Kinematic, Neuromuscular and Bicep Femoris In Vivo Mechanics during the Nordic Hamstring Exercise and Variations of the Nordic Hamstring Exercise
Abstract
1. Introduction
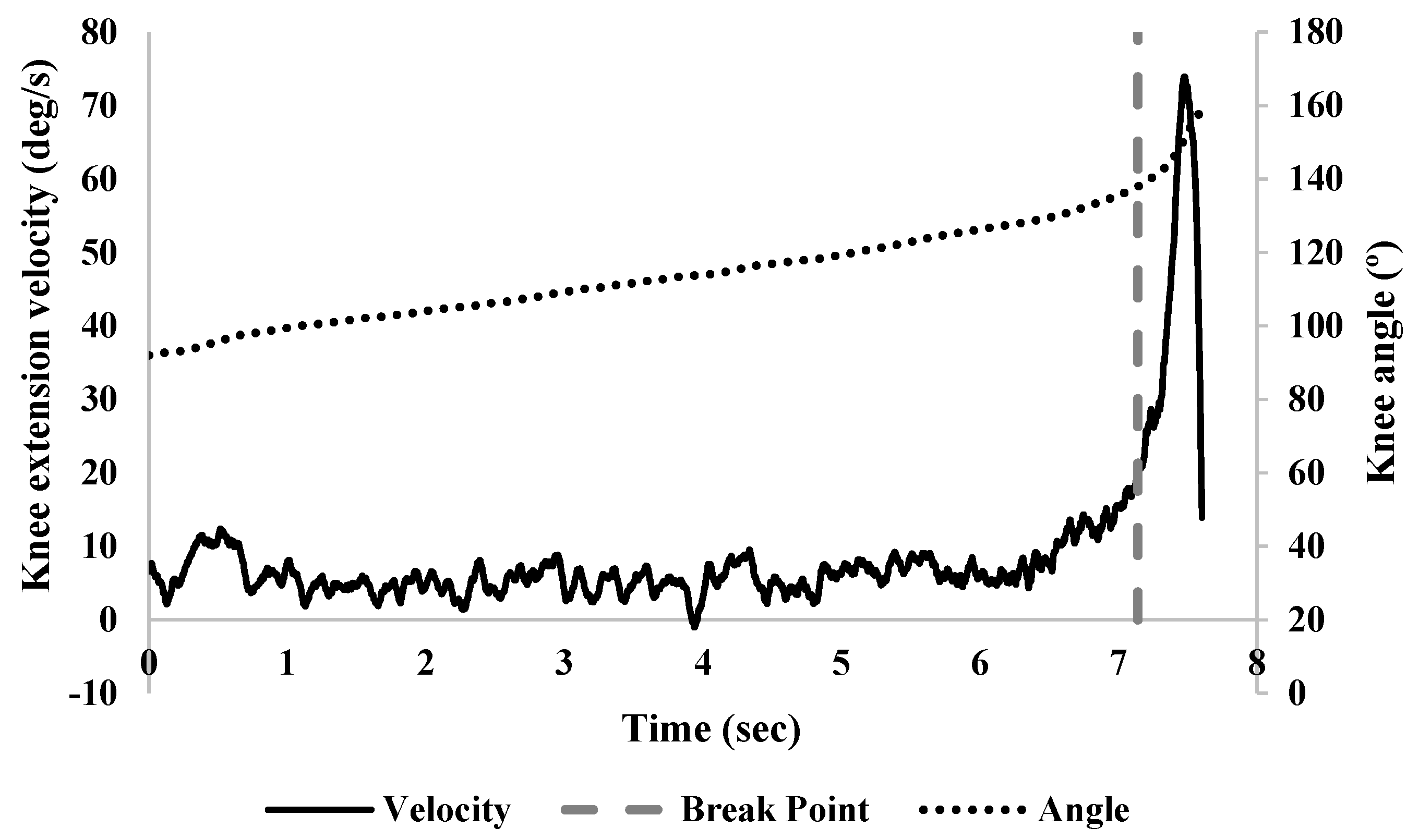
2. Materials and Methods
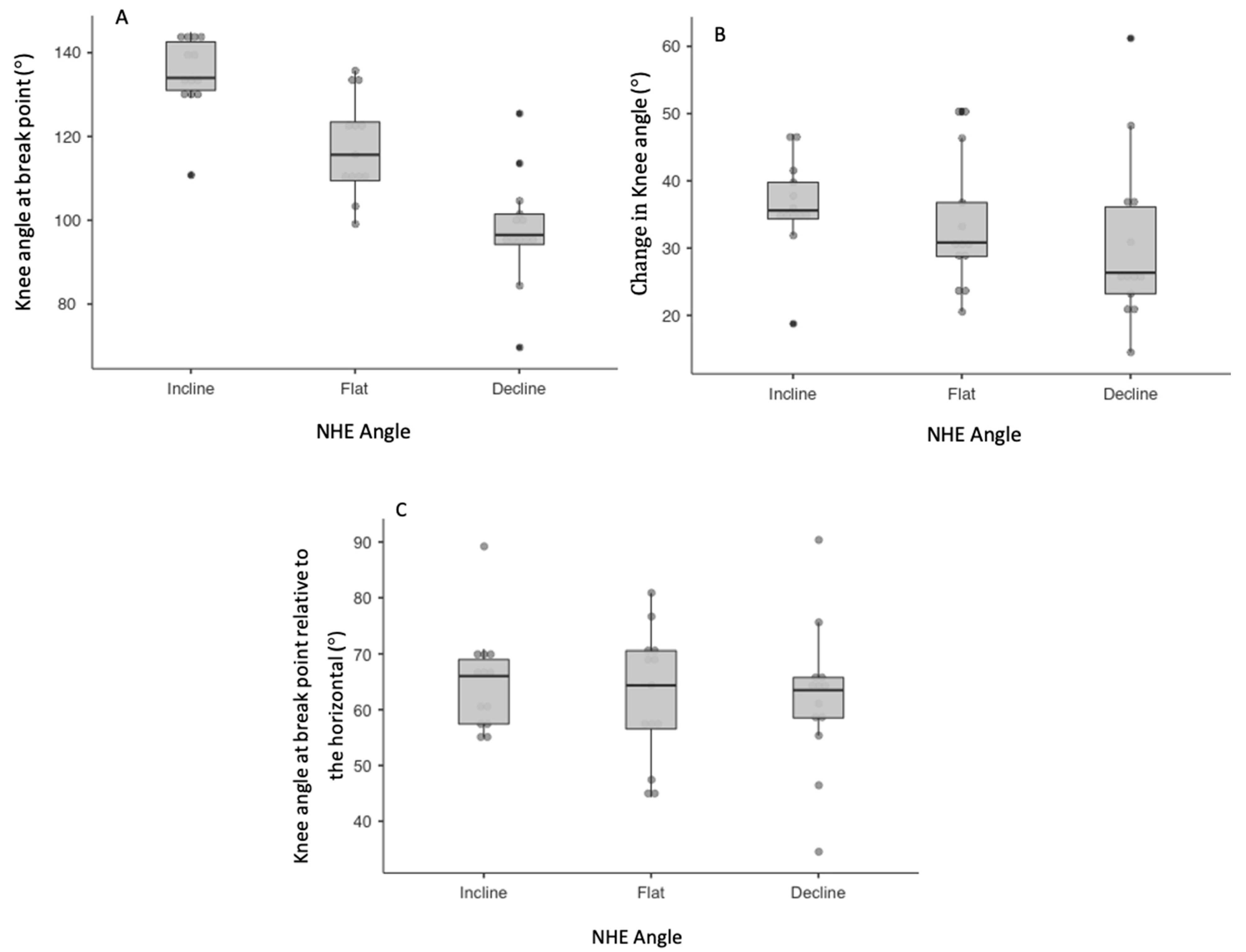
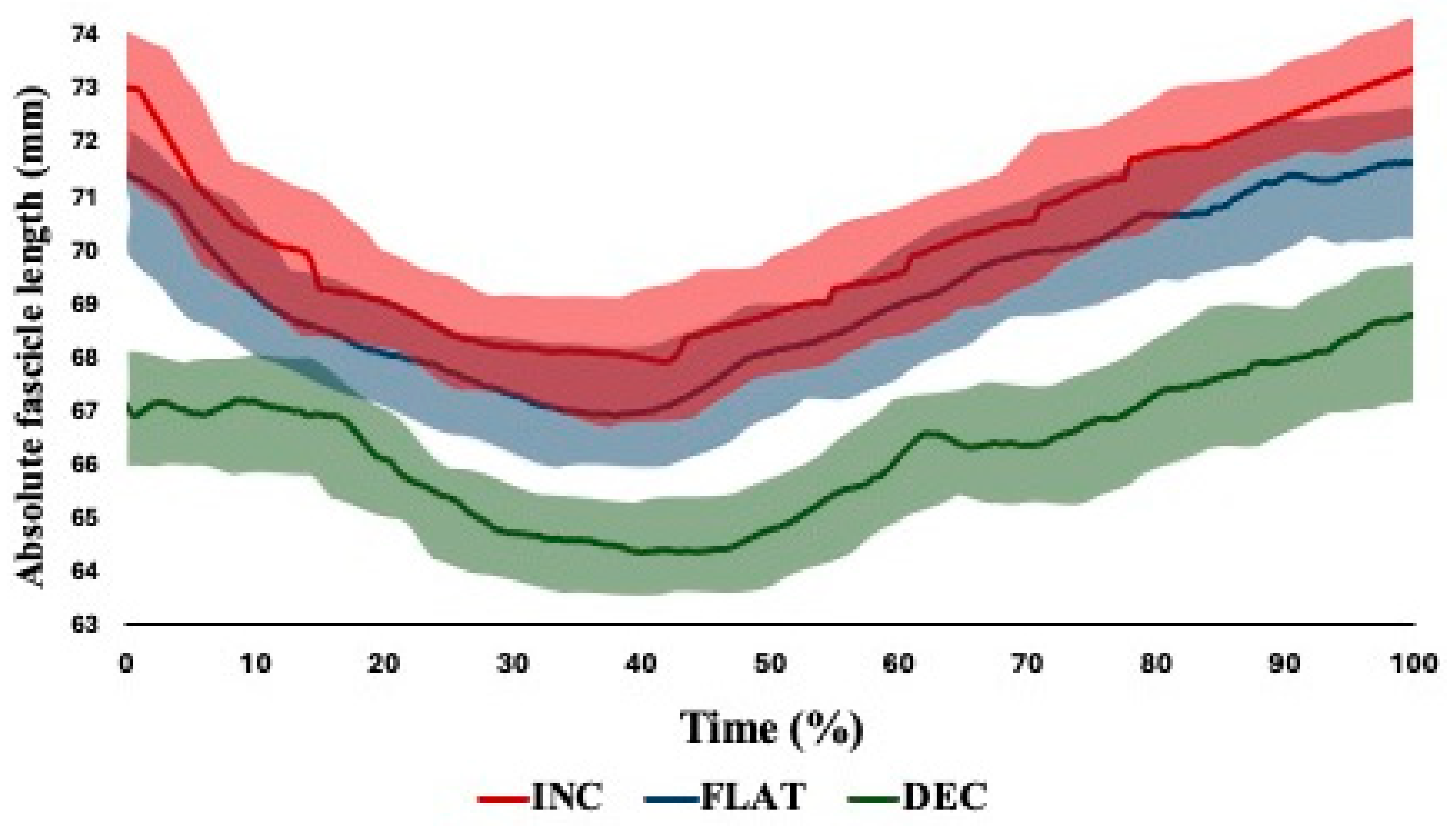
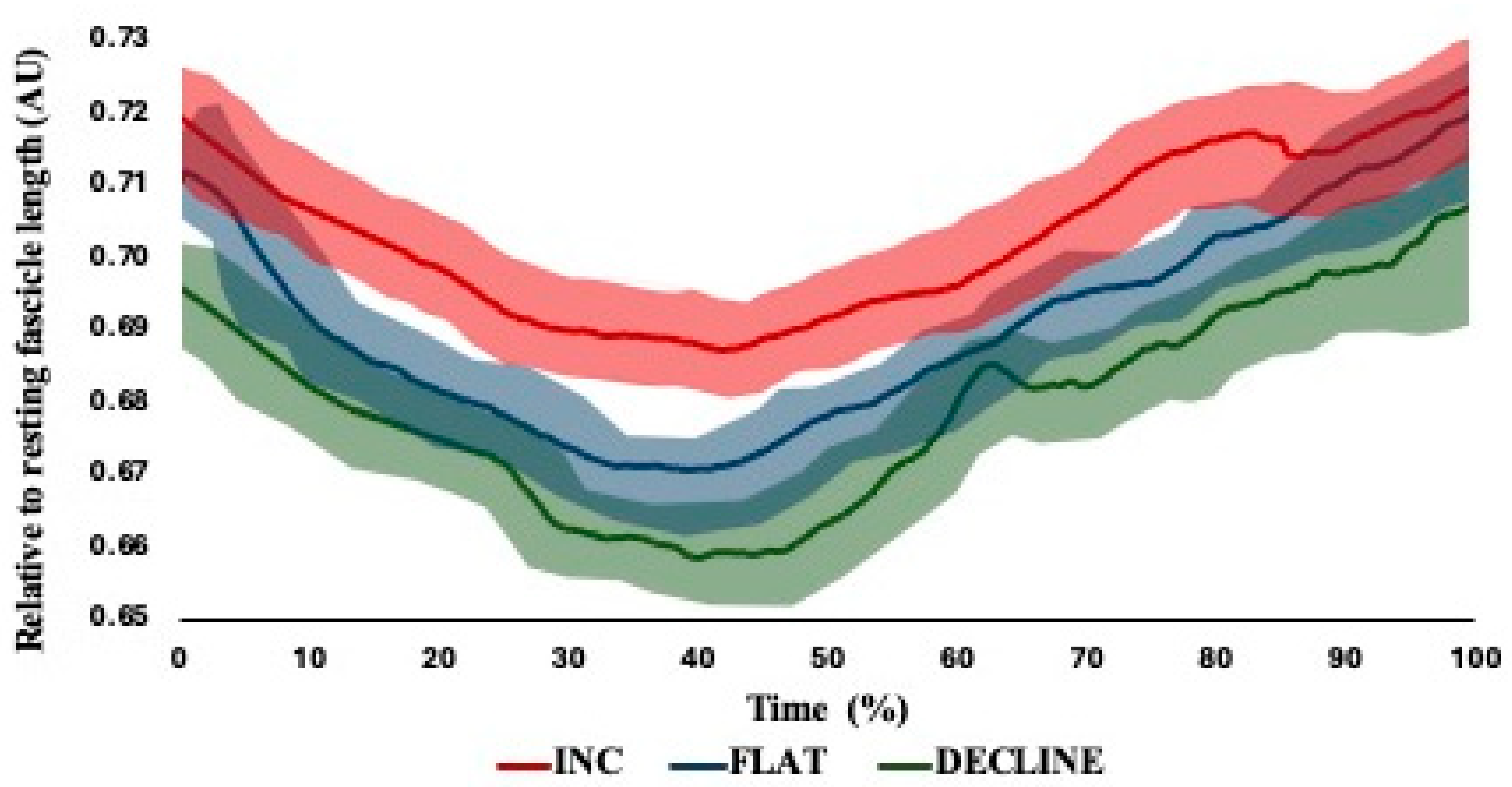
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Pairwise Post Hoc | Cohen’s d (95% CI) | Effect Size Descriptor | ||
|---|---|---|---|---|
| Knee angle (°) at break point | Incline vs. Flat | 0.001 $ | 1.39 (0.14–2.59) | Moderate |
| Incline vs. Decline | <0.001 $$ | 2.82 (1.20–4.38) | Large | |
| Flat vs. Decline | 0.002 $ | 1.30 (0.06–2.49) | Moderate | |
| Change in knee angle (°) | Incline vs. Flat | 0.667 | 0.29 (−0.81–1.38) | Trivial |
| Incline vs. Decline | 0.337 | 0.51 (−0.61–1.61) | Small | |
| Flat vs. Decline | 0.792 | 0.22 (−0.88–1.31) | Trivial | |
| Knee angle (°) at break point relative to the horizontal | Incline vs. Flat | 0.801 | 0.37 (−0.74–1.46) | Small |
| Incline vs. Decline | 0.780 | 0.42 (−0.69–1.51) | Small | |
| Flat vs. Decline | 0.997 | 0.04 (1.05–1.13) | Trivial | |
| Relative MTU length (%) at break point | Incline vs. Flat | 0.090 | 0.70 (−0.44–1.81) | Small |
| Incline vs. Decline | 0.006 $ | 1.14 (−0.07–2.30) | Moderate | |
| Flat vs. Decline | 0.270 | 0.54 (−0.58–1.64) | Small | |
| Change in relative MTU length (%) | Incline vs. Flat | 0.984 | 0.06 (−1.03–1.15) | Trivial |
| Incline vs. Decline | 0.945 | 0.12 (−0.97–1.21) | Trivial | |
| Flat vs. Decline | 0.991 | 0.04 (−1.05–1.13) | Trivial |
| Pairwise Post Hoc | Cohen’s d (95% CI) | Effect Size Descriptor | ||
|---|---|---|---|---|
| Starting fascicle length (mm) | Incline vs. Flat | 0.004 | 1.78 (0.44–3.06) | Large |
| Incline vs. Decline | 0.000 | 3.75 (1.12–5.33) | Large | |
| Flat vs. Decline | 0.000 | 2.12 (1.08–3.11) | Large | |
| Shortest fascicle length (mm) | Incline vs. Flat | 0.007 | 1.37 (0.12–2.57) | Moderate |
| Incline vs. Decline | 0.001 | 2.76 (1.16–4.30) | Large | |
| Flat vs. Decline | 0.001 | 2.27 (0.81–3.68) | Large | |
| Fascicle length at break point (mm) | Incline vs. Flat | 0.001 | 2.25 (0.79–3.65) | Large |
| Incline vs. Decline | 0.000 | 3.74 (1.83–5.60) | Large | |
| Flat vs. Decline | 0.000 | 2.55 (1.01–4.03) | Large | |
| Total dynamic change in fascicle length (mm) | Incline vs. Flat | 0.005 | 1.42 (0.16–2.63) | Moderate |
| Incline vs. Decline | 0.001 | 2.72 (1.13–4.25) | Large | |
| Flat vs. Decline | 0.005 | 1.61 (0.31–2.86) | Large |
References
- Bourne, M.N.; Timmins, R.G.; Opar, D.A.; Pizzari, T.; Ruddy, J.D.; Sims, C.; Williams, M.D.; Shield, A.J. An Evidence-Based Framework for Strengthening Exercises to Prevent Hamstring Injury. Sports Med. 2018, 48, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, M.; Ripley, N.; McMahon, J.; Evans, M.; Haff, G.G.; Comfort, P. The Effect of Nordic Hamstring Exercise Intervention Volume on Eccentric Strength and Muscle Architecture Adaptations: A Systematic Review and Meta-analyses. Sports Med. 2019, 50, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Duhig, S.J.; Bourne, M.N.; Buhmann, R.L.; Williams, M.D.; Minett, G.M.; Roberts, L.A.; Timmins, R.G.; Sims, C.K.E.; Shield, A.J. Effect of concentric and eccentric hamstring training on sprint recovery, strength and muscle architecture in inexperienced athletes. J. Sci. Med. Sport 2019, 22, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.; Opar, D.A.; Williams, M.D.; Bourne, M.; Timmins, R. Razor Hamstring Curl and Nordic Hamstring Exercise architectural adaptations: Impact of Exercise Selection and Intensity. Scand. J. Med. Sci. Sports 2019, 29, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, B.J.; Beller, R.; Hahn, D. Biceps Femoris Long Head Muscle Fascicles Actively Lengthen During the Nordic Hamstring Exercise. Front. Sports Act. Living 2021, 3, 669813. [Google Scholar] [CrossRef]
- Van Hooren, B.; Vanwanseele, B.; van Rossom, S.; Teratsias, P.; Willems, P.; Drost, M.; Meijer, K. Muscle forces and fascicle behavior during three hamstring exercises. Scand. J. Med. Sci. Sports 2022, 32, 997–1012. [Google Scholar] [CrossRef]
- Ekstrand, J.; Bengtsson, H.; Walden, M.; Davison, M.; Hagglund, M. Still poorly adopted in male professional football: But teams that used the Nordic Hamstring Exercise in team training had fewer hamstring injuries—A retrospective survey of 17 teams of the UEFA Elite Club Injury Study during the 2020–2021 season. Br. Med. J. Open Sport Exerc. Med. 2022, 8, e001368. [Google Scholar] [CrossRef]
- Ekstrand, J.; Hallén, A.; Gauffin, H.; Bengtsson, H. Low adoption in women’s professional football: Teams that used the Nordic Hamstring Exercise in the team training had fewer match hamstring injuries. BMJ Open Sport Exerc. Med. 2023, 9, e001523. [Google Scholar] [CrossRef]
- Sarabon, N.; Marusič, J.; Marković, G.; Kozinc, Z. Kinematic and electromyographic analysis of variations in Nordic hamstring exercise. PLoS ONE 2019, 14, e0223437. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.M.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Lee Li, C.; Yung, P.; Chan, K. The reliability and validity of a video-based method for assessing hamstring strength in football players. J. Exerc. Sci. Fit. 2017, 15, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Sconce, E.; Jones, P.; Turner, E.; Comfort, P.; Graham-Smith, P. The validity of the nordic hamstring lower for a field-based assessment of eccentric hamstring strength. J. Sport Rehabil. 2015, 24, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ripley, N.; Comfort, P.; McMahon, J. Comparison between methods to estimate bicep femoris fascicle length from three estimation equations using a 10 cm ultrasound probe. Meas. Phys. Educ. Exerc. Sci. 2022, 27, 43–50. [Google Scholar] [CrossRef]
- Ripley, N.; Comfort, P.; McMahon, J.J. Comparison between Short, Medium, and Long Fields of View in Estimating Bicep Femoris Fascicle Length. Muscles 2024, 3, 153–165. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Hawkins, D.; Hull, M.L. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J. Biomech. 1990, 23, 487–494. [Google Scholar] [CrossRef]
- Farris, D.J.; Lichtwark, G.A. UltraTrack: Software for semi-automated tracking of muscle fascicles in sequences of B-mode ultrasound images. Comput. Methods Programs Biomed. 2016, 128, 111–118. [Google Scholar] [CrossRef]
- Van Hooren, B.; Teratsias, P.; Hodson-Tole, E.F. Ultrasound imaging to assess skeletal muscle architecture during movements: A systematic review of methods, reliability, and challenges. J. Appl. Physiol. 2020, 128, 978–999. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropratic Mediint 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Rhea, M.R. Determining the Magnitude of Treatment Effects in Strength Training Research Through the Use of the Effect Size. J. Strength Cond. Res. 2004, 18, 918–920. [Google Scholar]
- Guex, K.; Degache, F.; Morisod, C.; Sailly, M.; Millet, G.P. Hamstring Architectural and Functional Adaptations Following Long vs. Short Muscle Length Eccentric Training. Front. Physiol. 2016, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J.; Cannavan, D.; Coleman, D.R.; Horne, S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J. Appl. Physiol. (1985) 2007, 103, 1565–1575. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J. Effects of range of motion on muscle development during resistance training interventions: A systematic review. SAGE Open Med. 2020, 8, 2050312120901559. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, A.; Lahti, J.; Giacomo, J.-P.; Gerus, P.; Cronin, N.; Morin, J. Impact of Hip Flexion Angle on Unilateral and Bilateral Nordic Hamstring Exercise Torque and High-Density Electromyography Activity. J. Orthop. Sports Phys. Ther. 2019, 49, 584–592. [Google Scholar] [CrossRef]
- Bourne, M.N.; Opar, D.A.; Williams, M.D.; Shield, A.J. Eccentric Knee Flexor Strength and Risk of Hamstring Injuries in Rugby Union: A Prospective Study. Am. J. Sports Med. 2015, 43, 2663–2670. [Google Scholar] [CrossRef]
- Shield, A.; Bourne, M. Hamstring Injury Prevention Practices in Elite Sport: Evidence for Eccentric Strength vs. Lumbo-Pelvic Training. Sports Med. 2018, 48, 513–524. [Google Scholar] [CrossRef]
- Timmins, R.G.; Bourne, M.N.; Shield, A.J.; Williams, M.D.; Lorenzen, C.; Opar, D.A. Short biceps femoris fascicles and eccentric knee flexor weakness increase the risk of hamstring injury in elite football (soccer): A prospective cohort study. Br. J. Sports Med. 2016, 50, 1524–1535. [Google Scholar] [CrossRef]
- Franchi, M.V.; Fitze, D.P.; Raiteri, B.J.; Hahn, D.; Spörri, J. Ultrasound-derived Biceps Femoris Long-Head Fascicle Length: Extrapolation Pitfalls. Med. Sci. Sports Exerc. 2019, 52, 233–243. [Google Scholar] [CrossRef]
- Ando, R.; Nosaka, K.; Inami, T.; Tomita, A.; Watanabe, K.; Blazevich, A.J.; Akima, H. Difference in fascicle behaviors between superficial and deep quadriceps muscles during isometric contractions. Muscle Nerve 2016, 53, 797–802. [Google Scholar] [CrossRef]
- Ando, R.; Nosaka, K.; Tomita, A.; Watanabe, K.; Blazevich, A.J.; Akima, H. Vastus intermedius vs vastus lateralis fascicle behaviors during maximal concentric and eccentric contractions. Scand. J. Med. Sci. Sports 2018, 28, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Burden, A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. 2010, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Avrillon, S.; Guilhem, G.; Barthelemy, A.; Hug, F. Coordination of hamstrings is individual specific and is related to motor performance. J. Appl. Physiol. 2018, 125, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Avrillon, S.; Hug, F.; Guilhem, G. Bilateral differences in hamstring coordination in previously injured elite athletes. J. Appl. Physiol. 2020, 128, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Deslauriers, A.R. Regional heterogeneity in muscle fiber strain: The role of fiber architecture. Front. Physiol. 2014, 5, 303. [Google Scholar] [CrossRef]
- Hegyi, A.; Csala, D.; Peter, A.; Finni, T.; Cronin, N.J. High-density electromyography activity in various hamstring exercises. Scand. J. Med. Sci. Sports 2019, 29, 34–43. [Google Scholar] [CrossRef]
- Hegyi, A.; Gonçalves, B.; Finni, T.; Cronin, N. Individual Region- and Muscle-specific Hamstring Activity at Different Running Speeds. Med. Sci. Sports Exerc. 2019, 51, 2274–2285. [Google Scholar] [CrossRef]
- Hegyi, A.; Peter, A.; Finni, T.; Cronin, N.J. Region-dependent hamstrings activity in Nordic hamstring exercise and stiff-leg deadlift defined with high-density electromyography. Scand. J. Med. Sci. Sports 2018, 28, 992–1000. [Google Scholar] [CrossRef]


| Mean (SD) | CV% (95% CI); Descriptor | ICC (95% CI); Descriptor | ||
|---|---|---|---|---|
| Knee angle (°) at break point | Incline | 135.08 (9.22) | 3.67 (2.26–5.08); Good | 0.877 (0.626–0.975); Moderate |
| Flat | 117.67 (11.87) | 4.44 (2.73–6.15); Good | 0.965 (0.877–0.993); Good | |
| Decline | 98.04 (13.28) | 4.64 (2.86–6.42); Good | 0.943 (0.809–0.989); Good | |
| Change in knee angle (°) | Incline | 36.34 (7.02) | 4.89 (3.01–6.77); Good | 0.787 (0.628–0.955); Moderate |
| Flat | 33.44 (9.87) | 4.88 (3.00–6.76); Good | 0.908 (0.706–0.982); Moderate | |
| Decline | 30.53 (12.65) | 4.81 (2.96–6.66); Good | 0.951 (0.831–0.991); Good | |
| Knee angle (°) at break point relative to the horizontal | Incline | 64.98 (9.22) | 6.39 (3.93–8.85); Good | 0.822 (0.725–0.985); Moderate |
| Flat | 61.27 (11.82) | 8.80 (5.42–12.18); Moderate | 0.912 (0.857–0.963); Good | |
| Decline | 61.94 (13.31) | 8.60 (5.29–11.91); Moderate | 0.933 (0.849–0.990); Good | |
| Relative MTU length (%) at break point | Incline | 100.95 (2.78) | 5.61 (3.45–7.77); Good | 0.877 (0.626–0.975); Moderate |
| Flat | 98.64 (2.55) | 6.56 (4.04–9.08); Good | 0.812 (0.477–0.961); Poor | |
| Decline | 96.77 (3.39) | 5.48 (3.37–7.59); Good | 0.809 (0.472–0.960); Poor | |
| Change in relative MTU length (%) | Incline | 7.73 (0.83) | 6.45 (3.97–8.93); Good | 0.777 (0.408–0.953); Poor |
| Flat | 7.63 (2.03) | 7.78 (4.79–10.77); Moderate | 0.814 (0.482–0.961); Poor | |
| Decline | 7.52 (2.19) | 5.29 (3.26–7.32); Good | 0.631 (0.176–0.914); Poor |
| Mean (SD) | CV% (95% CI); Descriptor | ICC (95% CI); Descriptor | ||
|---|---|---|---|---|
| Peak bicep femoris sEMG (μV) | Incline | 413.49 (148.75) | 6.40 (3.94–8.86); Good | 0.857 (0.773–0.889); Good |
| Flat | 364.70 (124.14) | 8.85 (5.45–12.25); Moderate | 0.822 (0.680–0.880); Moderate | |
| Decline | 324.98 (109.76) | 7.17 (4.41–9.93); Good | 0.848 (0.749–0.887); Moderate | |
| Peak semitendinosus sEMG (μV) | Incline | 352.45 (121.87) | 7.21 (4.44–9.98); Good | 0.817 (0.669–0.879); Moderate |
| Flat | 380.83 (153.72) | 6.81 (4.19–9.43); Good | 0.808 (0.646–0.876); Moderate | |
| Decline | 380.27 (153.89) | 8.97 (5.52–12.42); Moderate | 0.830 (0.700–0.882); Moderate |
| Incline | Flat | Decline | |
|---|---|---|---|
| ICC (95% CI) Descriptor | 0.677 (0.525–0.709) Moderate | 0.717 (0.646–0.786) Moderate | 0.679 (0.633–0.725) Moderate |
| CMC (95% CI) Descriptor | 0.669 (0.638–0.701) Moderate | 0.701 (0.642–0.740) Moderate | 0.672 (0.628–0.715) Moderate |
| Starting fascicle length (mm) | 73.02 ± 0.87 | 71.43 ± 0.92 | 67.12 ± 1.16 |
| Shortest fascicle length (mm) | 67.89 ± 0.92 | 66.93 ± 0.37 | 64.40 ± 1.53 |
| Fascicle length at break point (mm) | 73.38 ± 0.93 | 71.63 ± 0.59 | 68.76 ± 1.48 |
| Fascicle length excursion (mm) | 10.81 ± 1.18 | 9.22 ± 1.06 | 7.11 ± 1.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripley, N.; Fahey, J.; Comfort, P.; McMahon, J. Kinematic, Neuromuscular and Bicep Femoris In Vivo Mechanics during the Nordic Hamstring Exercise and Variations of the Nordic Hamstring Exercise. Muscles 2024, 3, 310-322. https://doi.org/10.3390/muscles3030027
Ripley N, Fahey J, Comfort P, McMahon J. Kinematic, Neuromuscular and Bicep Femoris In Vivo Mechanics during the Nordic Hamstring Exercise and Variations of the Nordic Hamstring Exercise. Muscles. 2024; 3(3):310-322. https://doi.org/10.3390/muscles3030027
Chicago/Turabian StyleRipley, Nicholas, Jack Fahey, Paul Comfort, and John McMahon. 2024. "Kinematic, Neuromuscular and Bicep Femoris In Vivo Mechanics during the Nordic Hamstring Exercise and Variations of the Nordic Hamstring Exercise" Muscles 3, no. 3: 310-322. https://doi.org/10.3390/muscles3030027
APA StyleRipley, N., Fahey, J., Comfort, P., & McMahon, J. (2024). Kinematic, Neuromuscular and Bicep Femoris In Vivo Mechanics during the Nordic Hamstring Exercise and Variations of the Nordic Hamstring Exercise. Muscles, 3(3), 310-322. https://doi.org/10.3390/muscles3030027








