Abstract
The zebrafish model is an emerging model for the study of the complex behavioural patterns noted in depression and neurological disorders. Confinement and memory loss are linked with cognition and mental health impairment, where confinement paradigms are assessed using other behavioural responses based on novel tanks or T tanks. Since zebrafish are exploratory animals, the impact during confinement cannot be evaluated using a novel tank or T tank. The present study investigates the response of the zebrafish to acute confinement and assesses its memory-based learning behaviour through parameters such as movement, swimming speed, and time spent inside the confined space. The movement and swimming speed of the fishes in confinement showed no significant difference. When confined inside a space, the fish showed their anxiety with erratic movements or bouts of freezing, which declined by 83%, during the six days of confinement and the escape time from the confinement space also decreased by 58%. The impact of anxiety, resulting in clockwise and counter-clockwise movement, also reduced after three days. Our results summarise that the decrease in anxiety can help the fish in habituating itself to a forced condition. This experiment on zebrafish behavioural biology is used to assess the cognitive behaviour against confinement, and it emphasizes the learning of behavioural adaptions under both crowded and solitary conditions.
1. Introduction
Zebrafish are considered one of the best model organisms to study developmental biology, toxicology, pharmacology, and behavioural research [1]. It is recognised by several researchers [2] as one of the best models for cognitive research regarding memory [3], aggressive–submissive behaviour [4], food searching behaviour, shoaling behaviour, etc. Many approaches have been employed to study learning and memory, with respect to anxiety and other cognitive impairments, in zebrafish. Behaviour responses are considered as the reflection of neural activity and its modulation by external stimuli [5]. Memory, learning, and social behaviours are found to be major hallmarks of various neurodegenerative, psychological, and cognitive disorders [6]. Several paradigms have been developed to quantify and understand zebrafish behaviour and cognition [7,8,9,10,11]. It is also reported that the complexity of behaviours in zebrafish is very high, and hence, significant research must be conducted to completely understand these behaviours [12]. Cognitive behavioural studies in zebrafish can be correlated to human neurological disorders and can help in the understanding of the various aspects of the disease symptoms [13,14,15].
The memory and anxiety-based behavioural assays were performed on zebrafish to determine the effects of the toxicity of specific drugs/compounds on their memory and learning [16,17,18]. When zebrafish are introduced into a potentially dangerous environment or stimuli, complex behaviours will be provoked. These can be observed as erratic movements, freezing bouts, opercular movement, coded colour change, geotaxis, thigmotaxis, and scototaxis [19]. A tolerable level of isolation from, or deprivation of, society would produce mental abnormalities, such as hallucinations, anxiety states, depression, and paranoid symptoms in humans. The correlation of anxiety with learning is important for understanding cognitive impairment, since it has been reported that confinement is also a key feature contributing to memory loss during the early stages of Alzheimer’s disease [20,21,22]. The escaping behaviour of zebrafish from a moving trawl net is observed, and the group of fishes learned to escape from the net in a faster way through social learning [23]. The disruption of several neurological compounds and neurotransmitters, which is the underlying cause of neurological disorders, arises due to stress caused by social isolation [24,25,26]. Moreover, a slowing in EEG frequency was observed during solitary confinement due to social deprivation [27]. Anxiety-induced or stressed zebrafish subjected to confinement showed stress-induced hyperthermia as an emotional fever [18]. The spatiotemporal exploratory activity of the shortfin zebrafish in the open tank after the short period of confinement is significantly different from that exhibited by the control group [28]. Nociceptive behaviour in zebrafish was observed to be anxiety-eliciting in the confinement space [29].
Anxiety is the most notably observed behaviour of zebrafish when they are introduced into a new environment. Increasing evidence suggests that the anxiety-like behaviour of zebrafish is evolutionarily conserved and comparable with mammalian models [30,31]. Additionally, this anxiety behaviour regulates aversive learning and emotionality [20,32]. Anxiety and stress can be caused by various factors, including handling, capture, net chasing, physical disturbances, novel environment, exposure to a predator, addiction withdrawal, fish crowding, confinement, social stressors, environmental stressors, and infections.
In this study, we have used a simple assay to assess and understand the memory repertoire of zebrafish to space confinement and its related anxiety. Memory and anxiety-based behavioural assays shall be performed in zebrafish to determine the effects of toxicity of specific drugs/compounds on their memory and learning. The correlation of anxiety with learning is important for understanding cognitive impairment, since it has been reported that confinement is also a key feature that contributes to memory loss during the early stages of Alzheimer’s disease [33]. In this study, we have used a simple assay to assess and understand the behavioural repertoire of zebrafish to space confinement and its relation with anxiety and memory.
2. Methodology
2.1. Animal Husbandry
Adult wild AB-type zebrafish (about 1.5 years old) bought from a local aquarium were kept in quarantine for 2 weeks and maintained in a re-circulating stand-alone system (Aquaneering, San Diego, CA, USA) at 28 °C with 10:14 h dark:light cycle. The pH and conductivity were maintained at 7.1 to 7.8 and 1000–1400 µS/cm, respectively. All the fishes were fed thrice a day with commercial fish feed and with Artemia nauplii [34]. The handling and experimentation on animals were carried out under the protocol of the Institutional Bioethical Committee (ID IBSC/2013/DBT-IDB/RRK-009) and complied with the ARRIVE guidelines, carried out following the U.K. Animals (Scientific Procedures) Act, 1986, and its associated guidelines.
2.2. Experimental Setup
A standard cuboid shape (25 cm length × 17 cm width × 12 cm height) with a 100 mm dia Petri dish at the centre was used for this experiment (3.5 L filled with water). The Petri dish was provisioned with an over-lid, which can be manually opened and closed without disturbing the water in the tank. A 100 mm dia Petri dish was used to evaluate the effect of a confined space on anxiety and memory. Initially, the adult zebrafish (n = 6) were kept in the closed Petri dish for 15 min for acclimatisation, after which the Petri dish was opened manually and the movement of the zebrafish was video recorded (top view) until the fish escaped from the confined space (Petri dish). The opening of the lid was considered as the stimuli for the zebrafish to move out of the confined space (Figure 1A). The memory to how to escape from the confinement zone was observed through the swimming and turning behaviour. Changes from stress and anxiety behaviour to slow swimming behaviour on consecutive days is observed as learned escaping behaviour until the application of the stimuli (lid open) (Table 1). All apparatuses rested on a level stable surface.
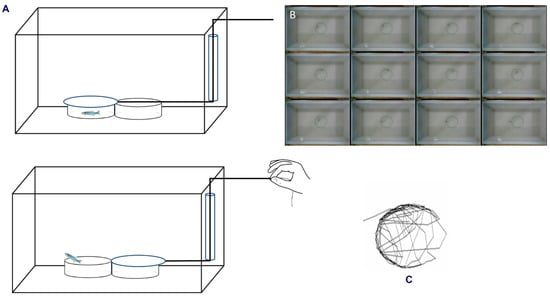
Figure 1.
(A) Illustration of the experimental design showing acclimatisation of zebrafish in the confined space for 12–15 min, after which the dish was opened for the observation of the behaviour. This setup was used to study the habituation of zebrafish in confinement; (B) representation of the movement of zebrafish in the confined space, including crossing over the huddle during the experiment. (C) The movement pattern tracked using the tracker software.

Table 1.
Ethogram describing the behaviour category and its definition, as observed in the present study.
2.3. Behaviour Analysis
Using adult zebrafish, the experiment was repeatedly performed, and measurements were recorded for 6 days. The quantification of the behaviour of zebrafish was assessed based on parameters such as total distance travelled, total time spent, and the swimming speed of the fish [35]. The clockwise and counter-clockwise movement also indicated the zebrafish escape behaviours [36]. All behavioural recordings were analysed, and a graph was plotted using Tracker software (video analysis and modelling tool, Version 4.11.0) [37,38] (Figure 1B). The x and y coordinates at each time point—(x1, y1) as time point A, and (x2, y2) as time point B—were obtained, and the distance travelled was calculated using Formula (1), followed by the calculation of the swimming speed using Formula (2).
Distance = √((x2 − x1)2 + (y2 − y1)2)
Velocity = distance/time
2.4. Statistical Analysis
All other data were calculated as means ± standard deviation (SD) using the tool Origin 8.0, and the comparison within populations was made using ANOVA (Graph Pad Prism Version 6.0, San Diego, CA, USA). The correlation–regression curve and a percentage analysis were performed, wherever applicable. A statistical significance of 0.05 is used in the study.
3. Results
The video recordings were captured using a Canon SX 50 camera at a rate of 30 frames per second; the swimming behaviour was recorded until the fish came out of the confinement space (as shown in Figure 1B) and was tracked using the manual tracking method in the video analysis and modelling tool Tracker-Version 5.1.5 [39]. Initially, the fish were kept in the confined environment for acclimatisation for 12–15 min, following which a stimuli was induced by opening the confinement area for the fish to escape. The fish showed a startled swim response as soon as the stimuli was induced, and the escape of fish from the confined space was observed. The zebrafish moves at an optimal speed of 39 cm/s, and the threshold was set to 45 cm/s for erratic movement. The fish’s speed, upon reaching less than 5 cm/s, was considered as freezing.
3.1. Response Time for Escaping from Confinement
The total time spent inside the confined space showed a change in pattern from day 1 to day 6. The average time for the fish to escape on day 1, day 2, day 3, day 4, day 5 and day 6 is 12.78 ± 6.38, 11.67 ± 7.33, 9.18 ± 2.18, 8.8 ± 2.37, 5.55 ± 1.95, and 5.12 ± 3.11 s, respectively. There was no significant difference between each day for the time that was spent inside the confined space F (5, 29) = 0.426, p = 0.827. A negative correlation was observed between escape response time and treatment days with an R-value of −0.95286, p = 0.0005 (Figure 2A).
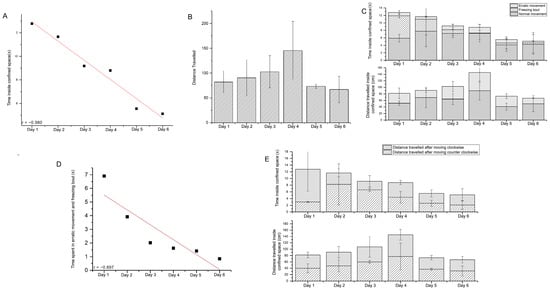
Figure 2.
(A) Regression curve showing the negative correlation between trial days (increase) and time spent inside the confined space (decrease). (B) The movement of the zebrafish is represented as the mean distance travelled ± SD for 6 days. (C) Image representing mean ± SD for time and movement of the zebrafish during the confinement period, with erratic movement and freezing bouts. (D) Regression curve showing the negative correlation between trial days (increase) and time spent in anxiety (decrease) during confinement. (E) Image representing mean ± SD for time and movement of the zebrafish during the confinement period, with respect to clockwise and anticlockwise movement.
3.2. Effect of Confined Space on the Movement of Zebrafish
The movement of the zebrafish was assessed for the time it spent inside the confined space, along with the 6-day trial. The total distance moved each day for day 1 to day 6 is 81.80 ± 37.75, 90.56 ± 24.44, 107.28 ± 45.18, 145.49 ± 42.00, 73.16 ± 15.97, and 66.91 ± 21.84, respectively (Figure 2B). There was no significant difference during the 6-day trial for distance travelled inside the experimental area, showing F (5, 29) = 0.7303, p = 0.6065.
3.3. Effect of Confined Space on Anxiety
The impact of the freezing bout and erratic movement was considered as a representation of anxiety. The time duration in which the zebrafish had no movement is used as one parameter of anxiety. During the freezing bout, the fish seems to be in a non-movable condition. The total time observed as anxiety inside the confined space for day 1 to day 6 is 6.01 ± 5.85, 3.2 ± 3.1, 1 ± 0.67, 0.08 ± 0.01, 0.52 ± 0.35, and 0.42 ± 0.38333, respectively. The total distance travelled during the anxiety period from day 1 to day 6 is 30.26 ± 15.76, 21.72 ± 5.39, 38.50 ± 15.67, 55.57 ± 30.17, 32.11 ± 6.84, and 16.96 ± 1.72, respectively (Figure 2C). The duration spent with erratic movement and freezing bouts was negatively correlated with the days of trial and showed a decrease in anxiety by an R-value of −0.897, p = 0.0151 (Figure 2D).
3.4. Effect of Confined Space on Rotational Movement
The counter-clockwise and clockwise movements were taken into consideration to determine the turning movement behaviour inside a confined space. There is no significant difference between counter-clockwise and clockwise movement concerning the travelled time, distance, and velocity. The time spent after moving counter-clockwise from day 1 to day 6 is 3.00 ± 0.17, 8.23 ± 6.13, 6.60 ± 0.50, 4.40 ± 1.70, 2.62 ± 0.88, and 2.10 ± 1.30, respectively; similarly, the time spent after moving clockwise was 9.77 ± 6.57, 3.43 ± 1.20, 2.58 ± 1.68, 4.40 ± 0.67, 2.93 ± 1.07, and 3.02 ± 1.82, respectively. The distance travelled after moving counter-clockwise from day 1 to day 6 is 39.77 ± 14.00, 47.25 ± 18.60, 59.95 ± 4.59, 76.91 ± 41.91, 36.75 ± 3.27, and 31.48 ± 15.70, respectively. Similarly, the distance travelled after moving clockwise from day 1 to day 6 is 42.03 ± 8.79, 43.32 ± 17.79, 47.33 ± 32.42, 68.58 ± 16.97, 36.42 ± 7.15, and 35.43 ± 10.65 cm, respectively (Figure 2E).
4. Discussion
There is an urgent need for novel bio behavioural assays using alternative model organisms, especially those species with sufficient physiological complexity, which is similar to that of humans, and a high throughput screening capacity, such as zebrafish [40,41]. In most cognitive diseases, confinement condition plays an important role in the progression of mild cognitive impairment and increases the risk of cognitive decline [33]. Developing a strategic system is essential to understand the relationship between confined space, anxiety, and cognition [42]. The behaviour pattern of zebrafish inside a confined space and its relationship with escape memory has been assessed in the present study, thereby correlating the relationship between anxiety and memory concerning habituation.
In the present study, the behaviour responses for learning, memory, and space confinement were assessed to understand their relationship using zebrafishes. Novel tank studies on zebrafish maintained in cylinder-based confinement for a short period exhibited a rapid increase in exploratory behaviour to rapidly habituate to the environment, showing that zebrafish can easily habituate to an environment [43]. These results suggest that the effect of confinement cannot be assessed directly by the novel tank test, but rather the assessment effect must be studied in the confined environment only. The system was designed to evaluate both space confinement and memory, in which the fish was allowed to swim in a Petri dish as part of a confined space, and the study continued for six days to assess the learning capacity of the zebrafish.
The current study showed that the travelled duration in the experiment area gradually decreased, showing a total reduction of 58% at the end of 6-day trial. This provides an insight into the memory of zebrafish during the 6-day trial period, considering the confinement for 15 min as acute stress. The distance gradually decreased each day, and the fish’s memory was improved by increasing the possibility of escaping from the obstacle sooner.
There was no significant variation or correlation between distance travelled and swimming speed during the study period. The standard deviation was also taken into consideration to rule out the possibility of the impact of acclimatisation in the initial period and the later period of the study. There was a higher deviation of 9.0 s and 4.4 s on day 6, showing an increase in consistency and a decrease in standard deviation. These results imply that the learning and memory capacity of the fish to escape from the Petri dish at their earliest increased during the study period, and was not influenced by distance travelled and velocity.
Anxiety in zebrafish is observed as freezing bouts and erratic movement [44,45]. Freezing is defined as a total absence of movement, except for the gills and eyes, for 2 s or longer. The freezing bout, as a form of anxiety, was significantly observed in zebrafish when they were confined to space in a white background [12]. The fish remained in the freeze state for a longer time on day 1, but gradually, the freezing period was reduced. A significant decrease in exploration or elevated erratic movements represents the behavioural profile indicative of high stress and anxiety [46,47]. Acute stress can increase the movement and aggressiveness of zebrafish [48]. The cause of erratic movement and its influence is observed between day 1 and day 4, showing the anxiety-like erratic movement effect, with no influence on days 5 and 6. When comparing the study duration with anxiety (erratic movement and freeze bout) behaviour, there is a significant negative correlation between them, with a decrease in anxiety-like conditions by 83%.
Studying the counter-clockwise and clockwise turning frequencies helps to determine the impact of anxiety on movement [36]. The turning frequency is positively correlated with the anxiety of the zebrafish, with increased erratic movement due to anxiety. The increase in turning frequency is directly proportional to the erratic movement. Further analysis also shows that when the erratic movement is reduced on days 5 and 6, the turning frequency also decreases, showing anxiety reduction. From these results, days 1–4 can be considered as the training period for the fish, and days 5–6 are considered as the resultant behaviour of learning. Therefore, habituation facilitates a reduction in anxiety of the fish during the 6-day trial.
5. Conclusions
In conclusion, this study implied that a decrease in stress and anxiety may lead to better habituation, even under stressed conditions. The confinement of isolated fish increases their stress level by a higher percentage than do other stressors. The learning and memory of the fishes in the 6-day trial were observed within 4 days of the training period, and exhibited habituation behaviour on the next two days, in order to overcome the confined environment for survival. Training over the 4 days facilitated the reduction in anxiety of the fish, which is implied by the decrease in freezing bouts and erratic movement during the duration of the study. The turning frequency also decreased, showing the relationship between learning, memory, and anxiety. With the present study, we have developed a simple system for demonstrating the behaviour paradigm for cognitive-related studies. In addition, this study shows the relationship between a confined space, anxiety, and the habituation of the fish in a controlled condition. The study can further be developed for different time segments of acclimation to understand the learning curve under a degree of stress and anxiety. Changes in stress levels influence memory and learning, and thus, there are many possibilities for investigating new learning behaviours, social interactions, predator encountering, etc., in the context of confinement.
Author Contributions
Conceptualization, C.R.W.A. and R.K.R.; methodology, C.R.W.A. and R.K.R.; software, C.R.W.A. and R.K.R.; validation, C.R.W.A. and R.K.R.; formal analysis, C.R.W.A. and R.K.R.; writing—original draft preparation, writing—review and editing, C.R.W.A. and R.K.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Ethics Committee, Sathyabama Institute of Science and Technology (protocol code SU/CLATR/IAEC/XVII/173/2021, 13 March 2021).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, S. Linking Genes to Brain, Behavior and Neurological Diseases: What Can We Learn from Zebrafish? Genes. Brain. Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef]
- Roberts, A.C.; Bill, B.R.; Glanzman, D.L. Learning and Memory in Zebrafish Larvae. Front. Neural Circuits 2013, 7, 126. [Google Scholar] [CrossRef]
- Fernandes, Y.; Talpos, A.; Gerlai, R. Towards the Characterization of Short-Term Memory of Zebrafish: Effect of Fixed versus Random Reward Location. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 189–195. [Google Scholar] [CrossRef]
- Vuaden, F.C.; Savio, L.E.B.; Piato, A.L.; Pereira, T.C.; Vianna, M.R.; Bogo, M.R.; Bonan, C.D.; Wyse, A.T.S. Long-Term Methionine Exposure Induces Memory Impairment on Inhibitory Avoidance Task and Alters Acetylcholinesterase Activity and Expression in Zebrafish (Danio Rerio). Neurochem. Res. 2012, 37, 1545–1553. [Google Scholar] [CrossRef]
- Girdhar, K.; Gruebele, M.; Chemla, Y.R. The Behavioral Space of Zebrafish Locomotion and Its Neural Network Analog. PLoS ONE 2015, 10, e0128668. [Google Scholar] [CrossRef]
- Stewart, A.; Kadri, F.; Dileo, J.; Chung, K.M.; Cachat, J.; Goodspeed, J.; Suciu, C.; Roy, S.; Gaikwad, S.; Wong, K.; et al. The Developing Utility of Zebrafish in Modeling Neurobehavioral Disorders. Int. J. Comp. Psychol. 2010, 23, 104–120. [Google Scholar] [CrossRef]
- Dabrowski, K.; Miller, M. Contested Paradigm in Raising Zebrafish (Danio Rerio). Zebrafish 2018, 15, 295–309. [Google Scholar] [CrossRef]
- Pather, S.; Gerlai, R. Shuttle Box Learning in Zebrafish (Danio Rerio). Behav. Brain Res. 2009, 196, 323–327. [Google Scholar] [CrossRef]
- Xu, X.; Scott-Scheiern, T.; Kempker, L.; Simons, K. Active Avoidance Conditioning in Zebrafish (Danio Rerio). Neurobiol. Learn. Mem. 2007, 87, 72–77. [Google Scholar] [CrossRef]
- Aoki, R.; Tsuboi, T.; Okamoto, H. Y-Maze Avoidance: An Automated and Rapid Associative Learning Paradigm in Zebrafish. Neurosci. Res. 2015, 91, 69–72. [Google Scholar] [CrossRef]
- Mathur, P.; Lau, B.; Guo, S. Conditioned Place Preference Behavior in Zebrafish. Nat. Protoc. 2011, 6, 338–345. [Google Scholar] [CrossRef]
- Blaser, R.E.; Chadwick, L.; McGinnis, G.C. Behavioral Measures of Anxiety in Zebrafish (Danio Rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef]
- Morales, E.E.; Wingert, R.A. Zebrafish as a Model of Kidney Disease. In Results and Problems in Cell Differentiation; Springer: New York, NY, USA, 2017; Volume 60, pp. 55–75. ISBN 9789529253333. [Google Scholar]
- Stewart, A.; Wong, K.; Cachat, J.; Gaikwad, S.; Kyzar, E.; Wu, N.; Hart, P.; Piet, V.; Utterback, E.; Elegante, M.; et al. Zebrafish Models to Study Drug Abuse-Related Phenotypes. Rev. Neurosci. 2011, 22, 95–105. [Google Scholar] [CrossRef]
- Becker, T.S.; Rinkwitz, S. Zebrafish as a Genomics Model for Human Neurological and Polygenic Disorders. Dev. Neurobiol. 2012, 72, 415–428. [Google Scholar] [CrossRef]
- Gaikwad, S.; Stewart, A.; Hart, P.; Wong, K.; Piet, V.; Cachat, J.; Kalueff, A.V. Acute Stress Disrupts Performance of Zebrafish in the Cued and Spatial Memory Tests: The Utility of Fish Models to Study Stress-Memory Interplay. Behav. Process. 2011, 87, 224–230. [Google Scholar] [CrossRef]
- Ninkovic, J.; Bally-Cuif, L. The Zebrafish as a Model System for Assessing the Reinforcing Properties of Drugs of Abuse. Methods 2006, 39, 262–274. [Google Scholar] [CrossRef]
- Rey, S.; Huntingford, F.A.; Boltaña, S.; Vargas, R.; Knowles, T.G.; Mackenzie, S. Fish Can Show Emotional Fever: Stress-Induced Hyperthermia in Zebrafish. Proceedings. Biol. Sci. 2015, 282, 658–667. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar]
- Jesuthasan, S. Fear, Anxiety, and Control in the Zebrafish. Dev. Neurobiol. 2012, 72, 395–403. [Google Scholar] [CrossRef]
- Avdesh, A.; Martin-Iverson, M.T.; Mondal, A.; Chen, M.; Askraba, S.; Morgan, N.; Lardelli, M.; Groth, D.M.; Verdile, G.; Martins, R.N. Evaluation of Color Preference in Zebrafish for Learning and Memory. J. Alzheimer’s Dis. 2012, 28, 459–469. [Google Scholar] [CrossRef]
- Cerutti, D.; Levin, E. Behavioral Neuroscience of Zebrafish, Methods of Behavior Analysis in Neuroscience, 2nd ed.Taylor & Francis Group LLC: Oxfordshire, UK, 2008; pp. 293–310. ISBN 9781420052343. [Google Scholar]
- Lindeyer, C.M.; Reader, S.M. Social Learning of Escape Routes in Zebrafish and the Stability of Behavioural Traditions. Anim. Behav. 2010, 79, 827–834. [Google Scholar] [CrossRef]
- Zelikowsky, M.; Hui, M.; Karigo, T.; Choe, A.; Yang, B.; Blanco, M.R.; Beadle, K.; Gradinaru, V.; Deverman, B.E.; Anderson, D.J. The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell 2018, 173, 1265–1279.e19. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The Neuroendocrinology of Social Isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Hawkley, L.C. Social Isolation and Health, with an Emphasis on Underlying Mechanisms. Perspect. Biol. Med. 2003, 46, S39–S52. [Google Scholar] [CrossRef]
- Borbély, A.A.; Baumann, F.; Brandeis, D.; Strauch, I.; Lehmann, D. Sleep Deprivation: Effect on Sleep Stages and EEG Power Density in Man. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 483–493. [Google Scholar] [CrossRef]
- Stewart, A.M.; Gaikwad, S.; Kyzar, E.; Kalueff, A.V. Understanding Spatio-Temporal Strategies of Adult Zebrafish Exploration in the Open Field Test. Brain Res. 2012, 1451, 44–52. [Google Scholar] [CrossRef]
- Lopez-Luna, J.; Al-Jubouri, Q.; Al-Nuaimy, W.; Sneddon, L.U. Impact of Stress, Fear and Anxiety on the Nociceptive Responses of Larval Zebrafish. PLoS ONE 2017, 12, e0181010. [Google Scholar] [CrossRef]
- Amo, R.; Aizawa, H.; Takahoko, M.; Kobayashi, M.; Takahashi, R.; Aoki, T.; Okamoto, H. Identification of the Zebrafish Ventral Habenula as a Homolog of the Mammalian Lateral Habenula. J. Neurosci. 2010, 30, 1566–1574. [Google Scholar] [CrossRef]
- Lee, A.; Mathuru, A.S.; Teh, C.; Kibat, C.; Korzh, V.; Penney, T.B.; Jesuthasan, S. The Habenula Prevents Helpless Behavior in Larval Zebrafish. Curr. Biol. 2010, 20, 2211–2216. [Google Scholar] [CrossRef]
- Agetsuma, M.; Aizawa, H.; Aoki, T.; Nakayama, R.; Takahoko, M.; Goto, M.; Sassa, T.; Amo, R.; Shiraki, T.; Kawakami, K.; et al. The Habenula Is Crucial for Experience-Dependent Modification of Fear Responses in Zebrafish. Nat. Neurosci. 2010, 13, 1354–1356. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Wilson, R.S.; Beck, T.L.; Bienias, J.L.; Bennett, D.A. Mild Cognitive Impairment in Different Functional Domains and Incident Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1479–1484. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular Care and Maintenance of a Zebrafish (Danio Rerio) Laboratory: An Introduction. J. Vis. Exp. 2012, 2012, e4196. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Sison, M.; Gerlai, R. Behavioral Performance Altering Effects of MK-801 in Zebrafish (Danio Rerio). Behav. Brain Res. 2011, 220, 331–337. [Google Scholar] [CrossRef]
- Brown, D. Combining Computational Physics with Video Analysis in Tracker. 2007. Available online: https://www.compadre.org/osp/items/detail.cfm?ID=9686 (accessed on 6 October 2022).
- Wee, L.K.; Tat, L.L. Video Analysis and Modeling Tool for Physics Education: A workshop for Redesigning Pedagogy. arXiv: Physics Education. 2012. Available online: https://www.semanticscholar.org/paper/Video-Analysis-and-Modeling-Tool-for-Physics-A-for-Wee-Lee/b86be2b53e910bd44f41d7c08a48ff1ecfd5b64c (accessed on 6 October 2022).
- Sivaji, K.; Kannan, R.R. Polysorbate 80 Coated Gold Nanoparticle as a Drug Carrier for Brain Targeting in Zebrafish Model. J. Clust. Sci. 2019, 30, 897–906. [Google Scholar] [CrossRef]
- Burne, T.; Scott, E.; van Swinderen, B.; Hilliard, M.; Reinhard, J.; Claudianos, C.; Eyles, D.; McGrath, J. Big Ideas for Small Brains: What Can Psychiatry Learn from Worms, Flies, Bees and Fish? Mol. Psychiatry 2011, 16, 7–16. [Google Scholar] [CrossRef]
- Gerlai, R. High-Throughput Behavioral Screens: The First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules 2010, 15, 2609–2622. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar]
- Piato, L.; Maria, E.; Rosemberg, D.B.; Rico, E.P.; Mussulini, B.H.M.; Bonan, C.D.; Dias, R.D.; Blaser, R.E.; Souza, D.O.; De, D.L. Differences in Spatio-Temporal Behavior of Zebrafish in the Open Tank Paradigm after a Short-Period Confinement into Dark and Bright Environments. PLoS ONE 2011, 6, e19397. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole Induced Changes in Zebrafish Behavior, Neural Activity and c-Fos Expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Bass, S.L.S.; Gerlai, R. Zebrafish (Danio Rerio) Responds Differentially to Stimulus Fish: The Effects of Sympatric and Allopatric Predators and Harmless Fish. Behav. Brain Res. 2008, 186, 107–117. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Ritter, F.; Kreutz, L.C.; Quevedo, R.M.; da Silva, L.B.; Bedin, A.C.; Finco, J.; Cericato, L. Whole-Body Cortisol Increases after Direct and Visual Contact with a Predator in Zebrafish, Danio Rerio. Aquaculture 2007, 272, 774–778. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic Effects of Nicotine in Zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Champagne, D.L.; Hoefnagels, C.C.M.; de Kloet, R.E.; Richardson, M.K. Translating Rodent Behavioral Repertoire to Zebrafish (Danio Rerio): Relevance for Stress Research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).