Cellulose through the Lens of Microfluidics: A Review
Abstract
:1. Introduction
2. Cellulose and Microfluidics
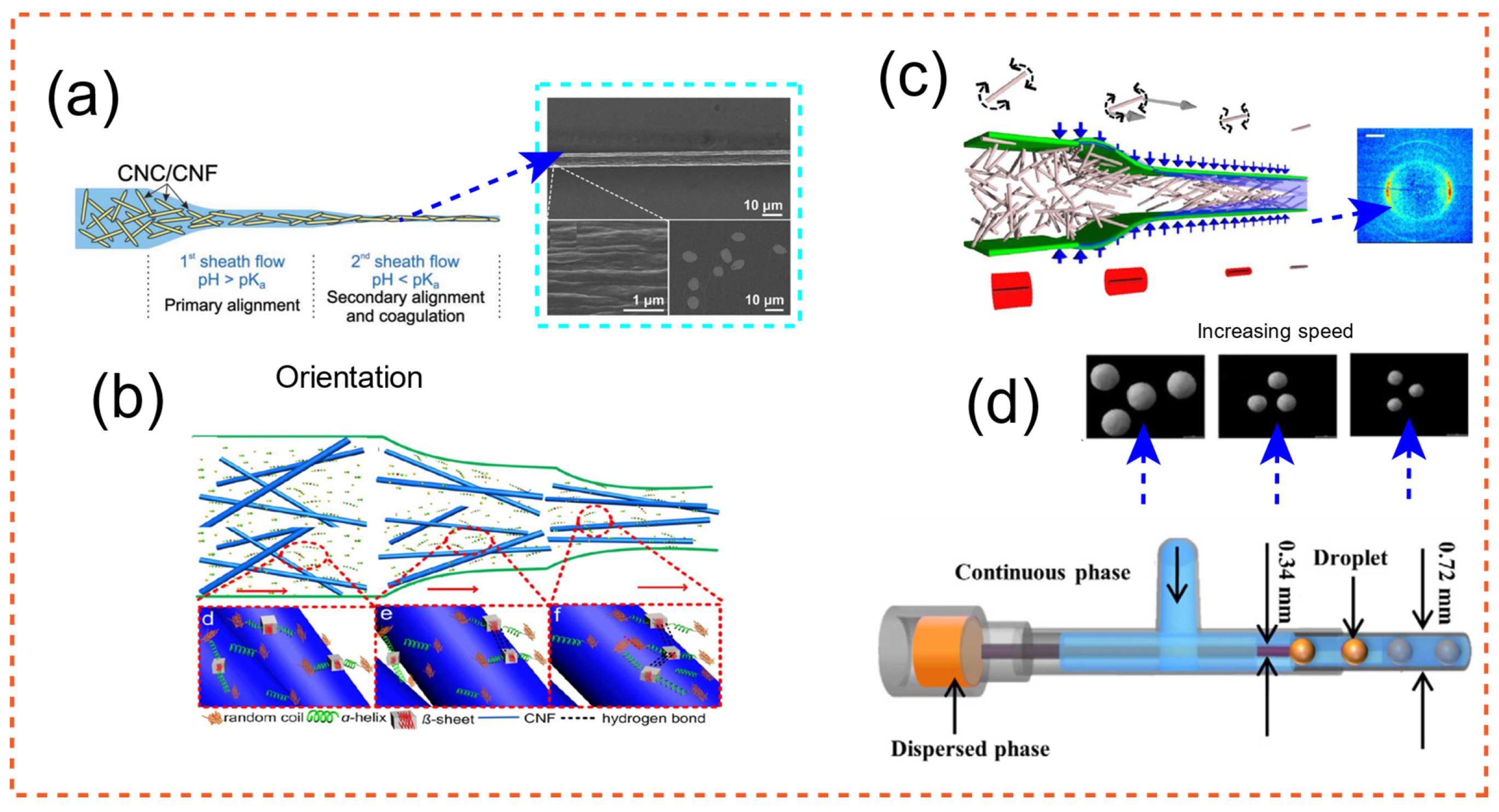
2.1. Design of Cellulose with Microfluidics
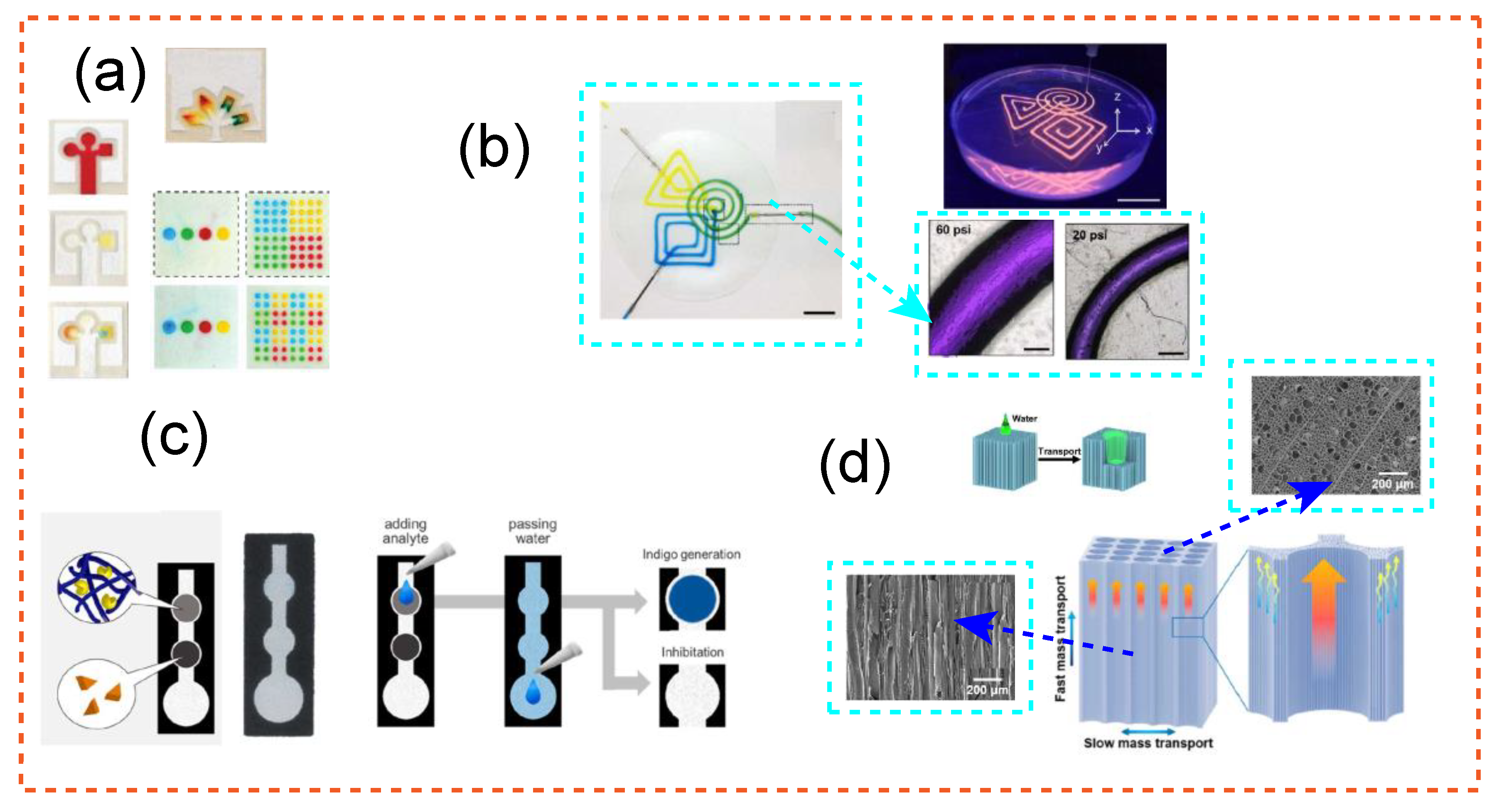
2.2. Cellulose as a Microfluidic Building Block
2.3. Advanced Integration of Cellulose in Microfluidcs
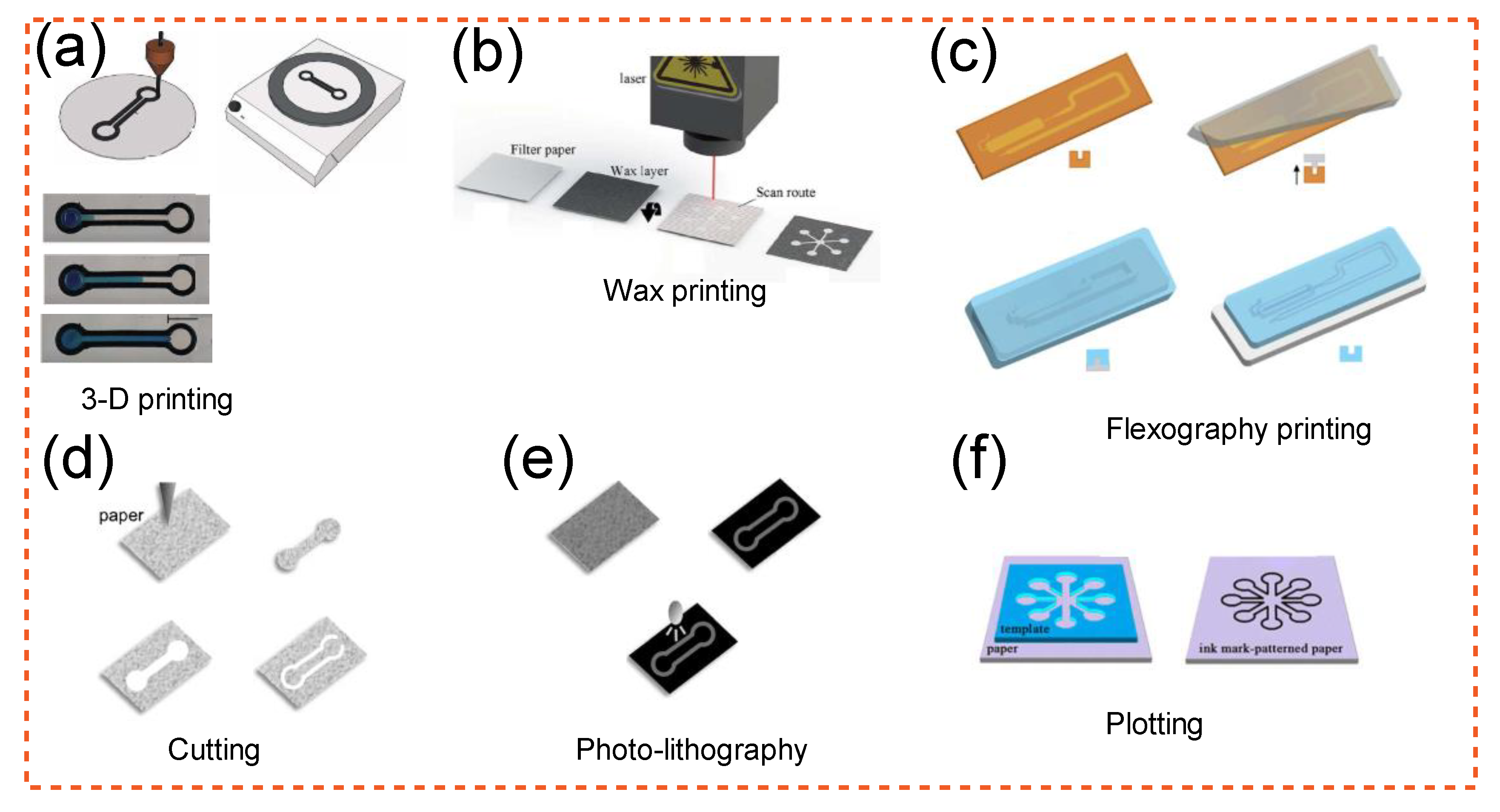
2.4. Using Microfluidics to Shape Cellulose-Based Products
3. Conclusions and Projections in the Future
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gahrooee, T.R.; Moud, A.A.; Danesh, M.; Hatzikiriakos, S.G. Rheological Characterization of CNC-CTAB Network below and above Critical Micelle Concentration (CMC). Carbohydr. Polym. 2021, 257, 117552. [Google Scholar] [CrossRef]
- Moud, A.A.; Arjmand, M.; Yan, N.; Nezhad, A.S.; Hejazi, S.H. Colloidal behavior of cellulose nanocrystals in presence of sodium chloride. ChemistrySelect 2018, 3, 4969–4978. [Google Scholar] [CrossRef]
- Moud, A.A.; Arjmand, M.; Liu, J.; Yang, Y.; Sanati-Nezhad, A.; Hejazi, S.H. Cellulose nanocrystal structure in the presence of salts. Cellulose 2019, 26, 9387–9401. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.-Y.; Li, Y. Highly Efficient and Superfast Cellulose Dissolution by Green Chloride Salts and Its Dissolution Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 18446–18454. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar] [CrossRef]
- Xing, X.; Li, W.; Zhang, J.; Wu, H.; Guan, Y.; Gao, H. TEMPO-oxidized cellulose hydrogel for efficient adsorption of Cu2+ and Pb2+ modified by polyethyleneimine. Cellulose 2021, 28, 7953–7968. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y. Grafting of regenerated cellulose films with fibrous polymer and modified into phosphate and sulfate groups: Application for removal of a model azo-dye. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126173. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
- Duan, J.; He, X.; Zhang, L. Magnetic cellulose–TiO2 nanocomposite microspheres for highly selective enrichment of phosphopeptides. Chem. Commun. 2015, 51, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Laib, R.; Amokrane-Nibou, S.; Dahdouh, N.; Mansouri, T.E.M.; Rekhila, G.; Trari, M.; Nibou, D. Removal of the cationic textile dye by Recycled newspaper pulp and its cellulose microfibers extracted: Characterization, release, and adsorption studies. Iran. J. Chem. Chem. Eng. 2021, 40, 133–141. [Google Scholar]
- Lin, W.-H.; Jana, S.C. Analysis of porous structures of cellulose aerogel monoliths and microparticles. Microporous Mesoporous Mater. 2021, 310, 110625. [Google Scholar] [CrossRef]
- Selman, M.H.; Hemayatkar, M.; Deelder, A.M.; Wuhrer, M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal. Chem. 2011, 83, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Mwandira, W.; Nakashima, K.; Togo, Y.; Sato, T.; Kawasaki, S. Cellulose-metallothionein biosorbent for removal of Pb (II) and Zn (II) from polluted water. Chemosphere 2020, 246, 125733. [Google Scholar] [CrossRef] [PubMed]
- Buruaga-Ramiro, C.; Valenzuela, S.V.; Valls, C.; Roncero, M.B.; Pastor, F.J.; Díaz, P.; Martínez, J. Bacterial cellulose matrices to develop enzymatically active paper. Cellulose 2020, 27, 3413–3426. [Google Scholar] [CrossRef]
- Yeap, E.W.; Ng, D.Z.; Prhashanna, A.; Somasundar, A.; Acevedo, A.J.; Xu, Q.; Salahioglu, F.; Garland, M.V.; Khan, S.A. Bottom-up structural design of crystalline drug-excipient composite microparticles via microfluidic droplet-based processing. Cryst. Growth Des. 2017, 17, 3030–3039. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Abd Razak, S.I. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef] [PubMed]
- Khine, Y.Y.; Stenzel, M.H. Surface modified cellulose nanomaterials: A source of non-spherical nanoparticles for drug delivery. Mater. Horiz. 2020, 7, 1727–1758. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–761. [Google Scholar]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. In Journal of Applied Polymer Science: Applied Polymer Symposium (United States); ITT Rayonier Inc.: Shelton, WA, USA, 1983. [Google Scholar]
- Blok, A.E.; Bolhuis, D.P.; Kibbelaar, H.V.; Bonn, D.; Velikov, K.P.; Stieger, M. Comparing rheological, tribological and sensory properties of microfibrillated cellulose dispersions and xanthan gum solutions. Food Hydrocoll. 2021, 121, 107052. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient cleavage of strong hydrogen bonds in sugarcane bagasse by ternary acidic deep eutectic solvent and ultrasonication to facile fabrication of cellulose nanofibers. Cellulose 2021, 28, 6159–6182. [Google Scholar] [CrossRef]
- Wei, X.; Lin, T.; Duan, M.; Du, H.; Yin, X. Cellulose nanocrystal-based liquid crystal structures and the unique optical characteristics of cellulose nanocrystal films. BioResources 2021, 16, 2116. [Google Scholar] [CrossRef]
- Teh, K.C.; Foo, M.L.; Ooi, C.W.; Chew, I.M.L. Sustainable and cost-effective approach for the synthesis of lignin-containing cellulose nanocrystals from oil palm empty fruit bunch. Chemosphere 2021, 267, 129277. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Jiang, Y.; Ji, X.; Kong, F.; Lin, T.; Shao, H.; Han, W. Flexible cellulose nanofiber/Bi2Te3 composite film for wearable thermoelectric devices. J. Power Sources 2020, 479, 229044. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Huang, R.; Zhang, Y.; Jia, C.; Zhao, H.; Chen, W.; Xue, Y. Fabrication and characterization of cellulose nanofiber aerogels prepared via two different drying techniques. Polymers 2020, 12, 2583. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Wang, Y.; Mu, X.; Ling, S.; Yu, H.; Chen, W.; Guo, C.; Watson, M.C.; Yu, Y. Stimuli-responsive composite biopolymer actuators with selective spatial deformation behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 14602–14608. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Ma, M.; Xu, F. Modulation of assembly and dynamics in colloidal hydrogels via ionic bridge from cellulose nanofibrils and poly (ethylene glycol). ACS Macro Lett. 2015, 4, 829–833. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic properties of poly (vinyl alcohol) hydrogels with cellulose nanocrystals fabricated through sodium chloride addition: Rheological evidence of double network formation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125577. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Gel Development Using Cellulose Nanocrystals. Ph.D. Thesis, Univeristy of Calgary, Calgary, AB, Canada, 2020. [Google Scholar]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Nonlinear viscoelastic characterization of charged cellulose nanocrystal network structure in the presence of salt in aqueous media. Cellulose 2020, 27, 5729–5743. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and water-resistant properties of eco-friendly chitosan membrane reinforced with cellulose nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.; Pinheiro, I.; Gouveia, R.; Thim, G.; Lona, L. Functionalized cellulose nanocrystals as reinforcement in biodegradable polymer nanocomposites. Polym. Compos. 2018, 39, E9–E29. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Qin, X.; Zhang, Y.; Sinko, R.; Keten, S. Achieving enhanced interfacial adhesion and dispersion in cellulose nanocomposites via amorphous interfaces. Macromolecules 2018, 51, 10304–10311. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Clarkson, C.M.; Shrestha, S.; El Awad Azrak, S.M.; Mavlan, M.; Youngblood, J.P. High-performance waterborne polyurethane coating based on a blocked isocyanate with cellulose nanocrystals (CNC) as the polyol. ACS Appl. Polym. Mater. 2019, 2, 385–393. [Google Scholar] [CrossRef]
- Biswas, P.; Mamatha, S.; Naskar, S.; Rao, Y.S.; Johnson, R.; Padmanabham, G. 3D extrusion printing of magnesium aluminate spinel ceramic parts using thermally induced gelation of methyl cellulose. J. Alloy. Compd. 2019, 770, 419–423. [Google Scholar] [CrossRef]
- Hudelja, H.; Konegger, T.; Wicklein, B.; Čretnik, J.; Akhtar, F.; Kocjan, A. Freeze-casting of highly porous cellulose-nanofiber-reinforced γ-Al2O3 monoliths. Open Ceram. 2021, 5, 100069. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- de Morais Zanata, D.; Battirola, L.C.; do Carmo Gonçalves, M. Chemically cross-linked aerogels based on cellulose nanocrystals and polysilsesquioxane. Cellulose 2018, 25, 7225–7238. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and porous nanocellulose aerogels with high loadings of metal–organic-framework particles for separations applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lei, T.; Wu, Q. Facile preparation of mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Physical, viscoelastic and mechanical properties. Cellulose 2013, 20, 2947–2958. [Google Scholar] [CrossRef]
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J. Biomater. Appl. 2015, 29, 882–893. [Google Scholar] [CrossRef]
- Park, J.H.; Noh, J.; Schütz, C.; Salazar-Alvarez, G.; Scalia, G.; Bergström, L.; Lagerwall, J. Macroscopic control of helix orientation in films dried from cholesteric liquid crystalline cellulose nanocrystal suspensions. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2014, 15, 1477–1484. [Google Scholar] [CrossRef]
- Stephen, M.J.; Straley, J.P. Physics of liquid crystals. Rev. Mod. Phys. 1974, 46, 617. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Lehr, C.; Schütz, C.; Sanctuary, R.; Osipov, M.A.; Baller, J.; Lagerwall, J.P. Fractionation of cellulose nanocrystals: Enhancing liquid crystal ordering without promoting gelation. NPG Asia Mater. 2018, 10, 455–465. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Sandquist, D. New horizons for microfibrillated cellulose. Appita Technol. Innov. Manuf. Environ. 2013, 66, 156–162. [Google Scholar]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Huang, C.; Yu, H.; Abdalkarim, S.Y.H.; Li, Y.; Chen, X.; Yang, X.; Zhou, Y.; Zhang, L. A comprehensive investigation on cellulose nanocrystals with different crystal structures from cotton via an efficient route. Carbohydr. Polym. 2021, 276, 118766. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Critical insights into the reinforcement potential of cellulose nanocrystals in polymer nanocomposites. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100761. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Nuruddin, M.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films. ACS Appl. Mater. Interfaces 2018, 11, 1376–1383. [Google Scholar] [CrossRef]
- De La Cruz, J.A.; Liu, Q.; Senyuk, B.; Frazier, A.W.; Peddireddy, K.; Smalyukh, I.I. Cellulose-based reflective liquid crystal films as optical filters and solar gain regulators. ACS Photonics 2018, 5, 2468–2477. [Google Scholar] [CrossRef]
- Giese, M.; Blusch, L.K.; Khan, M.K.; MacLachlan, M.J. Functional materials from cellulose-derived liquid-crystal templates. Angew. Chem. Int. Ed. 2015, 54, 2888–2910. [Google Scholar] [CrossRef]
- Lagerwall, J.P.; Schütz, C.; Salajkova, M.; Noh, J.; Park, J.H.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Z.; Wang, Y.; Zhao, Y. Bioinspired conductive cellulose liquid-crystal hydrogels as multifunctional electrical skins. Proc. Natl. Acad. Sci. USA 2020, 117, 18310–18316. [Google Scholar] [CrossRef] [PubMed]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, S.; Lyu, S.; Fu, F. Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind. Crops Prod. 2018, 122, 438–447. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Zeng, H.; Betti, M.; Chen, L. Highly porous, hydrophobic, and compressible cellulose nanocrystals/poly (vinyl alcohol) aerogels as recyclable absorbents for oil–water separation. ACS Sustain. Chem. Eng. 2019, 7, 11118–11128. [Google Scholar] [CrossRef]
- Cheng, Q.-Y.; Guan, C.-S.; Wang, M.; Li, Y.-D.; Zeng, J.-B. Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr. Polym. 2018, 199, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic inks based on cellulose nanofibrils and cross-linkable xylans for 3D printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, J.; Cheng, Q.; Wei, P.; Cheng, G.J.; Chang, C. Additive printing of recyclable anti-counterfeiting patterns with sol–gel cellulose nanocrystal inks. Nanoscale 2021, 13, 11808–11816. [Google Scholar] [CrossRef]
- Ebers, L.-S.; Laborie, M.-P. Direct ink writing of fully bio-based liquid crystalline lignin/hydroxypropyl cellulose aqueous inks: Optimization of formulations and printing parameters. ACS Appl. Bio. Mater. 2020, 3, 6897–6907. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhao, J.; Li, Y.; Lu, K. Synthesis of cellulose nanocrystals-armored fluorinated polyacrylate latexes via Pickering emulsion polymerization and their film properties. Colloids Surf. B Biointerfaces 2020, 192, 111071. [Google Scholar] [CrossRef]
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-enhanced cellulose nanocrystal Pickering emulsions. J. Colloid Interface Sci. 2015, 439, 139–148. [Google Scholar] [CrossRef]
- Wang, W.; Du, G.; Li, C.; Zhang, H.; Long, Y.; Ni, Y. Preparation of cellulose nanocrystals from asparagus (Asparagus officinalis L.) and their applications to palm oil/water Pickering emulsion. Carbohydr. Polym. 2016, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, L.; Xu, H.; Feng, X.; Wang, B.; Pukánszky, B.; Mao, Z.; Sui, X. Poly (lactic acid)/cellulose nanocrystal composites via the Pickering emulsion approach: Rheological, thermal and mechanical properties. Int. J. Biol. Macromol. 2019, 137, 197–204. [Google Scholar] [CrossRef]
- Jutakridsada, P.; Pimsawat, N.; Sillanpää, M.; Kamwilaisak, K. Olive oil stability in Pickering emulsion preparation from eucalyptus pulp and its rheology behaviour. Cellulose 2020, 27, 6189–6203. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.; Kapar, S.; Ataeian, P.; da Silva Bernardes, J.; Berry, R.; Zhao, W.; Zhou, G.; Tam, K.C. Microencapsulation of phase change materials with polystyrene/cellulose nanocrystal hybrid shell via Pickering emulsion polymerization. ACS Sustain. Chem. Eng. 2019, 7, 17756–17767. [Google Scholar] [CrossRef]
- Angkuratipakorn, T.; Sriprai, A.; Tantrawong, S.; Chaiyasit, W.; Singkhonrat, J. Fabrication and characterization of rice bran oil-in-water Pickering emulsion stabilized by cellulose nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 310–319. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly [2-(dimethylamino) ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jung, J.; Simonsen, J.; Zhao, Y. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocoll. 2018, 84, 229–237. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. Cellulose nanocrystals from ultrasound process stabilizing O/W Pickering emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, G.; Ma, Y.; Liu, Y.; Huang, X.; Zheng, Q.; Yue, P.; Yang, M. Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity. Int. J. Biol. Macromol. 2021, 170, 24–32. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, Y.; Lai, W.; Huang, F.; Ou, A.; Qin, R.; Liu, X.; Wang, X. Ester crosslinking enhanced hydrophilic cellulose nanofibrils aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Boston, MA, USA, 2019. [Google Scholar]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Microfluid. Based Microsyst. 2010, 305–376. [Google Scholar] [CrossRef]
- Lebedev, A.; Miraghaie, R.; Kotta, K.; Ball, C.E.; Zhang, J.; Buchsbaum, M.S.; Kolb, H.C.; Elizarov, A. Batch-reactor microfluidic device: First human use of a microfluidically produced PET radiotracer. Lab Chip 2013, 13, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiotis, A.; Karadimitriou, N.; Zarikos, I.; Steeb, H. Pore-scale effects during the transition from capillary-to viscosity-dominated flow dynamics within microfluidic porous-like domains. Sci. Rep. 2021, 11, 3891. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.L.; Paredes, S.; Sridhar, A.; Michel, B.; Brunschwiler, T. Radial hierarchical microfluidic evaporative cooling for 3-d integrated microprocessors. In Proceedings of the 4th European Conference on Microfluidics, Limerick, Ireland, 10–12 December 2014. [Google Scholar]
- Liu, Z.; Liu, X.; Jiang, S.; Zhu, C.; Ma, Y.; Fu, T. Effects on droplet generation in step-emulsification microfluidic devices. Chem. Eng. Sci. 2021, 246, 116959. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Park, S.; Chen, L.; Dong, X.; Young, E.W.; Liu, X. NanoPADs and nanoFACEs: An optically transparent nanopaper-based device for biomedical applications. Lab Chip 2020, 20, 3322–3333. [Google Scholar] [CrossRef]
- Markin, C.J.; Mokhtari, D.A.; Sunden, F.; Appel, M.J.; Akiva, E.; Longwell, S.; Sabatti, C.; Herschlag, D.; Fordyce, P.M. Revealing enzyme functional architecture via high-throughput microfluidic enzyme kinetics. Science 2021, 373, eabf8761. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-Y.; Park, S.-Y. Highly-porous uniformly-sized amidoxime-functionalized cellulose beads prepared by microfluidics with N-methylmorpholine N-oxide. Cellulose 2021, 28, 5401–5419. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y.; Wu, Y.; Xu, C.; Zhong, M.; Lai, H.; Huang, J. Fabrication of a microfluidic paper-based analytical device by silanization of filter cellulose using a paper mask for glucose assay. Analyst 2014, 139, 4593–4598. [Google Scholar] [CrossRef] [Green Version]
- Pokhrel, P.; Jha, S.; Giri, B. Selection of appropriate protein assay method for a paper microfluidics platform. Pract. Lab. Med. 2020, 21, e00166. [Google Scholar] [CrossRef]
- Song, J.; Babayekhorasani, F.; Spicer, P.T. Soft bacterial cellulose microcapsules with adaptable shapes. Biomacromolecules 2019, 20, 4437–4446. [Google Scholar] [CrossRef]
- Lari, A.S.; Khatibi, A.; Zahedi, P.; Ghourchian, H. Microfluidic-assisted production of poly (ɛ-caprolactone) and cellulose acetate nanoparticles: Effects of polymers, surfactants, and flow rate ratios. Polym. Bull. 2020, 78, 5449–5466. [Google Scholar] [CrossRef]
- Wise, H.G.; Takana, H.; Ohuchi, F.; Dichiara, A.B. Field-Assisted Alignment of Cellulose Nanofibrils in a Continuous Flow-Focusing System. ACS Appl. Mater. Interfaces 2020, 12, 28568–28575. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Ke, H.; Zhou, M.; Li, X. Experimental investigation of annular two-phase flow splitting at a microimpacting T-junction. Chem. Eng. Sci. 2014, 118, 154–163. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.-P.; Ju, X.-J.; Xie, R.; Yang, L.; Liang, B.; Chu, L.-Y. Microfluidic preparation of monodisperse ethyl cellulose hollow microcapsules with non-toxic solvent. J. Colloid Interface Sci. 2009, 336, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Pinheiro, K.M.; Baldo, T.A.; Bressan, L.P.; da Silva, J.A.; Coltro, W.K. Microchip-Based Devices for Bioanalytical Applications. In Tools and Trends in Bioanalytical Chemistry; Springer: Berlin/Heidelberg, Germany, 2022; pp. 467–482. [Google Scholar]
- Jaitpal, S.; Chavva, S.; Mabbott, S. Towards point-of-care detection of microRNAs using paper-based microfluidics. In Proceedings of the Optical Diagnostics and Sensing XXI: Toward Point-of-Care Diagnostics, Online, 6–11 March 2021; p. 116510C. [Google Scholar]
- Ng, J.S.; Hashimoto, M. 3D-PAD: Paper-Based Analytical Devices with Integrated Three-Dimensional Features. Biosensors 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Malec, A.; Kokkinis, G.; Haiden, C.; Giouroudi, I. Biosensing system for concentration quantification of magnetically labeled E. coli in water samples. Sensors 2018, 18, 2250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chang, H.; Neuzil, P. DEP-on-a-chip: Dielectrophoresis applied to microfluidic platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olm, F.; Lim, H.C.; Schallmoser, K.; Strunk, D.; Laurell, T.; Scheding, S. Acoustophoresis Enables the Label-Free Separation of Functionally Different Subsets of Cultured Bone Marrow Stromal Cells. Cytom. Part A 2021, 99, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.M.; Hemmert, J.W.; White, H.S. Magnetic field-controlled microfluidic transport. J. Am. Chem. Soc. 2002, 124, 462–467. [Google Scholar] [CrossRef]
- Siegel, A.C.; Shevkoplyas, S.S.; Weibel, D.B.; Bruzewicz, D.A.; Martinez, A.W.; Whitesides, G.M. Cofabrication of electromagnets and microfluidic systems in poly (dimethylsiloxane). Angew. Chem. 2006, 118, 7031–7036. [Google Scholar] [CrossRef]
- Lu, L.; Fan, S.; Niu, Q.; Peng, Q.; Geng, L.; Yang, G.; Shao, H.; Hsiao, B.S.; Zhang, Y. Strong silk fibers containing cellulose nanofibers generated by a bioinspired microfluidic chip. ACS Sustain. Chem. Eng. 2019, 7, 14765–14774. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, W.; Ren, M.; Ren, X. Fabrication of porous cellulose microspheres with controllable structures by microfluidic and flash freezing method. Mater. Lett. 2020, 262, 127193. [Google Scholar] [CrossRef]
- Håkansson, K.M.; Fall, A.B.; Lundell, F.; Yu, S.; Krywka, C.; Roth, S.V.; Santoro, G.; Kvick, M.; Wittberg, L.P.; Wågberg, L. Hydrodynamic alignment and assembly of nanofibrils resulting in strong cellulose filaments. Nat. Commun. 2014, 5, 4018. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Håkansson, K.M.; Gowda, V.K.; Lundell, F.; Hagström, B.; Köhnke, T. Continuous assembly of cellulose nanofibrils and nanocrystals into strong macrofibers through microfluidic spinning. Adv. Mater. Technol. 2019, 4, 1800557. [Google Scholar] [CrossRef]
- Benvidi, A.; Banaei, M.; Tezerjani, M.D.; Molahosseini, H.; Jahanbani, S. Impedimetric PSA aptasensor based on the use of a glassy carbon electrode modified with titanium oxide nanoparticles and silk fibroin nanofibers. Microchim. Acta 2018, 185, 50. [Google Scholar] [CrossRef]
- Hu, K.; Gupta, M.K.; Kulkarni, D.D.; Tsukruk, V.V. Ultra-robust graphene oxide-silk fibroin nanocomposite membranes. Adv. Mater. 2013, 25, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Steven, E.; Saleh, W.R.; Lebedev, V.; Acquah, S.F.; Laukhin, V.; Alamo, R.G.; Brooks, J.S. Carbon nanotubes on a spider silk scaffold. Nat. Commun. 2013, 4, 2435. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Qian, Z.; Li, J.; Sun, H.; Han, Y.; Xia, X.; Zhou, J.; Wang, C.; Wang, Y.; Wang, C. Synthetic engineering of spider silk fiber as implantable optical waveguides for low-loss light guiding. ACS Appl. Mater. Interfaces 2017, 9, 14665–14676. [Google Scholar] [CrossRef]
- Lu, L.; Fan, S.; Geng, L.; Yao, X.; Zhang, Y. Low-loss light-guiding, strong silk generated by a bioinspired microfluidic chip. Chem. Eng. J. 2021, 405, 126793. [Google Scholar] [CrossRef]
- Kinahan, M.E.; Filippidi, E.; Köster, S.; Hu, X.; Evans, H.M.; Pfohl, T.; Kaplan, D.L.; Wong, J. Tunable silk: Using microfluidics to fabricate silk fibers with controllable properties. Biomacromolecules 2011, 12, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Konwarh, R.; Gupta, P.; Mandal, B.B. Silk-microfluidics for advanced biotechnological applications: A progressive review. Biotechnol. Adv. 2016, 34, 845–858. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, Y.; Lu, L.; Shao, H.; Qin, K.; Hu, X.; Xia, X. Recombinant spider silk from aqueous solutions via a bio-inspired microfluidic chip. Sci. Rep. 2016, 6, 36473. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Fan, S.; Geng, L.; Lin, J.; Yao, X.; Zhang, Y. Flow Analysis of Regenerated Silk Fibroin/Cellulose Nanofiber Suspensions via a Bioinspired Microfluidic Chip. Adv. Mater. Technol. 2021, 6, 2100124. [Google Scholar] [CrossRef]
- Rivera-Quintero, P.; Mercado, D.F.; Ballesteros-Rueda, L.M. Influence of the functionalization agent and crystalline phase of MnO2 Janus nanomaterials on the stability of aqueous nanofluids and its catalytic activity to promote asphaltene oxidation. Colloid Interface Sci. Commun. 2021, 45, 100525. [Google Scholar] [CrossRef]
- Zúñiga, M.C.; Steitz, J.A. The nucleotide sequence of a major glycine transfer RNA from the posterior silk gland of Bombyx mori L. Nucleic Acids Res. 1977, 4, 4175–4196. [Google Scholar] [CrossRef] [Green Version]
- Weitao, Z.; Jianxin, H.; Shan, D.; Shizhong, C.; Weidong, G. Electrospun silk fibroin/cellulose acetate blend nanofibres: Structure and properties. Iran. Polym. J. 2011, 20, 389–397. [Google Scholar]
- Zhou, W.; He, J.; Cui, S.; Gao, W. Preparation of electrospun silk fibroin/Cellulose Acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 2011, 12, 431–437. [Google Scholar] [CrossRef]
- Zhou, W.T.; He, J.X.; Cui, S.Z.; Gao, W.D. Nanofibrous membrane of silk fibroin/cellulose acetate blend for heavy metal ion adsorption. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2011; pp. 1431–1435. [Google Scholar]
- Du, S.; Zhang, J.; Zhou, W.T.; Li, Q.X.; Greene, G.W.; Zhu, H.J.; Li, J.L.; Wang, X.G. Interactions between fibroin and sericin proteins from Antheraea pernyi and Bombyx mori silk fibers. J. Colloid Interface Sci. 2016, 478, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Wu, Y.; Zhang, Y.; Zou, Y.; Dai, F.; Si, Y. Antibacterial Activity of Photoactive Silk Fibroin/Cellulose Acetate Blend Nanofibrous Membranes against Escherichia coli. ACS Sustain. Chem. Eng. 2020, 8, 16775–16780. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Dissolution and regeneration of silk from silkworm Bombyx mori in ionic liquids and its application to medical biomaterials. Int. J. Biol. Macromol. 2020, 143, 594–601. [Google Scholar] [CrossRef]
- Rivera-Galletti, A.; Gough, C.R.; Kaleem, F.; Burch, M.; Ratcliffe, C.; Lu, P.; Salas-De la Cruz, D.; Hu, X. Silk-Cellulose Acetate Biocomposite Materials Regenerated from Ionic Liquid. Polymers 2021, 13, 2911. [Google Scholar] [CrossRef]
- Arumugam, M.; Murugesan, B.; Pandiyan, N.; Chinnalagu, D.K.; Rangasamy, G.; Mahalingam, S. Electrospinning cellulose acetate/silk fibroin/Au-Ag hybrid composite nanofiber for enhanced biocidal activity against MCF-7 breast cancer cell. Mater. Sci. Eng. C 2021, 123, 112019. [Google Scholar] [CrossRef]
- Pignon, F.; Challamel, M.; De Geyer, A.; Elchamaa, M.; Semeraro, E.F.; Hengl, N.; Jean, B.; Putaux, J.-L.; Gicquel, E.; Bras, J. Breakdown and buildup mechanisms of cellulose nanocrystal suspensions under shear and upon relaxation probed by SAXS and SALS. Carbohydr. Polym. 2021, 260, 117751. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, J.; Bao, S.; Shao, G.; Wang, Z.; Qin, B.; Wang, T.; Fu, Y. Preparation and application of modified three-dimensional cellulose microspheres for paclitaxel targeted separation. J. Chromatogr. A 2021, 1655, 462487. [Google Scholar] [CrossRef] [PubMed]
- Pepicelli, M.; Binelli, M.R.; Studart, A.R.; Rühs, P.A.; Fischer, P. Self-grown bacterial cellulose capsules made through emulsion templating. ACS Biomater. Sci. Eng. 2021, 7, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Duong, D.D.; Kwak, J.; Song, H.S.; Lee, N.Y. Construction of microfluidic blood–brain barrier model assisted by 3D coculture on cellulose fiber. Microsyst. Technol. 2021, 27, 3917–3926. [Google Scholar] [CrossRef]
- Jayapiriya, U.; Goel, S. Influence of cellulose separators in coin-sized 3D printed paper-based microbial fuel cells. Sustain. Energy Technol. Assess. 2021, 47, 101535. [Google Scholar] [CrossRef]
- Sharratt, W.N.; Lopez, C.G.; Sarkis, M.; Tyagi, G.; O’Connell, R.; Rogers, S.E.; Cabral, J.T. Ionotropic Gelation Fronts in Sodium Carboxymethyl Cellulose for Hydrogel Particle Formation. Gels 2021, 7, 44. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, D.; Wu, X.; Zhao, Y.; Shang, L.; Ren, J.; Zhao, Y. Natural polysaccharide based complex drug delivery system from microfluidic electrospray for wound healing. Appl. Mater. Today 2021, 23, 101000. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Huang, R.; Huang, Z.; Hu, B.; Zheng, W.; Yang, G.; Jiang, X. Evaluation of the effect of the structure of bacterial cellulose on full thickness skin wound repair on a microfluidic chip. Biomacromolecules 2015, 16, 780–789. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Srivastava, V.R.; Chandra, P. Nanobioengineered Sensing Technologies Based on Cellulose Matrices for Detection of Small Molecules, Macromolecules, and Cells. Biosensors 2021, 11, 168. [Google Scholar] [CrossRef]
- Del Giudice, F.; Tassieri, M.; Oelschlaeger, C.; Shen, A.Q. When microrheology, bulk rheology, and microfluidics meet: Broadband rheology of hydroxyethyl cellulose water solutions. Macromolecules 2017, 50, 2951–2963. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Hu, F.; Cheng, Z.; Wang, B.; Chen, K. Isolation and rheological characterization of cellulose nanofibrils (CNFs) produced by microfluidic homogenization, ball-milling, grinding and refining. Cellulose 2021, 28, 3389–3408. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, J.; Cheng, Z.; Yuan, Z.; Wang, X.; Wang, B. Precisely controlled preparation of uniform nanocrystalline cellulose via microfluidic technology. J. Ind. Eng. Chem. 2021. [CrossRef]
- Carrick, C.; Larsson, P.A.; Brismar, H.; Aidun, C.; Wågberg, L. Native and functionalized micrometre-sized cellulose capsules prepared by microfluidic flow focusing. RSC Adv. 2014, 4, 19061–19067. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, X.; Huang, W.; Liu, P.; Zhang, L. Cellulose-based hydrogels with excellent microstructural replication ability and cytocompatibility for microfluidic devices. Cellulose 2013, 20, 1897–1909. [Google Scholar] [CrossRef]
- Zhang, L.; Deraney, R.N.; Tripathi, A. Adsorption and isolation of nucleic acids on cellulose magnetic beads using a three-dimensional printed microfluidic chip. Biomicrofluidics 2015, 9, 064118. [Google Scholar] [CrossRef] [Green Version]
- Wenzlik, D.; Ohm, C.; Serra, C.; Zentel, R. Preparation of cholesteric particles from cellulose derivatives in a microfluidic setup. Soft Matter 2011, 7, 2340–2344. [Google Scholar] [CrossRef]
- Miyashita, Y.; Iwasaka, M.; Kimura, T. Microcrystal-like cellulose fibrils as the diamagnetic director for microfluidic systems. J. Appl. Phys. 2014, 115, 17B519. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Li, H.; Sun, J.; Cai, H.; Cui, D. Development of a multilayer microfluidic device integrated with a PDMS-cellulose composite film for sample pre-treatment and immunoassay. Sens. Actuators A Phys. 2013, 193, 54–58. [Google Scholar] [CrossRef]
- Włodarczyk, E.; Zarzycki, P.K. Chromatographic behavior of selected dyes on silica and cellulose micro-TLC plates: Potential application as target substances for extraction, chromatographic, and/or microfluidic systems. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 259–281. [Google Scholar] [CrossRef]
- Ghorbani, M.; Aghdam, A.S.; Gevari, M.T.; Koşar, A.; Cebeci, F.Ç.; Grishenkov, D.; Svagan, A.J. Facile hydrodynamic cavitation ON CHIP via cellulose nanofibers stabilized perfluorodroplets inside layer-by-layer assembled SLIPS surfaces. Chem. Eng. J. 2020, 382, 122809. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, C.-W.; Han, S.-Y.; Lee, E.-A.; Cindradewi, A.W.; Kim, J.-K.; Kwon, G.-J.; Seo, Y.-H.; Youe, W.-J.; Gwon, J. Preparation and Properties of Wet-Spun Microcomposite Filaments from Cellulose Nanocrystals and Alginate Using a Microfluidic Device. BioResources 2021, 13, 1709. [Google Scholar] [CrossRef]
- Grate, J.W.; Mo, K.-F.; Shin, Y.; Vasdekis, A.; Warner, M.G.; Kelly, R.T.; Orr, G.; Hu, D.; Dehoff, K.J.; Brockman, F.J. Alexa fluor-labeled fluorescent cellulose nanocrystals for bioimaging solid cellulose in spatially structured microenvironments. Bioconjugate Chem. 2015, 26, 593–601. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, G.; Wang, J.; Xue, W.; Du, C.; Wu, G. Preparation of carboxymethyl cellulose based microgels for cell encapsulation. Express Polym. Lett. 2014, 8, 841–849. [Google Scholar] [CrossRef]
- Rao, L.T.; Dubey, S.K.; Javed, A.; Goel, S. Parametric Performance Investigation on Membraneless Microfluidic Paper Fuel Cell with Graphite Composed Pencil Stoke Electrodes. Int. J. Precis. Eng. Manuf. 2021, 22, 177–187. [Google Scholar] [CrossRef]
- Shen, L.-L.; Zhang, G.-R.; Venter, T.; Biesalski, M.; Etzold, B.J. Towards best practices for improving paper-based microfluidic fuel cells. Electrochim. Acta 2019, 298, 389–399. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, C.; Huang, Y.; Nie, Y.; Yang, J.; Shen, R.; Sun, D. Regenerated bacterial cellulose microfluidic column for glycoproteins separation. Carbohydr. Polym. 2016, 137, 271–276. [Google Scholar] [CrossRef]
- Yan, X.; Xu, A.; Zeng, L.; Gao, P.; Zhao, T. A paper-based microfluidic fuel cell with hydrogen peroxide as fuel and oxidant. Energy Technol. 2018, 6, 140–143. [Google Scholar] [CrossRef]
- Tzivelekis, C.; Selby, M.P.; Batet, A.; Madadi, H.; Dalgarno, K. Microfluidic chip fabrication and performance analysis of 3D printed material for use in microfluidic nucleic acid amplification applications. J. Micromech. Microeng. 2021, 31, 035005. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Chen, X.; Mo, D.; Gong, M. A Flexible Method for Nanofiber-based 3D Microfluidic Device Fabrication for Water Quality Monitoring. Micromachines 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Klasner, S.A.; Price, A.K.; Hoeman, K.W.; Wilson, R.S.; Bell, K.J.; Culbertson, C.T. Based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal. Bioanal. Chem. 2010, 397, 1821–1829. [Google Scholar] [CrossRef]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, D.; Amrouche, S.; Calafiura, P.; Estrade, V.; Farrell, S.; Germain, C.; Gligorov, V.; Golling, T.; Gray, H.; Guyon, I. The TrackML Particle Tracking Challenge. 2018. Available online: https://hal.inria.fr/hal-01680537v2/document (accessed on 26 December 2021).
- Abe, K.; Kotera, K.; Suzuki, K.; Citterio, D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal. Bioanal. Chem. 2010, 398, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Shen, W. Progress in patterned paper sizing for fabrication of paper-based microfluidic sensors. Cellulose 2010, 17, 649–659. [Google Scholar] [CrossRef]
- Wang, W.; Wu, W.-Y.; Zhu, J.-J. Tree-shaped paper strip for semiquantitative colorimetric detection of protein with self-calibration. J. Chromatogr. A 2010, 1217, 3896–3899. [Google Scholar] [CrossRef]
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Shehata, A.-A.M.; Filipe, C.D.; Pelton, R. Streaming potential sensing in paper-based microfluidic channels. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 16–18. [Google Scholar] [CrossRef]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, G.; Ding, Z.; Chang, C.-L.; Savran, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef]
- Zargaryan, A.; Farhoudi, N.; Haworth, G.; Ashby, J.F.; Au, S.H. Hybrid 3D printed-paper microfluidics. Sci. Rep. 2020, 10, 18379. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, H.; Fan, Y. Laser-induced selective wax reflow for paper-based microfluidics. RSC Adv. 2019, 9, 11460–11464. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Olmos, C.M.; Vaca, A.; Rosero, G.; Peñaherrera, A.; Perez, C.; de Sá Carneiro, I.; Vizuete, K.; Arroyo, C.R.; Debut, A.; Pérez, M.S. Epoxy resin mold and PDMS microfluidic devices through photopolymer flexographic printing plate. Sens. Actuators B Chem. 2019, 288, 742–748. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-paper micro-and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim. Acta 2016, 183, 1521–1542. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. based microfluidic devices by plasma treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef]
- Arce, C.; Llano, T.; García, P.; Alberto, C. Technical and environmental improvement of the bleaching sequence of dissolving pulp for fibre production. Cellulose 2020, 27, 4079–4090. [Google Scholar] [CrossRef]
- Berlioz, S.; Molina-Boisseau, S.; Nishiyama, Y.; Heux, L. Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 2009, 10, 2144–2151. [Google Scholar] [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective esterification and etherification of cellulose: A review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustain. Chem. Eng. 2013, 1, 858–870. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Drofenik, J.; Gaberscek, M.; Dominko, R.; Poulsen, F.W.; Mogensen, M.; Pejovnik, S.; Jamnik, J. Cellulose as a binding material in graphitic anodes for Li ion batteries: A performance and degradation study. Electrochim. Acta 2003, 48, 883–889. [Google Scholar] [CrossRef]
- Clasen, C.; Kulicke, W.-M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Yao, Y.; Dai, J.; Hu, L. Progress in 3D printing of carbon materials for energy-related applications. Adv. Mater. 2017, 29, 1603486. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J.r. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J. Behaviour of a starch as a viscosity modifier for aerial lime-based mortars. Carbohydr. Polym. 2010, 80, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Peng, Y.; Tan, H.; Jian, S.; Zhi, Z.; Guo, Y.; Qi, H.; Zhang, T.; He, X. Effect of hydroxypropyl-methyl cellulose ether on rheology of cement paste plasticized by polycarboxylate superplasticizer. Constr. Build. Mater. 2018, 160, 341–350. [Google Scholar] [CrossRef]
- Chatterjee, T.; O’Donnell, K.P.; Rickard, M.A.; Nickless, B.; Li, Y.; Ginzburg, V.V.; Sammler, R.L. Rheology of cellulose ether excipients designed for hot melt extrusion. Biomacromolecules 2018, 19, 4430–4441. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Liao, C.-W.; Chao, Y.-C.; Kuo, C. Fabrication and characterization of asymmetric Janus and ternary particles. ACS Appl. Mater. Interfaces 2010, 2, 3185–3191. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Distance-based tear lactoferrin assay on microfluidic paper device using interfacial interactions on surface-modified cellulose. ACS Appl. Mater. Interfaces 2015, 7, 24864–24875. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X. Fabrication of three-dimensional microfluidic channels in a single layer of cellulose paper. Microfluid. Nanofluidics 2014, 16, 819–827. [Google Scholar] [CrossRef]
- Ardalan, S.; Hosseinifard, M.; Vosough, M.; Golmohammadi, H. Towards smart personalized perspiration analysis: An IoT-integrated cellulose-based microfluidic wearable patch for smartphone fluorimetric multi-sensing of sweat biomarkers. Biosens. Bioelectron. 2020, 168, 112450. [Google Scholar] [CrossRef]
- Arun, R.K.; Gupta, V.; Singh, P.; Biswas, G.; Chanda, N. Selection of graphite pencil grades for the design of suitable electrodes for stacking multiple single-inlet paper-pencil fuel cells. ChemistrySelect 2019, 4, 152–159. [Google Scholar] [CrossRef]
- del Torno-de Román, L.; Navarro, M.; Hughes, G.; Esquivel, J.P.; Milton, R.D.; Minteer, S.D.; Sabaté, N. Improved performance of a paper-based glucose fuel cell by capillary induced flow. Electrochim. Acta 2018, 282, 336–342. [Google Scholar] [CrossRef]
- Jia, C.; Jiang, F.; Hu, P.; Kuang, Y.; He, S.; Li, T.; Chen, C.; Murphy, A.; Yang, C.; Yao, Y. Anisotropic, mesoporous microfluidic frameworks with scalable, aligned cellulose nanofibers. ACS Appl. Mater. Interfaces 2018, 10, 7362–7370. [Google Scholar] [CrossRef]
- Murase, R.; Kondo, S.; Kitamura, T.; Goi, Y.; Hashimoto, M.; Teramoto, Y. Cellulose nanofibers as a module for paper-based microfluidic analytical devices: Labile substance storage, processability, and reaction field provision and control. ACS Appl. Bio Mater. 2018, 1, 480–486. [Google Scholar] [CrossRef]
- Kumar, T.; Soares, R.R.; Dholey, L.A.; Ramachandraiah, H.; Aval, N.A.; Aljadi, Z.; Pettersson, T.; Russom, A. Multi-layer assembly of cellulose nanofibrils in a microfluidic device for the selective capture and release of viable tumor cells from whole blood. Nanoscale 2020, 12, 21788–21797. [Google Scholar] [CrossRef]
- Choi, S.; Moon, S.W.; Lee, S.H.; Kim, W.; Kim, S.; Kim, S.K.; Shin, J.-H.; Park, Y.-G.; Jin, K.-H.; Kim, T.G. A recyclable CNC-milled microfluidic platform for colorimetric assays and label-free aged-related macular degeneration detection. Sens. Actuators B Chem. 2019, 290, 484–492. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X. Experimental comparison of surface chemistries for biomolecule immobilization on paper-based microfluidic devices. J. Micromech. Microeng. 2019, 29, 124003. [Google Scholar] [CrossRef]
- Bao, W.; Fang, Z.; Wan, J.; Dai, J.; Zhu, H.; Han, X.; Yang, X.; Preston, C.; Hu, L. Aqueous gating of van der waals materials on bilayer nanopaper. ACS Nano 2014, 8, 10606–10612. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, M.; Singh, K.; Sharma, N.N.; Akhtar, J. Flexible Microfluidics Biosensor Technology. In Electrical and Electronic Devices, Circuits and Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 377–386. [Google Scholar]
- Solin, K.; Borghei, M.; Imani, M.; Kämäräinen, T.; Kiri, K.; Mäkelä, T.; Khakalo, A.; Orelma, H.; Gane, P.A.; Rojas, O.J. Bicomponent Cellulose Fibrils and Minerals Afford Wicking Channels Stencil-Printed on Paper for Rapid and Reliable Fluidic Platforms. ACS Appl. Polym. Mater. 2021, 3, 5536–5546. [Google Scholar] [CrossRef]
- Wang, X.; Yi, L.; Mukhitov, N.; Schrell, A.M.; Dhumpa, R.; Roper, M.G. Microfluidics-to-mass spectrometry: A review of coupling methods and applications. J. Chromatogr. A 2015, 1382, 98–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas, S.V.; de Souza, F.R.; Rodrigues Neto, J.C.; Vasconcelos, G.A.; Abdelnur, P.V.; Vaz, B.G.; Henry, C.S.; Coltro, W.K. Uncovering the formation of color gradients for glucose colorimetric assays on microfluidic paper-based analytical devices by mass spectrometry imaging. Anal. Chem. 2018, 90, 11949–11954. [Google Scholar] [CrossRef]
- Shiroma, L.Y.; Santhiago, M.; Gobbi, A.L.; Kubota, L.T. Separation and electrochemical detection of paracetamol and 4-aminophenol in a paper-based microfluidic device. Anal. Chim. Acta 2012, 725, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Downs, C. Progress in the development and integration of fluid flow control tools in paper microfluidics. Lab Chip 2017, 17, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Breedveld, V.; Hess, D.W. Flow control in fully enclosed microfluidics paper based analytical devices using plasma processes. Sens. Actuators B Chem. 2020, 320, 128606. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Kim, J.; Jin, S.H.; Park, K.-S.; Lee, C.-S. Flow control in paper-based microfluidic device for automatic multistep assays: A focused minireview. Korean J. Chem. Eng. 2016, 33, 2761–2770. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Ainla, A.; Güder, F.; Christodouleas, D.C.; Fernández-Abedul, M.T.; Whitesides, G.M. Integrating electronics and microfluidics on paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully inkjet-printed microfluidics: A solution to low-cost rapid three-dimensional microfluidics fabrication with numerous electrical and sensing applications. Sci. Rep. 2016, 6, 35111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible electronics based on micro/nanostructured paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Thouas, G.; Shen, W.; Whyte, G.; Garnier, G. Paper diagnostic for instantaneous blood typing. Anal. Chem. 2010, 82, 4158–4164. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Hu, J.; Wee, W.H.; Han, Y.L.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Direct writing electrodes using a ball pen for paper-based point-of-care testing. Analyst 2015, 140, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Crooks, R.M. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem. Soc. 2011, 133, 17564–17566. [Google Scholar] [CrossRef] [PubMed]
- San Park, T.; Yoon, J.-Y. Smartphone detection of Escherichia coli from field water samples on paper microfluidics. IEEE Sens. J. 2014, 15, 1902–1907. [Google Scholar] [CrossRef]
- Shin, S.; Hyun, J. Matrix-assisted three-dimensional printing of cellulose nanofibers for paper microfluidics. ACS Appl. Mater. Interfaces 2017, 9, 26438–26446. [Google Scholar] [CrossRef]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube based humidity sensor on cellulose paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Shin, S.; Kwak, H.; Shin, D.; Hyun, J. Solid matrix-assisted printing for three-dimensional structuring of a viscoelastic medium surface. Nat. Commun. 2019, 10, 4650. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Tauzin, L.J.; Baiyasi, R.; Wang, W.; Moringo, N.; Shuang, B.; Landes, C.F. Single particle tracking: From theory to biophysical applications. Chem. Rev. 2017, 117, 7331–7376. [Google Scholar] [CrossRef]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.-Y.; Kim, J.-W.; Fernandez-Nieves, A.; Martinez, C.J. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Panigrahi, D.; Sahu, P.K.; Swain, S.; Verma, R.K. Quality by design prospects of pharmaceuticals application of double emulsion method for PLGA loaded nanoparticles. SN Appl. Sci. 2021, 3, 638. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering emulsions containing cellulose microfibers produced by mechanical treatments as stabilizer in the food industry. Appl. Sci. 2019, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Ateş, S.; Durmaz, E.; Hamad, A. Evaluation possibilities of cellulose derivatives in food products. Kast. Univ. J. For. Fac. 2016, 16, 383–400. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.; Güell, C.; Henry, O.; Ferrando, M. Premix membrane emulsification to produce oil-in-water emulsions stabilized with various interfacial structures of whey protein and carboxymethyl cellulose. Food Hydrocoll. 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Diftis, N.; Kiosseoglou, V. Improvement of emulsifying properties of soybean protein isolate by conjugation with carboxymethyl cellulose. Food Chem. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Lv, X.; Song, Z.; Yu, J.; Su, Y.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Study on the demulsification of refinery oily sludge enhanced by microwave irradiation. Fuel 2020, 279, 118417. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of food-grade Pickering emulsions stabilized by a mixture of cellulose nanofibrils and nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Schuh, V.; Allard, K.; Herrmann, K.; Gibis, M.; Kohlus, R.; Weiss, J. Impact of carboxymethyl cellulose (CMC) and microcrystalline cellulose (MCC) on functional characteristics of emulsified sausages. Meat Sci. 2013, 93, 240–247. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Yang, X. Bacterial cellulose nanofibers improved the emulsifying capacity of soy protein isolate as a stabilizer for pickering high internal-phase emulsions. Food Hydrocoll. 2021, 112, 106279. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Zhang, W.; You, X.; Chauhan, I.; Mohanty, P.; Li, X. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int. J. Biol. Macromol. 2021, 166, 1586–1616. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Honarmand, M.; Dizani, M.; Moosavi, A.; Hannani, S.K. Shear-thinning droplet formation inside a microfluidic T-junction under an electric field. Acta Mech. 2021, 232, 2535–2554. [Google Scholar] [CrossRef]
- Andrieux, S.; Medina, L.; Herbst, M.; Berglund, L.A.; Stubenrauch, C. Monodisperse highly ordered chitosan/cellulose nanocomposite foams. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105516. [Google Scholar] [CrossRef]
- Chang, C.; Sustarich, J.; Bharadwaj, R.; Chandrasekaran, A.; Adams, P.D.; Singh, A.K. Droplet-based microfluidic platform for heterogeneous enzymatic assays. Lab Chip 2013, 13, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Ihmoudah, A.; Awad, M.M.; Rahman, A.; Butt, S.D. Numerical Study on Gas-Yield Power-Law Fluid in T-Junction Minichannel. In Proceedings of the International Conference on Nanochannels, Microchannels, and Minichannels, St. John’s, NL, Canada, 23–26 June 2019; p. V001T002A008. [Google Scholar]
- Lin, G.; Jiang, S.; Zhu, C.; Fu, T.; Ma, Y. Mass-transfer characteristics of CO2 absorption into aqueous solutions of N-methyldiethanolamine+ diethanolamine in a T-junction microchannel. ACS Sustain. Chem. Eng. 2019, 7, 4368–4375. [Google Scholar] [CrossRef]
- Mansour, M.H.; Kawahara, A.; Sadatomi, M. Experimental investigation of gas–non-Newtonian liquid two-phase flows from T-junction mixer in rectangular microchannel. Int. J. Multiph. Flow 2015, 72, 263–274. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; He, K.; Chen, J. Phase split of nitrogen/non-Newtonian fluid two-phase flow at a micro-T-junction. Int. J. Multiph. Flow 2011, 37, 1129–1134. [Google Scholar] [CrossRef]
- Mohsenian, S.; Ramiar, A.; Ranjbar, A. Numerical study of laminar non-Newtonian nanofluid flow in a T-Junction: Investigation of viscous dissipation and temperature dependent properties. Appl. Therm. Eng. 2016, 108, 221–232. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, S.; Kim, S.; Kim, J.H.; Lim, G. Controlled production of monodisperse polycaprolactone microspheres using flow-focusing microfluidic device. BioChip J. 2017, 11, 214–218. [Google Scholar] [CrossRef]
- Takayama, Y.; Matějka, L.; Kato, N. Dynamic gelation of shear-induced filamentous domains for cellulose ether assemblies due to polyion complexation. Carbohydr. Polym. 2020, 234, 115880. [Google Scholar] [CrossRef]
- Fan, W.Y.; Li, S.C.; Li, L.X.; Zhang, X.; Du, M.Q.; Yin, X.H. Hydrodynamics of gas/shear-thinning liquid two-phase flow in a co-flow mini-channel: Flow pattern and bubble length. Phys. Fluids 2020, 32, 092004. [Google Scholar] [CrossRef]
- Steegmans, M.L.J. Emulsification in Microfluidic Y-and T-Junctions. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar]
- Hughes, E.; Maan, A.A.; Acquistapace, S.; Burbidge, A.; Johns, M.L.; Gunes, D.Z.; Clausen, P.; Syrbe, A.; Hugo, J.; Schroen, K. Microfluidic preparation and self diffusion PFG-NMR analysis of monodisperse water-in-oil-in-water double emulsions. J. Colloid Interface Sci. 2013, 389, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Utada, A.S.; Shah, R.K.; Kim, J.W.; Weitz, D.A. Controllable monodisperse multiple emulsions. Angew. Chem. 2007, 119, 9128–9132. [Google Scholar] [CrossRef]
- Yu, Y.-L.; Zhang, M.-J.; Xie, R.; Ju, X.-J.; Wang, J.-Y.; Pi, S.-W.; Chu, L.-Y. Thermo-responsive monodisperse core–shell microspheres with PNIPAM core and biocompatible porous ethyl cellulose shell embedded with PNIPAM gates. J. Colloid Interface Sci. 2012, 376, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Druel, L.; Kenkel, A.; Baudron, V.; Buwalda, S.; Budtova, T. Cellulose aerogel microparticles via emulsion-coagulation technique. Biomacromolecules 2020, 21, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Miki, N. Hydrogel fiber cultivation method for forming bacterial cellulose microspheres. Micromachines 2018, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Alfassi, G.; Rein, D.M.; Cohen, Y. Cellulose emulsions and their hydrolysis. J. Chem. Technol. Biotechnol. 2019, 94, 178–184. [Google Scholar] [CrossRef]
- Parker, R.M.; Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Consani, G.; Abell, C.; Vignolini, S. Hierarchical self-assembly of cellulose nanocrystals in a confined geometry. ACS Nano 2016, 10, 8443–8449. [Google Scholar] [CrossRef]
- Levin, D.; Saem, S.; Osorio, D.A.; Cerf, A.; Cranston, E.D.; Moran-Mirabal, J.M. Green templating of ultraporous cross-linked cellulose nanocrystal microparticles. Chem. Mater. 2018, 30, 8040–8051. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Michelon, M.; Viganó, J.; Carvalho, M.S.; Cunha, R.L. Microfluidic approach to produce emulsion-filled alginate microgels. J. Food Eng. 2022, 315, 110812. [Google Scholar] [CrossRef]
- Miyagi, K.; Teramoto, Y. Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals. Nanomaterials 2021, 11, 2969. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bukusoglu, E.; Abbott, N.L. A practical guide to the preparation of liquid crystal-templated microparticles. Chem. Mater. 2017, 29, 53–61. [Google Scholar] [CrossRef]
- Parit, M.; Saha, P.; Davis, V.A.; Jiang, Z. Transparent and homogenous cellulose nanocrystal/lignin UV-protection films. ACS Omega 2018, 3, 10679–10691. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Marquis, M.l.; Renard, D.; Cathala, B. Microfluidic generation and selective degradation of biopolymer-based Janus microbeads. Biomacromolecules 2012, 13, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Li, Y.; Gevorkian, A.; Kumacheva, E. Compound droplets derived from a cholesteric suspension of cellulose nanocrystals. Soft Matter 2018, 14, 9713–9719. [Google Scholar] [CrossRef]
- Li, Y.; Suen, J.J.-Y.; Prince, E.; Larin, E.M.; Klinkova, A.; Thérien-Aubin, H.; Zhu, S.; Yang, B.; Helmy, A.S.; Lavrentovich, O.D. Colloidal cholesteric liquid crystal in spherical confinement. Nat. Commun. 2016, 7, 12520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, M.K.; Hauser, A.; Siqueira, G.; Libanori, R.; Vehusheia, S.L.; Schuerle, S.; Zimmermann, T.; Studart, A.R. Cellulose-Based Microparticles for Magnetically Controlled Optical Modulation and Sensing. Small 2020, 16, 1904251. [Google Scholar] [CrossRef]
- Chen, M.; Bolognesi, G.; Vladisavljević, G.T. Crosslinking Strategies for the Microfluidic Production of Microgels. Molecules 2021, 26, 3752. [Google Scholar] [CrossRef]
- Yu, J.; Huang, T.R.; Lim, Z.H.; Luo, R.; Pasula, R.R.; Liao, L.D.; Lim, S.; Chen, C.H. Production of hollow bacterial cellulose microspheres using microfluidics to form an injectable porous scaffold for wound healing. Adv. Healthc. Mater. 2016, 5, 2983–2992. [Google Scholar] [CrossRef]
- Tata Rao, L.; Rewatkar, P.; Dubey, S.K.; Javed, A.; Goel, S. Performance optimization of microfluidic paper fuel-cell with varying cellulose fiber papers as absorbent pad. Int. J. Energy Res. 2020, 44, 3893–3904. [Google Scholar] [CrossRef]
- Park, S.; Oh, Y.; Yun, J.; Yoo, E.; Jung, D.; Oh, K.K.; Lee, S.H. Cellulose/biopolymer/Fe3O4 hydrogel microbeads for dye and protein adsorption. Cellulose 2020, 27, 2757–2773. [Google Scholar] [CrossRef]
- Liu, Y.; Nambu, N.O.; Taya, M. Cell-laden microgel prepared using a biocompatible aqueous two-phase strategy. Biomed. Microdevices 2017, 19, 55. [Google Scholar] [CrossRef]
- Kaufman, G.; Mukhopadhyay, S.; Rokhlenko, Y.; Nejati, S.; Boltyanskiy, R.; Choo, Y.; Loewenberg, M.; Osuji, C.O. Highly stiff yet elastic microcapsules incorporating cellulose nanofibrils. Soft Matter 2017, 13, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.P.; Poling-Skutvik, R.; Osuji, C.O. Simple production of cellulose nanofibril microcapsules and the rheology of their suspensions. Soft Matter 2021, 17, 4517–4524. [Google Scholar] [CrossRef]
- Yeap, E.W.; Acevedo, A.J.; Khan, S.A. Microfluidic extractive crystallization for spherical drug/drug-excipient microparticle production. Org. Process Res. Dev. 2019, 23, 375–381. [Google Scholar] [CrossRef]
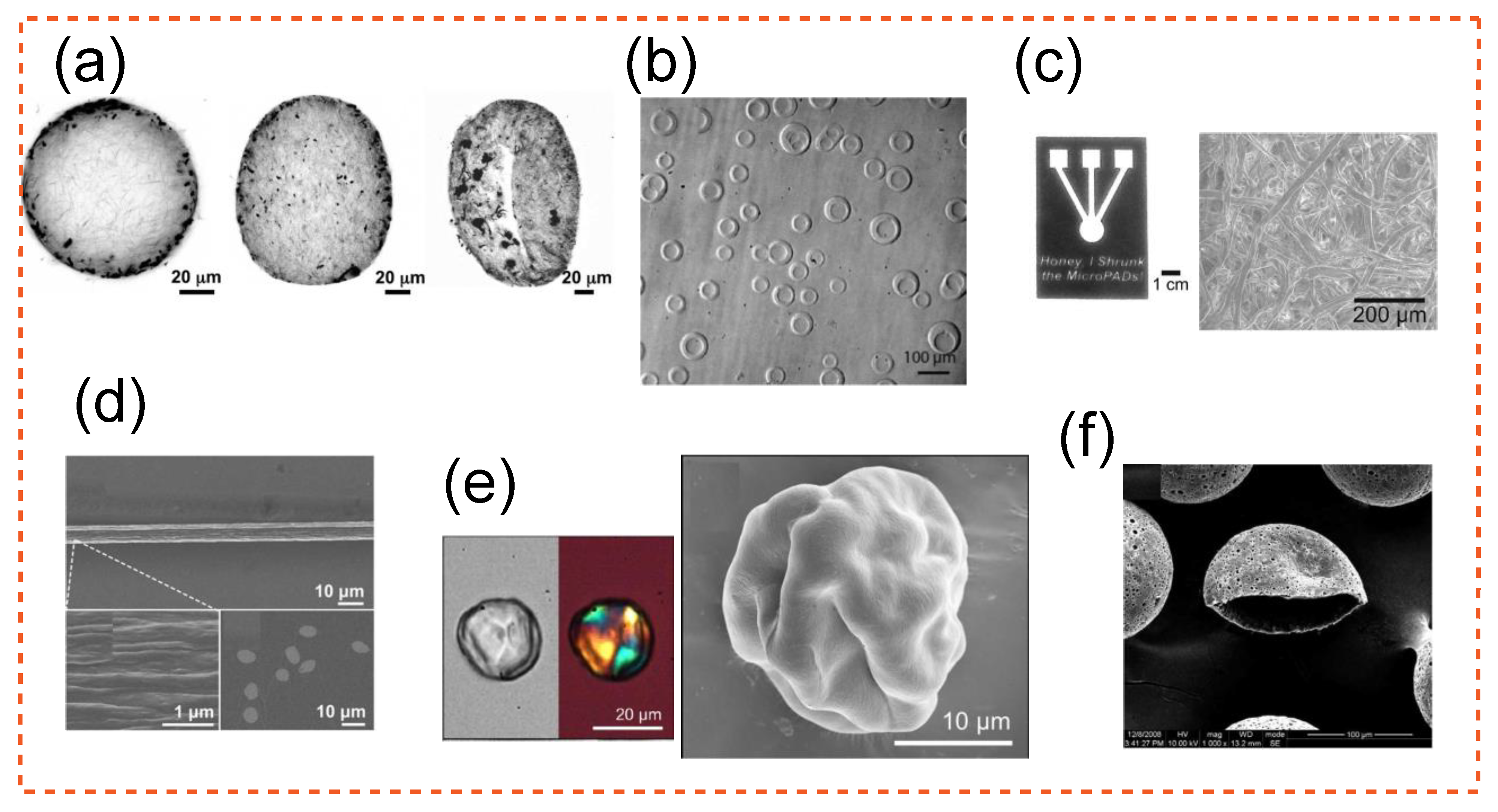
- Strong, E.B.; Schultz, S.A.; Martinez, A.W.; Martinez, N.W. Fabrication of miniaturized paper-based microfluidic devices (MicroPADs). Sci. Rep. 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhu, L.; Huang, C.; Jin, X. Direct electrospinning of ultrafine fibers with interconnected macropores enabled by in situ mixing microfluidics. ACS Appl. Mater. Interfaces 2016, 8, 34870–34878. [Google Scholar] [CrossRef]

| Study | Application | Highlight |
|---|---|---|
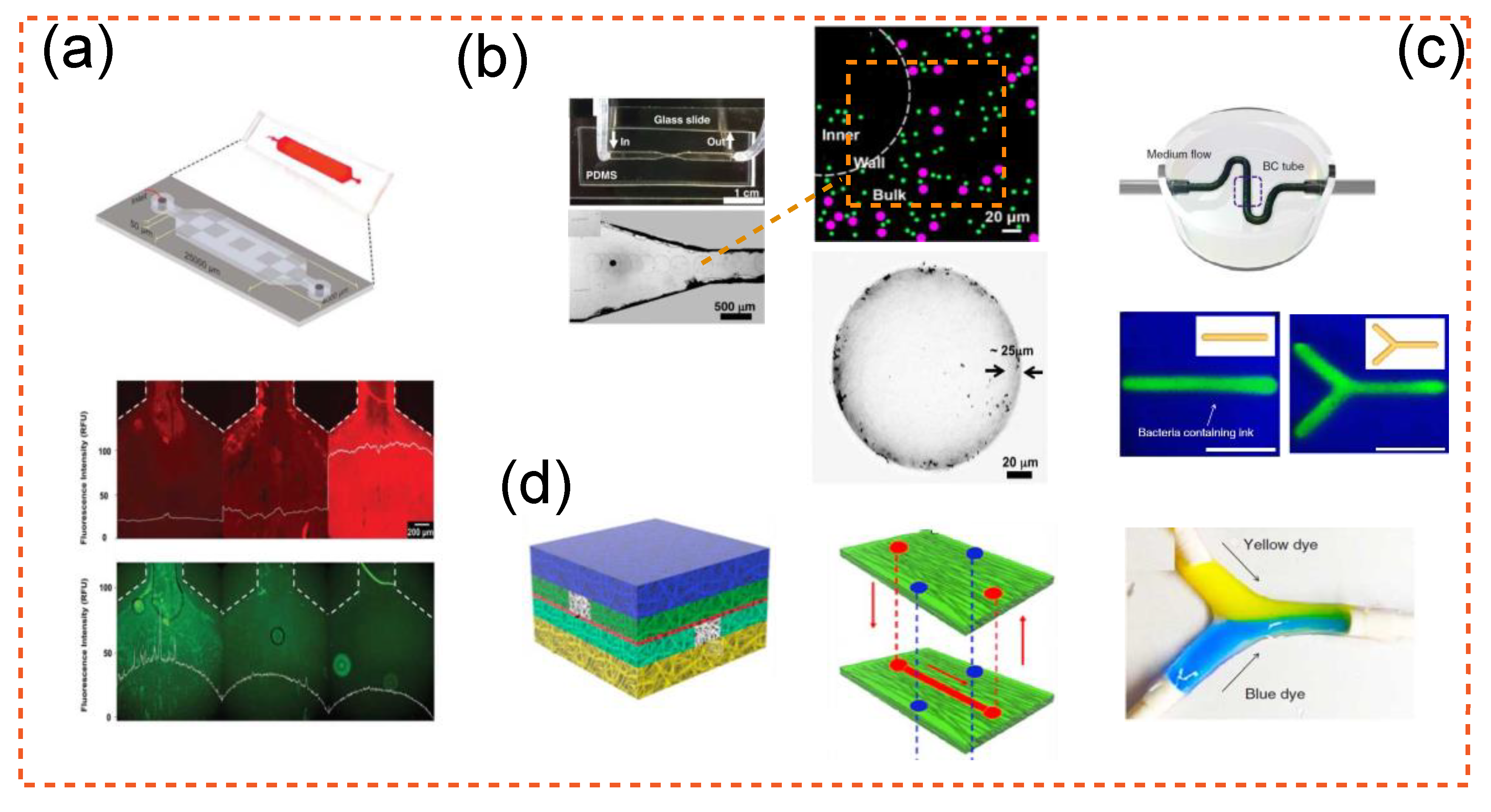
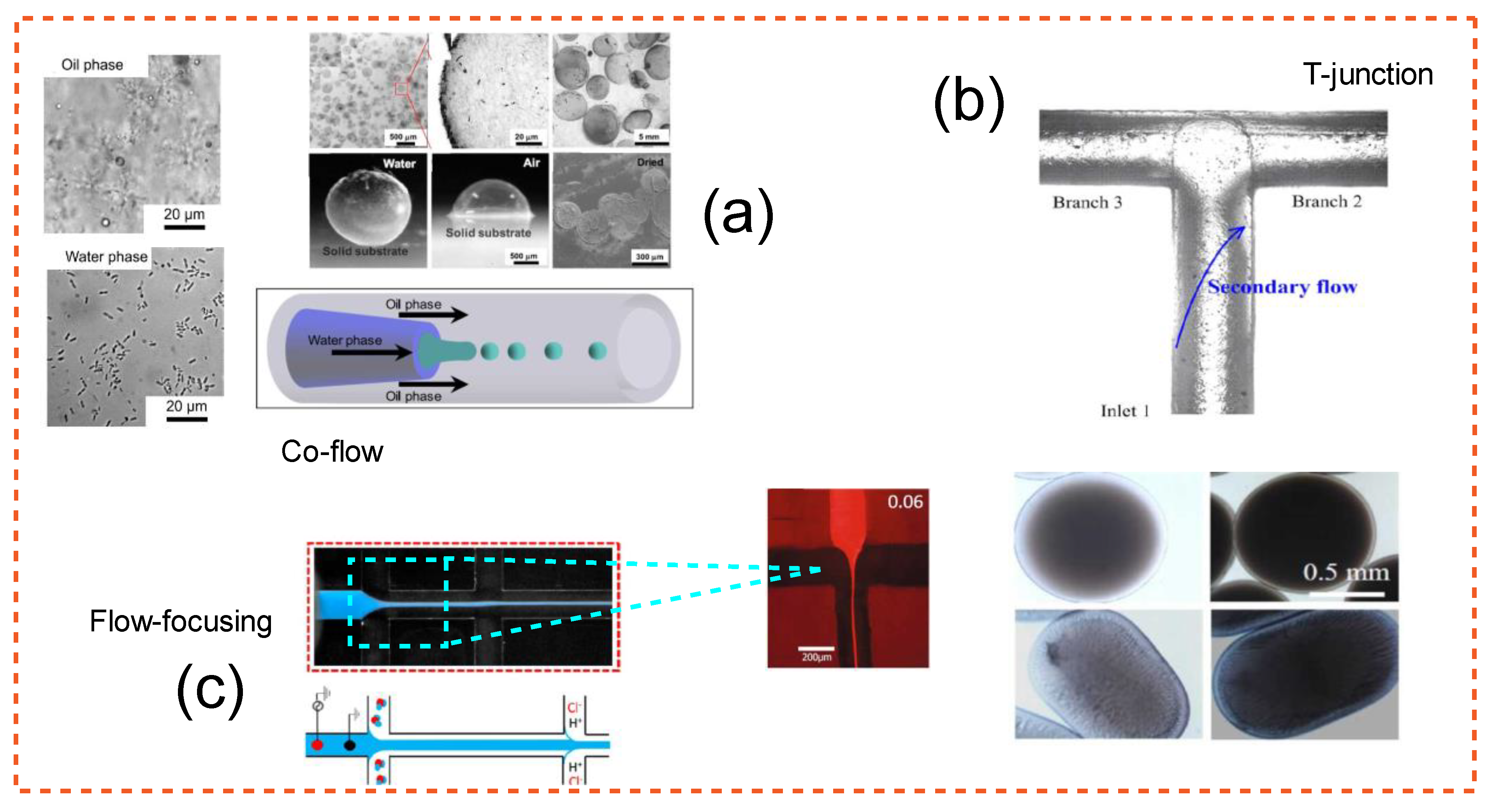
| Baek and Park [89] | Creation of uniformly sized porous cellulose beads | The creation of the cell/N-methyl morpholine N-oxide droplet in the ethylene glycol solution in the T-junction microfluidic chip could not be observed in situ using an optical microscope. As a model study, the form of a cellulose bead after coagulation was explored. |
| Pepicelli et al. [131] | Creation of cellulose-based biodegradable microcapsules. | Gluconacetobacter xylinus may live and flourish in a variety of environments. Cellulose is a major constituent of these self-secreted protective coatings (made with Gluconacetobacter xylinus). The results achieved mark the first step toward the fabrication of self-assembled degradable cellulose capsule. |
| Duong et al. [132] | Cellulose fiber membrane was sandwiched between two silicone elastomer poly(dimethylsiloxane) (PDMS) layers to mimic BBB | In vitro, a microfluidic system was created to replicate the human blood–brain barrier (BBB). BBB formation was assessed using cell survival, actin filament (F-actin) formation, and transepithelial electrical resistance (TEER). Overall, the model showed a simple to duplicate and low-cost framework for in vitro drug test. |
| Jayapiriya and Goel [133] | Creation of paper-based energy harvesting device | Using E. coli as the biocatalyst, a paper fuel cell can generate 11.8 W·cm−2 of electricity. Fuel cell construction that is both cost-effective and thrifty can be utilized to power a wide range of low-power point-of-care devices. |
| Sharratt et al. [134] | Creation of hydrogel microparticles | Hydrogel microparticles (HMPs) have a wide range of practical uses, from medication delivery to tissue development. The kinetics of gelation fronts are initially determined using 1D microfluidic studies. The effective diffusive coefficients rise with Fe3+ content and drop with NaCMC concentration. |
| Chen et al. [135] | Creation of core–shell microparticles | Polysaccharides have been shown to be useful in medication encapsulation and delivery. Authors offered a multicompartment polysaccharide core–shell microparticle that may be used to build a long-lasting dual-release system of active molecules for wound healing. Microparticles reduced inflammation while also promoting granulation tissue development, collagen deposition, and angiogenesis. |
| Liu et al. [96] | Creation of monodisperse ethyl cellulose (EC) hollow microcapsules | A simple and new approach is used to effectively create monodisperse ethyl cellulose hollow microcapsules. Microfluidic double emulsification and solvent diffusion are used in this method. Microcapsules manufactured in an iso-osmotic environment have a flawless spherical form and no collapse. |
| Li et al. [136] | Using bacterial cellulose for wound healing | Bacterial cellulose is a type of nano-biomaterial that may be used in tissue engineering. It is unknown how bacterial cellulose’s nanoscale structure impacts skin wound healing. The lower portion of bacterial cellulose film can encourage cell migration to aid in wound healing. |
| Zhao et al. [137] | Creation of cellulose-based flexible electronics | Cellulose is a natural biopolymer with several benefits such as low cost, ease of processing, and degradability. It is extensively used in flexible electronics as a substrate, dielectric material, gel electrolyte, and derived carbon-made material. |
| Mahapatra et al. [138] | Creation of cellulose-based sensing devices | For its unique features, including biocompatibility, cellulose has the potential to be used in the creation of cytosensors, and organisms in a variety of materials. |
| Del Giudice et al. [139] | Assessing morphological structure of hydroxyethyl cellulose with microfluidics | Non-modified hydroxyethyl cellulose acts as a linear uncharged polymer when dissolved in water, with an entangled mass concentration of 0.3 wt%. For the first time, authors presented the concentrations scaling for hydroxy ethyl cellulose solutions with the longest relaxation period. |
| Zeng et al. [140] | CNFs produced by microfluidic homogenization | The purpose of this research was to investigate and compare the shape and rheology of cellulose nanofibrils derived from bleached softwood kraft pulp. CNFs had the greatest viscous, bulk modulus, and loss modulus, as well as the largest aspect ratio. |
| Wang et al. [141] | Creation of uniform size CNCs via microfluidic technology | CNC is a novel form of molecular substance derived from biomass. CNCs with a good dividend and consistency were achieved by hydrolysis process in a microfluidic system using a 60% sulfuric acid solution at 35 °C for 40 min. |
| Lari et al. [93] | Creation of poly(ε-caprolactone) and cellulose acetate nanoparticles | The purpose of this study was to compare two types of microfluidic-assisted nanoparticles (NPs) based on poly(-caprolactone) (PCL) and cellulose acetate (CA). It was discovered that CA NPs had a smaller average diameter (37 nm) and a lower polydispersity index (PDI) (0.035) than PCL NPs. |
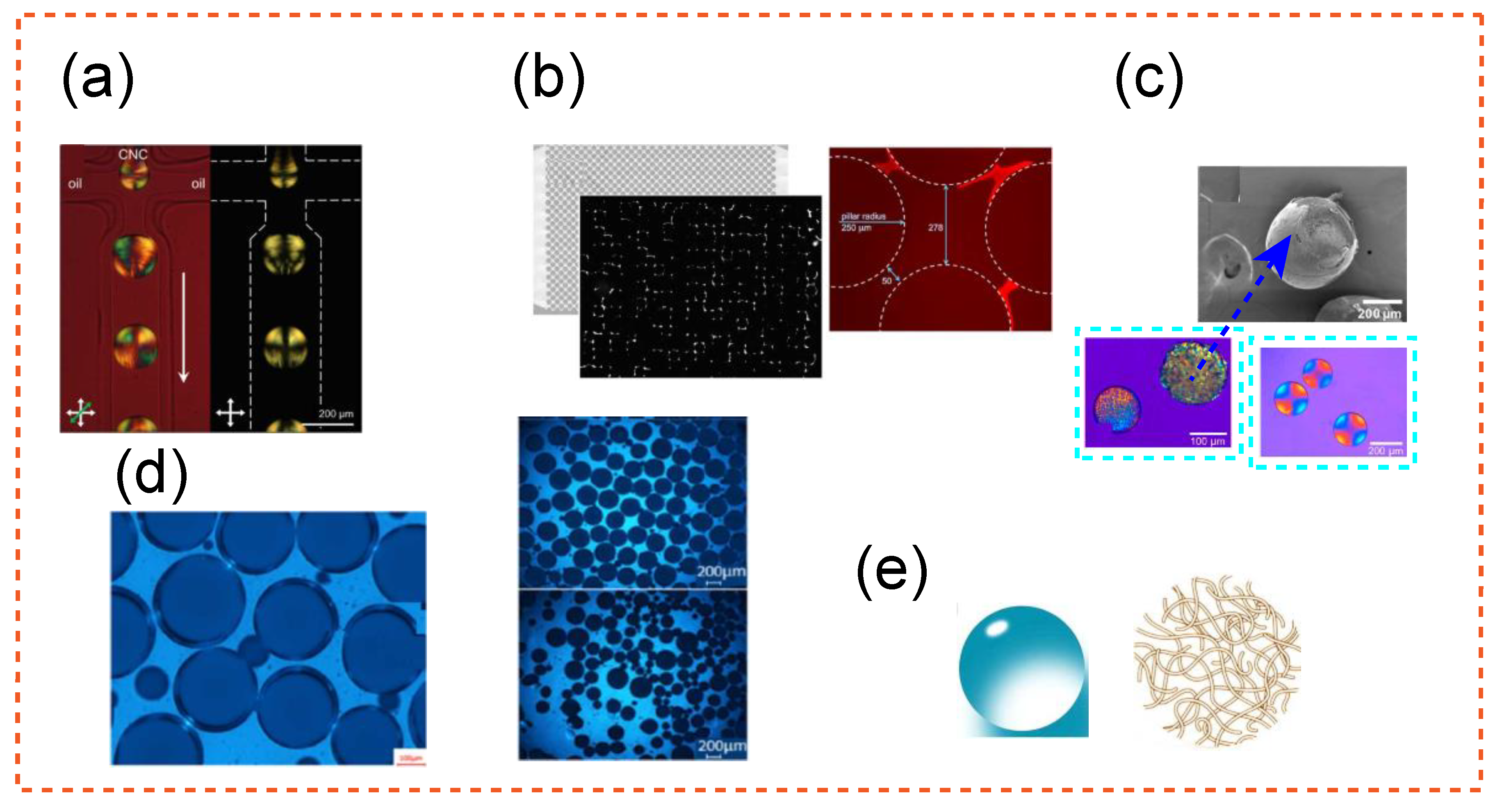
| Carrick et al. [142] | Creation of cellulose capsules | For medication delivery or controlled release capsules, cellulose capsules with a limited size distribution might be advantageous. Capsules were carboxymethylated to make them pH responsive and to expand roughly 10% when the pH was changed from 3 to 10. |
| Pei et al. [143] | Cross-linked cellulose hydrogel was used for making a chip | To create cellulose–collagen hybrid hydrogels, collagen, a critical extracellular component for cell culture, was cross-linked in the cellulose hydrogel. Researchers revealed that they have excellent structural reproduction ability, physical qualities, and cell culture cytocompatibility. |
| Zhang et al. [144] | Creation of a technology for adsorption and isolation of nucleic acids on cellulose magnetic beads | The use of a 3D-printed microfluidic chip enables the extraction of nucleic acids without the need of vortexes or centrifuges. Magnetic, interfacial, and viscous drag forces are described inside the chip’s microgeometries. Across a variety of HPV plasmid levels, an overall extraction efficiency of 61% is reported. |
| Wenzlik et al. [145] | In a microfluidic setting, cholesteric particles were made from cellulose derivatives | Co-flowing injection of drops of liquid crystalline mixes of cellulose derivatives into microspheres on the micrometre scale is used in the process. |
| Miyashita et al. [146] | The diamagnetic director for microfluidic systems is made up of microcrystal-like cellulose fibrils | Cellulose is a potential material for the development of biogenic optical systems that imitate the unique optical capabilities of living creatures. In a microfluidic laboratory, magnetic orientation tests on microcrystalline cellulose were performed. During the dispersed light intensity process, light intensity altered depending on the direction of the magnetic field. |
| Chen et al. [147] | A multilayer microfluidic device with a PDMS–cellulose composite film was developed | This paper describes an integrated multilayer microfluidic system that can pre-treat raw samples and detect them using immunoassays. Using the crossflow concept, a polydimethylsiloxane (PDMS)-cellulose composite film was employed to extract plasma from raw samples. |
| Włodarczyk and Zarzycki [148] | On silica and cellulose micro-TLC plates, the chromatographic behaviour of chosen colours was studied | The chromatographic behaviour of 18 colourants, including amaranth, black PN, bromophenol blue, and bromocresol green, was investigated. Data were gathered using silica and cellulose-coated microplates under thermostatic settings (303 K). Dyes are frequently utilized as colourants in food and industry, as well as sensing compounds in analytical and medicinal purposes. |
| Ghorbani et al. [149] | Creation of CNF- stabilized perfluoro droplets | In a variety of applications, hydrodynamic cavitation on microchips has been emphasised. Cavitating flow patterns may be used to promote a wide range of industrial and technical applications. Inside microfluidic devices, a novel technique involving cellulose nanocomposites perfluoro droplets was tested. |
| Park et al. [150] | Wet-spun microcomposite filaments were made with cellulose | To make microfilaments, cellulose nanocrystals were wet spun in a coagulation bath. The influence of sodium alginate on the characteristics of the micro composite filament was studied. The higher spinning rate of sodium alginate generated a rise in the alignment index of CNCs, leading to an improvement in the material’s tensile characteristics. |
| Grate et al. [151] | Creation of Alexa Fluor-labeled fluorescent CNCs | A group of researchers discovered a mechanism to attach Alexa Fluor dyes to cellulose nanocrystals while preserving the nanocrystal’s overall structure. Bioimaging tests revealed that the spatial positioning of solid cellulose deposits could be detected and their elimination over time under the action of Celluclast® enzymes or microorganisms could be monitored. |
| Ke et al. [152] | Microgels made from carboxymethyl cellulose for cell encapsulation | Carboxy methyl cellulose was modified with 4-hydroxybenzylamine (CMC-Ph) to create carboxy methyl cellulose-based microgels for use in scaffolds. The ATDC5 chondrocytic cell line was grown for up to 40 days after being encased in carboxy methyl cellulose microgels. |
| Rao et al. [153] | Creation of microfluidic paper fuel cell | MMPFCs (Membraneless Microfluidic Paper Fuel Cells) are promising technologies for harvesting energy for a variety of portable applications. Because of the built-in co-laminar flow and integrated capillary, the devices remove the need for membranes and additional pumps. |
| Shen et al. [154] | Creation of paper-based microfluidic fuel cells | Microfluidic fuel cells made of paper are emerging as possible renewable energy sources for small-scale electronic systems. The textural qualities of the paper channels have a considerable impact on the performance of paper fuel cells. The use of paper with a bigger mean pore width may result in a greater peak power density and open circuit voltage. |
| Shefa et al. [155] | A method of incorporation of curcumin (Cur) into a hydrogel system based on cellulose was developed | A freeze–thaw technique was used to create a Cur including physically crosslinked TEMPO-oxidized CNC–polyvinyl–alcohol curcumin– hydrogel, that produced curcumin to speed wound healing. L929 fibroblast cells incorporated curcumin within 4 h of incubation, according to in vitro experiments. |
| Chen et al. [156] | Separation of glycoproteins was achieved using bacterial cellulose microfluidic column | A simple technique was used to produce a regenerated bacterial cellulose column containing concanavalin A (Con A) lectin immobilised in a microfluidic device to evaluate and separate glycoproteins. Schiff-base formation was used to covalently link lectin Con A to the RBC matrix surface. |
| Study | Highlights |
|---|---|
| Lin et al. [198] | Three-dimensional microfluidic paper-based analytical devices (3D-µPADs) are a potential platform technology that enables for complicated fluid manipulation, parallel sample distribution, high throughput, and multiplex analysis assays. This technology can regulate the penetration depth of melted wax printed on both sides of a paper substrate, resulting in multilayer patterned channels in the substrate. |
| Martinez et al. [199] | A novel family of point-of-care diagnostic devices is PADs. They are affordable, simple to operate, and particularly developed for usage in poor nations. When completely developed, they may deliver faster and less expensive bioanalyses. |
| Yamada et al. [200] | On microfluidic PADs, “distance-based” detection patterns provide quantitative analysis without the need of signal output tools. Quantitative analysis is enabled by the distance-based quantified signal and the strong batch-to-batch production repeatability based on printing processes. |
| Li and Liu [201] | A wax-printing process is used to create 3D microfluidic channels inside a single sheet of cellulose paper. It enabled the production of up to four layers of paper channels in a 315-micrometer-thick substrate surface without the need for process optimization. |
| Ardalan et al. [202] | The smart wearable sweat patch (SWSP) is a non-invasive and in situ multi-sensing sweat biomarkers sensor that measures glucose, lactate, pH, chloride, and volume. A smartphone app was also created to use a detection algorithm to estimate the quantity of biomarkers. |
| Arun et al. [203] | The capillary-driven fluid flow of a combination of fuel and electrolyte drives the capillary-driven fluid flow of a microfluidic fuel cell. Various pencils are used to produce the graphite electrodes to study their influence on fuel cell performance. To improve performance, the paper fuel cell was also manufactured in different diameters and coupled as cell stacks. |
| Yan et al. [157] | H2O2 is used as both fuel and oxidant in a paper-based microfluidic fuel cell for portable electronics. It does have a peak energy capacity of 0.88 mW·cm−2. The fuel cell does not require precious-metal catalysts, and the fuel utilized is carbon free and environmentally friendly. |
| del Torno-de Román et al. [204] | The power and output current of a paper-based enzyme glucose/O2 fuel cell can be enhanced by adopting a quasi-steady flow. The fuel cell’s anode and cathode are composed of display carbon electrodes that have been correctly functionalized with protease inks. |
| Jia et al. [205] | Because of its hydrophilic properties, cellulose paper has been widely employed in microfluidic devices. Cellulose is placed in paper at random, with no specific direction or pathways. White wood possesses natural microchannels as well as a quick and anisotropic liquid and big solid particle movement. |
| Cai et al. [90] | By silanizing filter cellulose using a paper mask, authors created a new, low-cost, and straightforward approach for fabricating PADs. This procedure requires no expensive equipment and may be carried out by inexperienced persons. |
| Murase et al. [206] | Cellulose nanofiber can be utilized as a component in PADs. The thixotropic characteristic of TEMPO-oxidized CNCs aqueous dispersion allowed for inkjet printability, which aided manufacturing. |
| Kumar et al. [207] | Cancer diagnostics are not currently prioritised in resource-limited settings. However, budget-friendly and targeted screening test and diagnostic tools are in great need. Multi-layer cellulose nanofibril-based coverings on expendable microfluidics were tuned for targeted capture and efficient release of target cells. |
| Choi et al. [208] | The microfluidic cellulose microfibre chip was prototyped by injecting 10 percent CM solutions onto CNC-milled substrates. It can identify exudative age-related macular degeneration in human aqueous sense organs. |
| Fu and Liu [209] | PADs are typically mounted on a cellulose paper substrate. Covalent bonds with the target biomolecule can be achieved by modifying chemicals. The optimum performance for biosensing applications comes from potassium periodate (KIO4)-modified cellulose paper. |
| Lu et al. [114] | Spider silks have amazing mechanical qualities; hence, one of the areas of research in biomimetic fibres was the construction of structures with high silk fibres as optical waveguides. The fibres might be useful in biological media, bio photonics, and central nervous system interfaces. |
| Bao et al. [210] | Transistors built of van der Waals materials are allowed by an all-cellulose paper with CNF on the upper surface, which results in outstanding surface roughness and electrolyte absorption. These planar transistors can be employed as sensors in PADs, together with other components. |
| Yadav et al. [211] | Microfluidics has the potential to revolutionise point-of-care detection in smart healthcare. Paper as a substrate aids in reducing existing stiff wastes and inevitable pollution. Flexible microfluidic technology hardcopy provides a low-cost technical foundation for next-generation intelligent sensors. |
| Solin et al. [212] | Point-of-care diagnosis can benefit from microfluidic technologies. Authors looked at the fluidic structure due to stencil painting on flexible surfaces. Combining minerals with cellulose fibrils resulted in optimal printability and flow profiles. The findings demonstrate the use of these pathways for drug and chemical analysis. |
| Study | Material Used | Microfluidic Application |
|---|---|---|
| Shin and Hyun [226] | CNF | Construction material for microfluidics |
| Li and Liu [201] | CNF | Construction material for microfluidics |
| Jia et al. [205] | CNF | Construction material for microfluidics |
| Cai et al. [90] | Filter paper | Construction material for microfluidics, glucose assay |
| Yu et al. [271] | Bacterial cellulose | Production of cellulose microcapsules, wound healing |
| Nechyporchuk et al. [109] | CNF, CNC | Using microfluidics for spinning strong microfibers |
| Li et al. [136] | Bacterial cellulose | Microfluidics as a platform to examine wound dressing screening |
| Ardalan et al. [202] | Cotton thread | Cellulose-based microfluidic wearable patch |
| Tata Rao et al. [272] | Cellulose absorbent pads | Microfluidic paper–based fuel cells |
| Park et al. [273] | Bacterial cellulose | Cell culture and wound healing |
| Baek and Park [89] | Molten cellulose | Microfluidic set up was used to produce cellulose beads |
| Higashi and Miki [257] | Bacterial cellulose | Application for biochemical engineering and cell delivery systems |
| Song et al. [92] | Bacterial cellulose | Produce a framework for artificial cells |
| Pepicelli et al. [131] | Bacterial cellulose | Capsules for applications such as flavor, fragrance, agrochemicals, nutrients, and drug encapsulation |
| Zhang et al. [107] | Cellulose acetate | Remediation of water |
| Liu et al. [274] | Carboxy methyl cellulose | Preparing cell-laden microgels |
| Levin et al. [260] | CNCs | Porous microparticles for applications such as drug delivery or sorption agents. |
| Kaufman et al. [275] | CNF | Production of strong yet flexible microcapsule shells. |
| Dhand et al. [276] | TEMPO-oxidized CNF | Tune microparticles suspension to tailor complex fluid rheology |
| Carrick et al. [142] | cellulose pulp | Microencapsulation for drug delivery or controlled release capsules. |
| Yeap et al. [16] | Ethyl cellulose | Drug–excipient composite microparticles |
| Yeap et al. [277] | Ethyl cellulose | Production of monodisperse spherical drug particles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi Moud, A. Cellulose through the Lens of Microfluidics: A Review. Appl. Biosci. 2022, 1, 1-37. https://doi.org/10.3390/applbiosci1010001
Abbasi Moud A. Cellulose through the Lens of Microfluidics: A Review. Applied Biosciences. 2022; 1(1):1-37. https://doi.org/10.3390/applbiosci1010001
Chicago/Turabian StyleAbbasi Moud, Aref. 2022. "Cellulose through the Lens of Microfluidics: A Review" Applied Biosciences 1, no. 1: 1-37. https://doi.org/10.3390/applbiosci1010001