.K.; Petermann, K.K.; Timmermann, B.; Imhof, P.; Franz, A.; et al. A conserved kinase-based body-temperature sensor globally controls alternative splicing and gene expression. Mol. Cell. 2020, 78, 57–69.e4. [Google Scholar] [CrossRef]
- Ashburner, M. Drosophila: A Laboratory Handbook; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Rizki, T.M. The circulatory system and associated cells and tissues. In The Genetics and Biology of Drosophila; Ashburner, M., Wright, T.R.F., Eds.; Academic Press: New York, NY, USA, 1978; Volume 2b, pp. 397–452. [Google Scholar]
- Cooper, R.L.; McNabb, M.; Nadolski, J. The effects of a bacterial endotoxin LPS on synaptic transmission at the neuromuscular junction. Heliyon 2019, 5, e01430. [Google Scholar] [CrossRef]
- Zilberberg, N.; Ilan, N.; Gonzalez-Colaso, R.; Goldstein, S.A. Opening and closing of KCNKO potassium leak channels is tightly regulated. J. Gen. Physiol. 2000, 116, 721–734. [Google Scholar] [CrossRef]
- Zilberberg, N.; Ilan, N.; Goldstein, S.A. KCNKØ: Opening and closing the 2-P-domain potassium leak channel entails “C-type” gating of the outer pore. Neuron 2001, 32, 635–648. [Google Scholar] [CrossRef]
- Ilan, N.; Goldstein, S.A. Kcnko: Single, cloned potassium leak channels are multi-ion pores. Biophys. J. 2001, 80, 241–253. [Google Scholar]
- Kim, D. Physiology and pharmacology of two-pore domain potassium channels. Curr. Pharm. Des. 2005, 11, 2717–2736. [Google Scholar] [CrossRef]
- Tian, F.; Qiu, Y.; Lan, X.; Li, M.; Yang, H.; Gao, Z. A small-molecule compound selectively activates K2P channel TASK-3 by acting at two distant clusters of residues. Mol. Pharmacol. 2019, 96, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.; Zilberberg, N.; Goldstein, S.A. Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J. Biol. Chem. 2001, 276, 24449–24452. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Wischmeyer, E.; Xin Liu, G.; Preisig-Müller, R.; Daut, J.; Karschin, A.; Derst, C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J. Biol. Chem. 2000, 275, 16650–16667. [Google Scholar] [CrossRef]
- O’Connell, A.D.; Morton, M.J.; Hunter, M. Two-pore domain K+ channels-molecular sensors. Biochim. Biophys. Acta 2002, 1566, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Ringo, J.; Dowse, H. Modulation of Drosophila heartbeat by neurotransmitters. J. Comp. Physiol. B 1997, 167, 89–97. [Google Scholar] [CrossRef]
- Johnson, E.; Ringo, J.; Dowse, H. Native and heterologous neuropeptides are cardioactive in Drosophila melanogaster. J. Insect Physiol. 2000, 46, 1229–1236. [Google Scholar] [CrossRef]
- Nichols, R.; Kaminski, S.; Walling, E.; Zornik, E. Regulating the activity of a cardioacceleratory peptide. Peptides 1999, 20, 1153–1158. [Google Scholar] [CrossRef]
- Zornik, E.; Paisley, K.; Nichols, R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides 1999, 20, 45–51. [Google Scholar] [CrossRef]
- Titlow, J.S.; Rufer, J.; King, K.; Cooper, R.L. Pharmacological analysis of dopamine modulation in the Drosophila melanogaster larval heart. Physiol. Rep. 2013, 1, e00020. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Nichols, C.D.; Cooper, R.L. 5-HT stimulation of heart rate in Drosophila does not act through cAMP as revealed by pharmacogenetics. J. Appl. Physiol. 2013, 115, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Malloy, C.; Sifers, J.; Mikos, A.; Samadi, A.; Omar, A.; Hermanns, C.; Cooper, R.L. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A. Neuroethol. Sens. Neural Behav. Physiol. 2017, 203, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.I.; Wang, M.; Kao, H.H.; Cheng, Y.J.; Lin, Y.J.; Chen, R.H.; Chien, C.T. Activity-dependent retrograde laminin A signaling regulates synapse growth at Drosophila neuromuscular junctions. Proc. Natl. Acad. Sci. USA 2012, 109, 17699–17704. [Google Scholar] [CrossRef] [PubMed]
- Berke, B.; Wittnam, J.; McNeill, E.; Van Vactor, D.L.; Keshishian, H. Retrograde BMP signaling at the synapse: A permissive signal for synapse maturation and activity-dependent plasticity. J. Neurosci. 2013, 33, 17937–17950. [Google Scholar] [CrossRef]
- Chakkalakal, J.V.; Nishimune, H.; Ruas, J.L.; Spiegelman, B.M.; Sanes, J.R. Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development 2010, 137, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Marqués, G. Morphogens and synaptogenesis in Drosophila. J. Neurobiol. 2005, 64, 417–434. [Google Scholar] [CrossRef]
- Frank, C.A. Homeostatic plasticity at the Drosophila neuromuscular junction. Neuropharmacology 2014, 78, 63–74. [Google Scholar]
- Collins, C.A.; DiAntonio, A. Synaptic development: Insights from Drosophila. Curr. Opin. Neurobiol. 2007, 17, 35–42. [Google Scholar] [CrossRef]
- Li, H.; Peng, X.; Cooper, R.L. Development of Drosophila larval neuromuscular junctions: Maintaining synaptic strength. Neuroscience 2002, 115, 505–513. [Google Scholar]
- Xing, B.; Long, A.A.; Harrison, D.A.; Cooper, R.L. Developmental consequences of neuromuscular junctions with reduced presynaptic calcium channel function. Synapse 2005, 57, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.; Singh, G.K.; Osterwalder, T.; Roman, G.W.; Davis, R.L.; Keshishian, H. Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics 2008, 178, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Feliciangeli, S.; Chatelain, F.C.; Bichet, D.; Lesage, F. The family of K2P channels: Salient structural and functional properties. J. Physiol. 2015, 593, 2587–2603. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, E.R.; Taul, A.C.; Abul-Khoudoud, M.O.; Hensley, N.; Cooper, R.L. Effect of Doxapram, a K2p Channel Blocker, and pH on Heart Rate: Larval Drosophila Model. Appl. Biosci. 2023, 2, 406-420. https://doi.org/10.3390/applbiosci2030026
Elliott ER, Taul AC, Abul-Khoudoud MO, Hensley N, Cooper RL. Effect of Doxapram, a K2p Channel Blocker, and pH on Heart Rate: Larval Drosophila Model. Applied Biosciences. 2023; 2(3):406-420. https://doi.org/10.3390/applbiosci2030026
Chicago/Turabian StyleElliott, Elizabeth R., Alaina C. Taul, Maya O. Abul-Khoudoud, Nicole Hensley, and Robin L. Cooper. 2023. "Effect of Doxapram, a K2p Channel Blocker, and pH on Heart Rate: Larval Drosophila Model" Applied Biosciences 2, no. 3: 406-420. https://doi.org/10.3390/applbiosci2030026
APA StyleElliott, E. R., Taul, A. C., Abul-Khoudoud, M. O., Hensley, N., & Cooper, R. L. (2023). Effect of Doxapram, a K2p Channel Blocker, and pH on Heart Rate: Larval Drosophila Model. Applied Biosciences, 2(3), 406-420. https://doi.org/10.3390/applbiosci2030026






