Effects of ATP on Time Parameters of Contractility of Rats’ Slow and Fast Skeletal Muscles in Normal and Hypothermic Conditions
Abstract
1. Introduction
2. Results
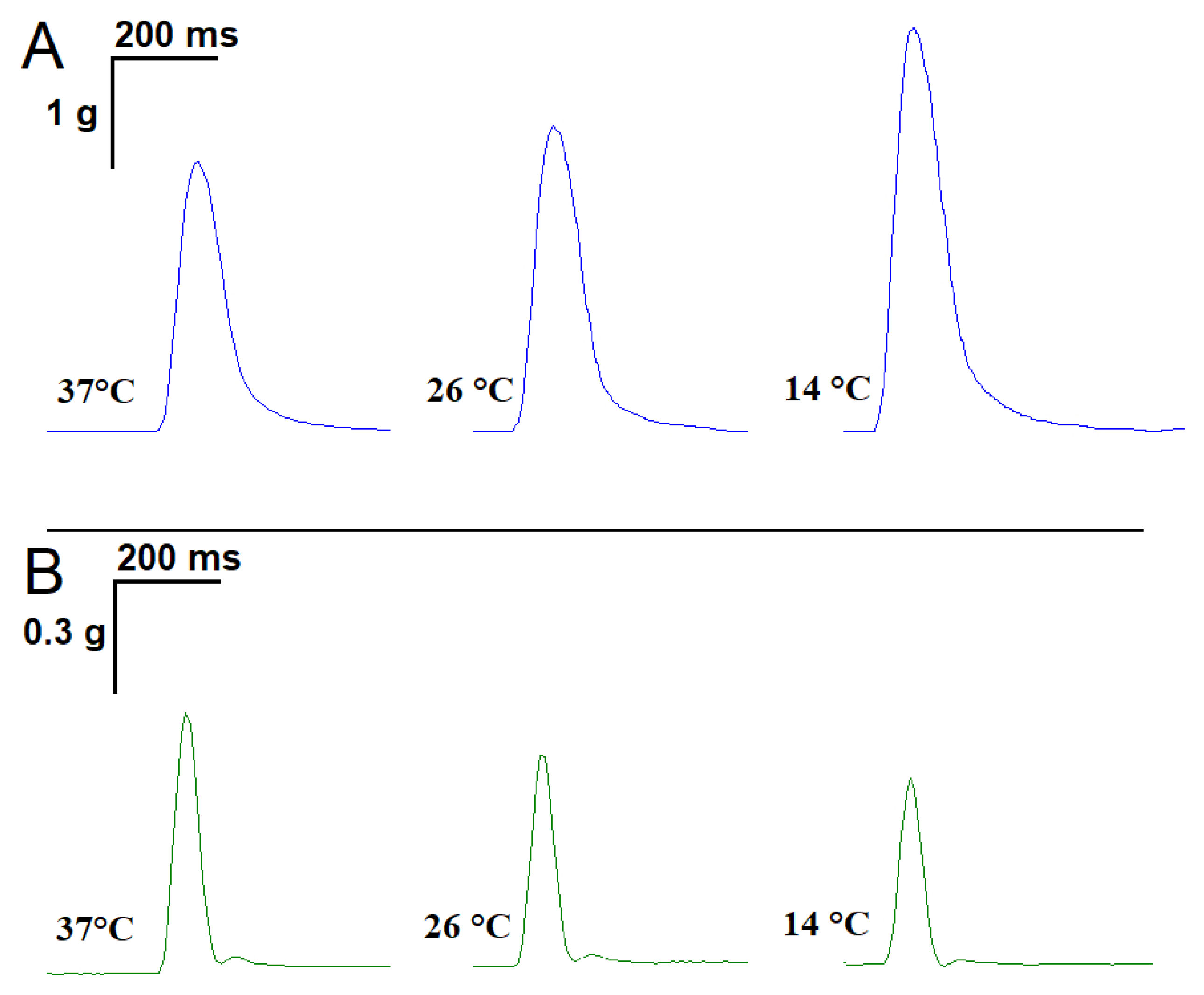
2.1. Influence of Temperature on the Time Parameters of Single Contractile Responses of m. soleus and m. EDL Induced by EFS
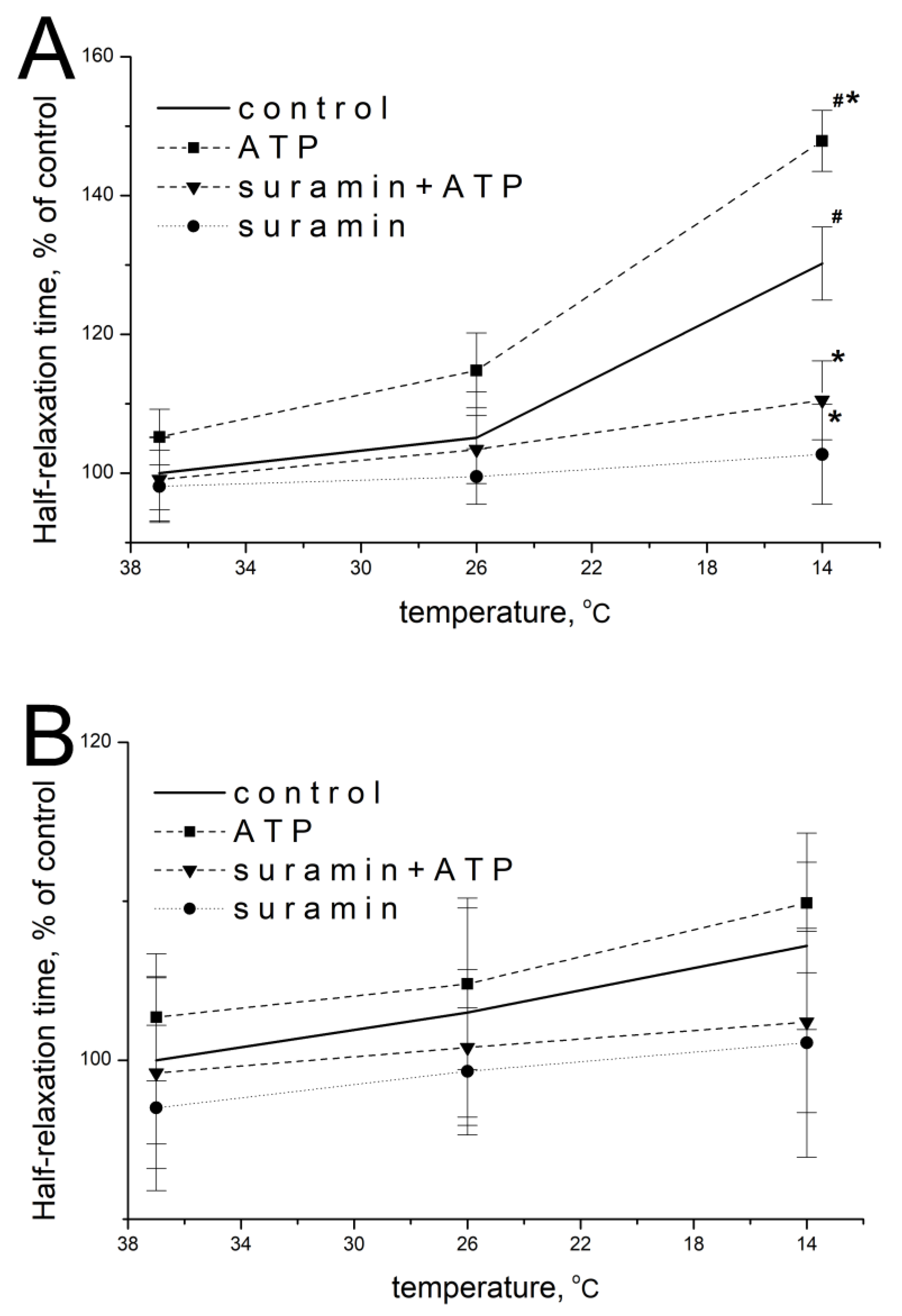
2.2. Effect of ATP and Suramin on Temporal Parameters of Single Contractile Responses of m. soleus and m. EDL Induced by EFS in Normal and Hypothermic Conditions
2.3. Effect of ATP at High Ca2+ Concentration
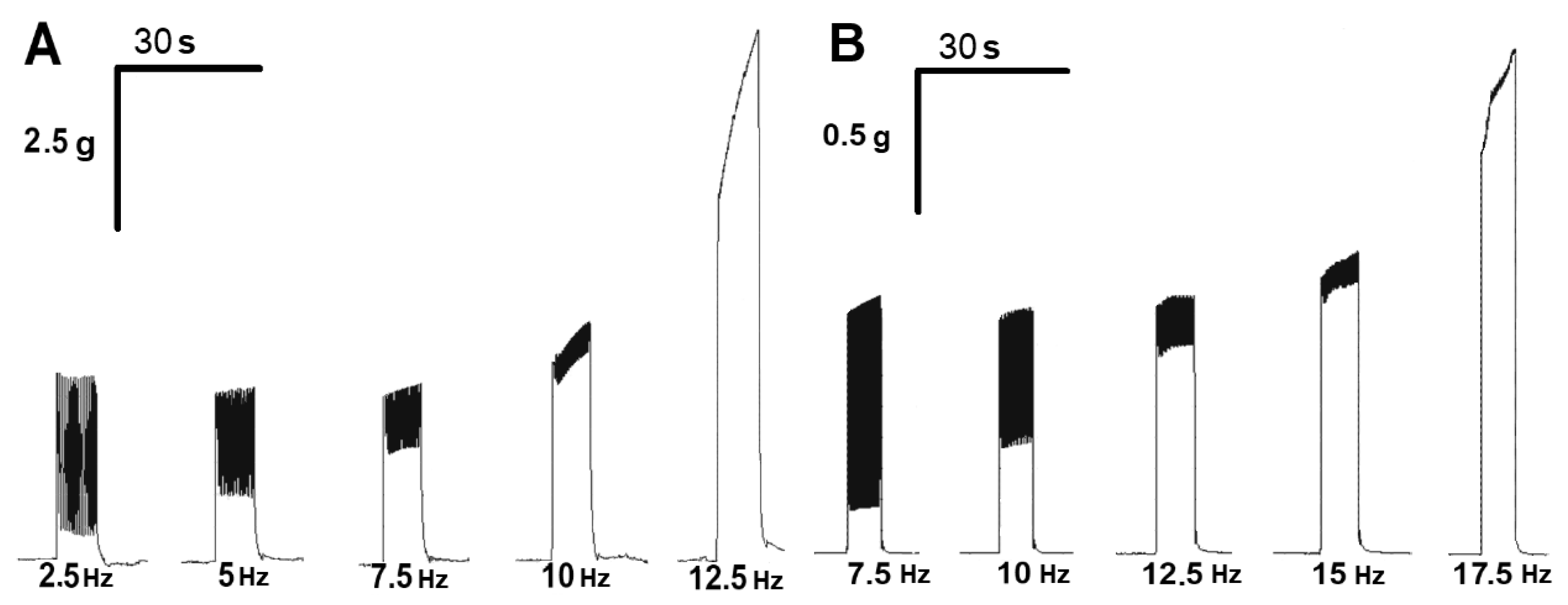
2.4. Effect of ATP on the Tetanic Contractile Responses of m. soleus and m. EDL Evoked by Electrical Stimulation in Normal and Hypothermic Conditions
2.5. Influence of Temperature on the Timing of Single Contractile Responses of m. soleus and m. EDL Induced by Carbachol
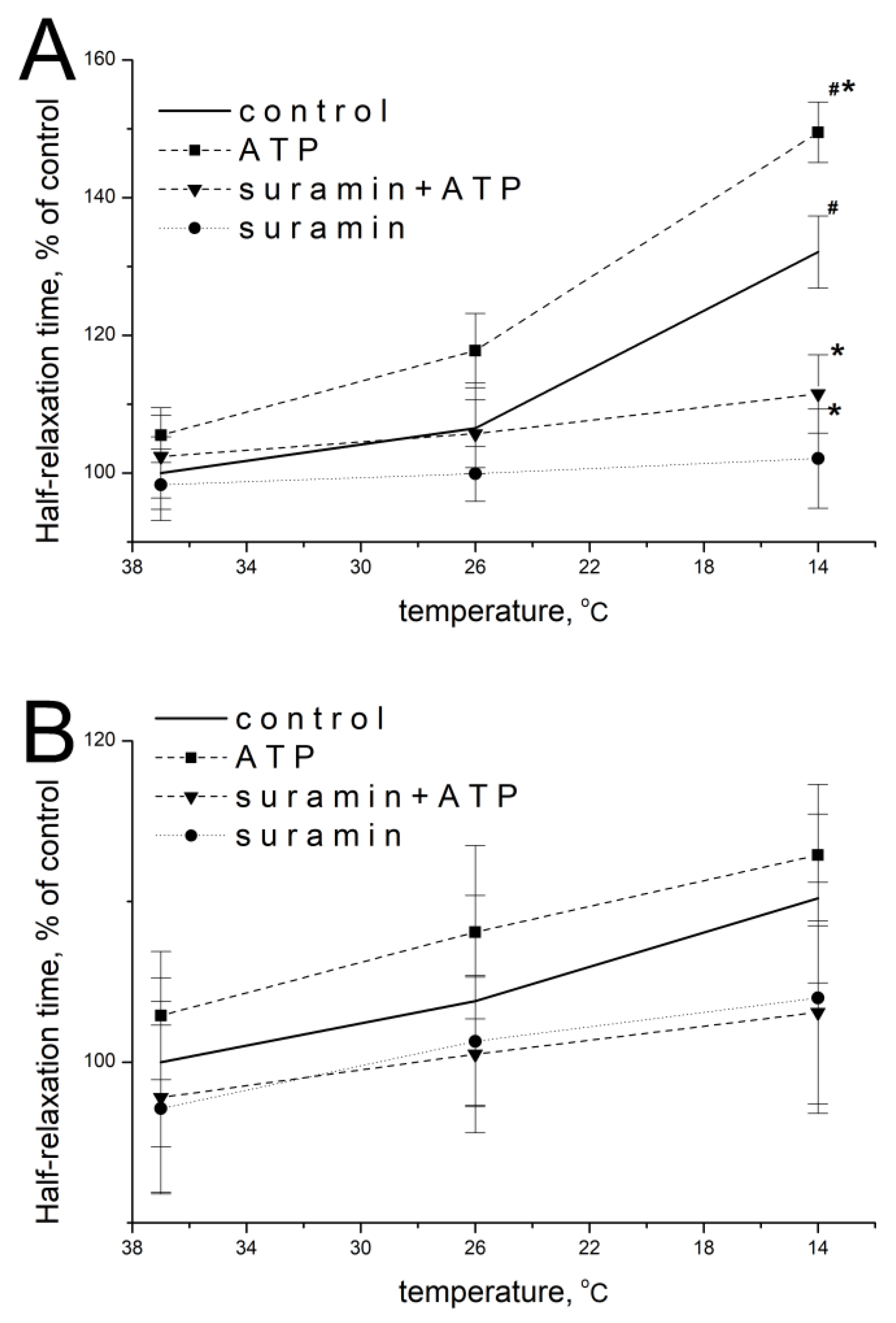
2.6. Effect of ATP and Suramin on Temporal Parameters of Single Contractile Responses of m. soleus and m. EDL Induced by Carbachol in Normal and Hypothermic Conditions
3. Discussion
4. Materials and Methods
4.1. Animals and Surgery
4.2. Single Contractions Induced by Electrical Field Stimulation
4.3. Contractions Induced by High-Frequency Electrical Field Stimulation
4.4. Carbachol-Induced Contractions
4.5. Assessment of the Temperature Dependency of Rat Skeletal Muscle Contraction
4.6. Experiments in Hypercalcium Environment
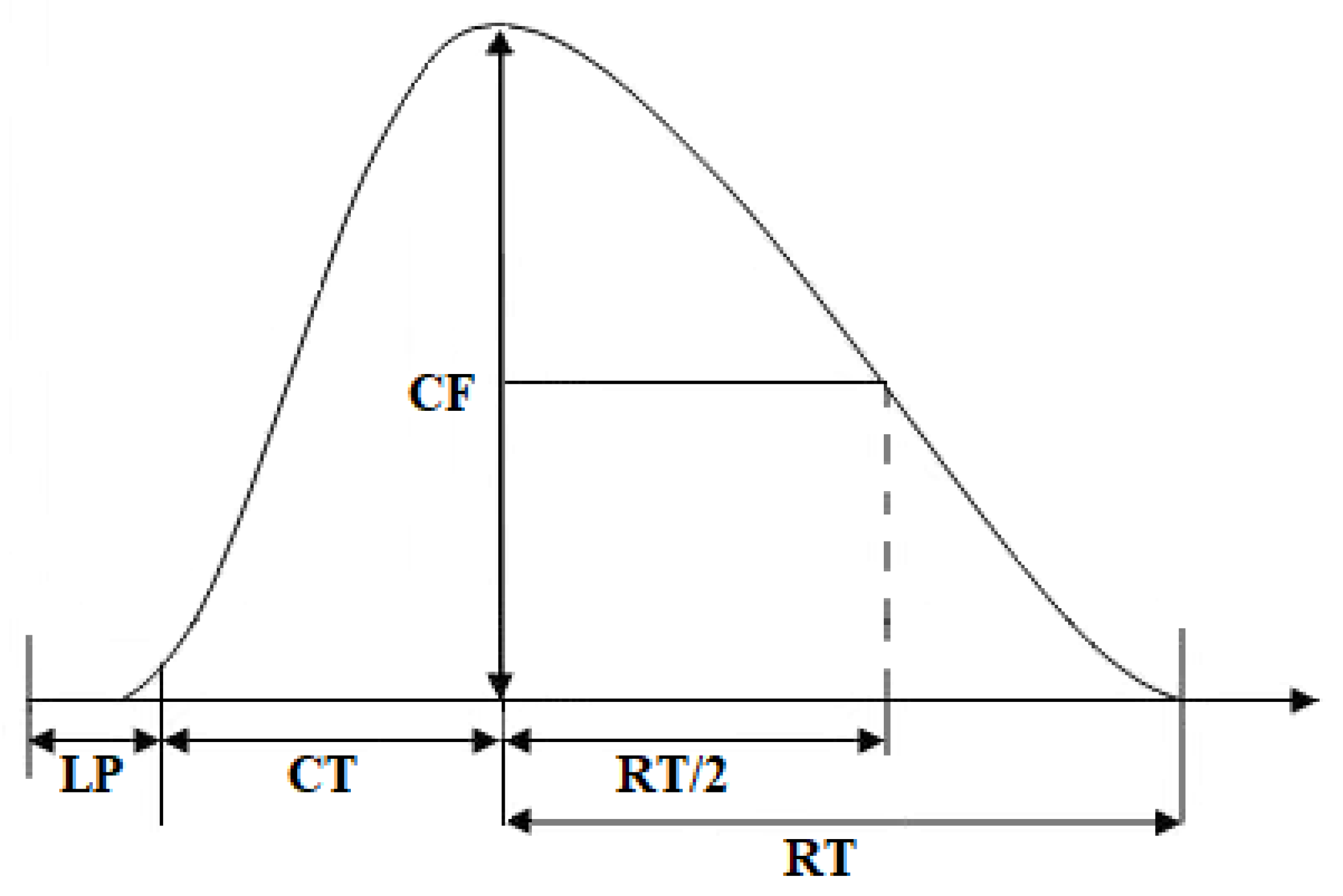
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angilletta, M.J., Jr. Thermal adaptation. In A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- James, R.S. A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J. Comp. Physiol. B 2013, 183, 723–733. [Google Scholar] [CrossRef]
- Castellani, J.W.; Tipton, M.J. Cold Stress Effects on Exposure Tolerance and Exercise Performance. Compr. Physiol. 2015, 6, 443–469. [Google Scholar] [PubMed]
- Bennett, A.F. Thermal dependence of locomotor capacity. Am. J. Physiol. 1990, 259, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Oksa, J. Temperature and neuromuscular function. Scand. J. Med. Sci. Sport. 2010, 20, 1–18. [Google Scholar] [CrossRef]
- Refinetti, R. Amplitude of the daily rhythm of body temperature in eleven mammalian species. J. Therm. Biol. 1999, 24, 477–481. [Google Scholar] [CrossRef]
- Wooden, K.M.; Walsberg, G.E. Body temperature and locomotor capacity in a heterothermic rodent. J. Exp. Biol. 2004, 207, 41–46. [Google Scholar] [CrossRef]
- Ducharme, M.B.; Van Helder, W.P.; Radomski, M.W. Tissue temperature profile in the human forearm during thermal stress at thermal stability. J. Appl. Physiol. 1991, 71, 1973–1978. [Google Scholar] [CrossRef]
- Ranatunga, K.W. Temperature dependence of mechanical power output in mammalian skeletal muscle. Exp. Physiol. 1998, 83, 371–376. [Google Scholar] [CrossRef]
- Bennett, A.F. Thermal dependence of muscle function. Am. J. Physiol. 1984, 247, 217–229. [Google Scholar] [CrossRef]
- Rall, J.A.; Woledge, R.C. Influence of temperature on mechanics and energetics of muscle contraction. Am. J. Physiol. 1990, 259, 197–203. [Google Scholar] [CrossRef]
- Marsh, R.L. Jumping ability of anurans. In Comparative Vertebrate Exercise Physiology; Jones, J.H., Ed.; Academic Press: San Diego, CA, USA, 1994; pp. 51–111. [Google Scholar]
- Syme, D.A. Functional properties of skeletal muscle. In Fish Physiology: Fish Biomechanics, Series; Shadwick, R.E., Lauder, G.V., Randall, D.J., Farrell, A.P., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2006; Volume 23, pp. 179–240. [Google Scholar]
- Seebacher, F.; James, R.S. Plasticity of muscle function in a thermoregulating ectotherm (Crocodylusporosus): Biomechanics and metabolism. Am. J. Physiol. 2008, 294, 1024–1032. [Google Scholar]
- Saltin, B.; Gagge, A.P.; Stolwijk, J.A. Muscle temperature during submaximal exercise in man. J. Appl. Physiol. 1968, 25, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.P.; Reardon, F.D.; Zaleski, W.; Reardon, M.L.; Haman, F.; Ducharm, M.B. Muscle temperature transients before, during, and after exercise measured using an intramuscular multisensor probe. J. Appl. Physiol. 2003, 94, 2350–2357. [Google Scholar] [CrossRef]
- Yaicharoen, P.; Wallman, K.; Morton, A.; Bishop, D. The effect of warm-up on intermittent sprint performance and selected thermoregulatory parameters. J. Sci. Med. Sport. 2012, 15, 451–456. [Google Scholar] [CrossRef]
- Huxley, A.F. Muscle. Annu. Rev. Physiol. 1964, 26, 131–152. [Google Scholar] [CrossRef]
- Rowlerson, A.M.; Spurway, N.C. Histochemical and immunohistochemical properties of skeletal muscle fibres from Rana and Xenopus. Histochem. J. 1988, 20, 657–673. [Google Scholar] [CrossRef]
- Grishin, S.N.; Ziganshin, A.U. Synaptic organization of tonic motor units in vertebrates. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. 2015, 9, 13–20. [Google Scholar] [CrossRef]
- Jimsheleishvili, S.; Marwaha, K.; Sherman, A.l. Physiology, Neuromuscular Transmission; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, 1462. [Google Scholar] [CrossRef] [PubMed]
- Pasnoor, M.; Dimachkie, M.M. Approach to Muscle and Neuromuscular Junction Disorders. Continuum 2019, 25, 1536–1563. [Google Scholar] [CrossRef]
- Apergis-Schoute, J.; Burnstock, G.; Nusbaum, M.P.; Parker, D.; Morales, M.A.; Trudeau, L.E.; Svensson, E. Editorial: Neuronal Ca-transmission. Front. Neural. Circuits 2019, 13, 19. [Google Scholar] [CrossRef]
- Svensson, E.; Apergis-Schoute, J.; Burnstock, G.; Nusbaum, M.P.; Parker, D.; Schiöth, H.B. General principles of neuronal co-transmission: Insights from multiple model systems. Front. Neural. Circuits 2019, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Dale, N. Purinergic signalling during development and ageing. Purinergic. Signal 2015, 11, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S. Purinergic signalling during myogenesis: A role for adenosine and its receptors. Acta Physiol. 2015, 214, 436–439. [Google Scholar] [CrossRef]
- Sokolova, E.M.; Grishin, S.N.; Shakirzyanova, A.V.; Talantova, M.V.; Giniatullin, R.A. Distinct receptors and different transduction mechanisms for ATP and adenosine at the frog motor nerve endings. Eur. J. Neurosci. 2003, 18, 1254–1264. [Google Scholar] [CrossRef]
- Burnstock, G.; Arnett, T.R.; Orriss, I.R. Purinergic signalling in the musculoskeletal system. Purinergic. Signal 2013, 9, 541–572. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.; Shakirzyanova, A.; Giniatullin, A.; Afzalov, R.; Giniatullin, R. Mechanisms of ATP action on motor nerve terminals at the frog neuromuscular junction. Eur. J. Neurosci. 2005, 21, 1271–1279. [Google Scholar] [CrossRef]
- Grishin, S.N.; Ziganshin, A.U. The role of purines in neuromuscular transmission. Biol. Membr. 2013, 30, 243–252. [Google Scholar]
- Ziganshin, A.U.; Khairullin, A.E.; Zobov, V.V.; Ziganshina, L.E.; Gabdrakhmanov, A.I.; Ziganshin, B.A.; Grishin, S.N. Effects of ATP and adenosine on contraction amplitude of rat soleus muscle at different temperatures. Muscle Nerve 2017, 55, 417–423. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Khairullin, A.E.; Teplov, A.Y.; Gabdrakhmanov, A.I.; Ziganshina, L.E.; Hoyle, C.H.V.; Ziganshin, B.A.; Grishin, S.N. The effects of ATP on the contractions of rat and mouse fast skeletal muscle. Muscle Nerve 2019, 59, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Khairullin, A.E.; Teplov, A.Y.; Grishin, S.N.; Farkhutdinov, A.M.; Ziganshin, A.U. The thermal sensitivity of purinergic modulation of contractile activity of locomotor and respiratory muscles in mice. Biophysics 2019, 64, 812–817. [Google Scholar] [CrossRef]
- Khairullin, A.E.; Ziganshin, A.U.; Grishin, S.N. Effect of hypothermia on purinergic synaptic modulation in the rat diaphragm. Biophysics 2020, 65, 1003–1008. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Kothari, M.J.; Shefner, J.M. Nerve, muscle, and neuromuscular junction electrophysiology at high temperature. Muscle Nerve 1997, 20, 431–436. [Google Scholar] [CrossRef]
- Ferretti, G. Cold and muscle performance. Int. J. Sport. Med. 1992, 1, 185–187. [Google Scholar] [CrossRef]
- Cowan, K.J.; Storey, K.B. Freeze-thaw effects on metabolic enzymes in wood frog organs. Cryobiology 2001, 43, 32–45. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Storey, K.B. Protein phosphatase type-1 from skeletal muscle of the freeze-tolerant wood frog. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 131, 27–36. [Google Scholar] [CrossRef]
- Boutilier, R.G.; St-Pierre, J. Adaptive plasticity of skeletal muscle energetics in hibernating frogs: Mitochondrial proton leak during metabolic depression. J. Exp. Biol. 2002, 15, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.; Heglund, N.C.; Cavagna, G.A. Energy transfer during stress relaxation of contracting frog muscle fibres. J. Physiol. 2001, 537, 923–939. [Google Scholar] [CrossRef]
- Nyitrai, M.; Rossi, R.; Adamek, N.; Pellegrino, M.A.; Bottinelli, R.; Geeves, M.A. What limits the velocity of fast-skeletal muscle contraction in mammals? J. Mol. Biol. 2006, 355, 432–442. [Google Scholar] [CrossRef]
- Saitongdee, P.; Becker, D.L.; Milner, P.; Knight, G.E.; Burnstock, G. Levels of gap junction proteins in coronary arterioles and aorta of hamsters exposed to the cold and during hibernation and arousal. J. Histochem. Cytochem. 2004, 52, 603–615. [Google Scholar] [CrossRef]
- Wallace, A.; Knight, G.E.; Cowen, T.; Burnstock, G. Changes in purinergicsignalling in developing and ageing rat tail artery: Importance for temperature control. Neuropharmacology 2006, 50, 191–208. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Rychkov, A.V.; Ziganshina, L.E.; Burnstock, G. Temperature dependency of P2 receptor-mediated responses. Eur. J. Pharmacol. 2002, 456, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.U.; Kamaliev, R.R.; Grishin, S.N.; Ziganshina, L.E.; Zefirov, A.L.; Burnstock, G. The influence of hypothermia on P2 receptor-mediated responses of frog skeletal muscle. Eur. J. Pharmacol. 2005, 21, 187–193. [Google Scholar] [CrossRef]
- Foldes, F.F.; Kuze, S.; Vizi, E.S.; Deery, A. The influence of temperature on neuromuscular performance. J. Neural. Transm. 1978, 43, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.J.; Raftery, M.A. Specific binding of ATP to extracellular sites on Torpedo acetylcholine receptor. Biochem 1993, 32, 7329–7333. [Google Scholar] [CrossRef] [PubMed]
- Giniatullin, R.A.; Sokolova, E.M. Modulating role of ATP in the neuromuscular junction. Russ. J. Physiol. 1998, 84, 1132–1138. [Google Scholar]
- Fu, W.M. Potentiation by ATP of the postsynaptic acetylcholine response at developing neuromuscular synapses in Xenopus cell cultures. J. Physiol. 1994, 477, 449–458. [Google Scholar] [CrossRef]
- Fu, W.M. Regulatory role of ATP at developing neuromuscular junctions. Prog. Neurobiol 1995, 47, 31–44. [Google Scholar] [CrossRef]
- Lu, Z.; Smith, D.O. Adenosine 5′-triphosphate increases acetyltholine channel-opening frequency in rat skeletal muscle. J. Physiol. 1991, 436, 45–56. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Khairullin, A.E.; Hoyle, C.H.V.; Grishin, S.N. Modulatory Roles of ATP and Adenosine in Cholinergic Neuromuscular Transmission. Int. J. Mol. Sci. 2020, 21, 6423. [Google Scholar] [CrossRef]
- Riley, D.A.; Van Dyke, J.M.; Vogel, V.; Curry, B.D.; Bain, J.L.W.; Schuett, R.; Costill, D.L.; Trappe, T.; Minchev, K.; Trappe, S. Soleus muscle stability in wild hibernating black bears. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, 369–379. [Google Scholar] [CrossRef]
- Eshpay, R.A.; Khairullin, A.E.; Karimova, R.G.; Nurieva, L.R.; Rizvanov, A.A.; Mukhamedyarov, M.A.; Ziganshin, A.U.; Grishin, S.N. Parameters of single and summated contractions of skeletal muscles in vivo and in vitro. Genes Cells 2015, 10, 123–126. [Google Scholar]
- McGeary, R.P.; Bennett, A.J.; Tran, Q.B.; Cosgrove, K.L.; Ross, B.P. Suramin: Clinical uses and structure-activity relationships. Mini Rev. Med. Chem. 2008, 8, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, G.; Braun, K.; Damer, M.; Ganso, M.; Hildebrandt, C.; Ullmann, H.; Kassack, M.U.; Nickel, P. Structure-activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr. Pharm. Des. 2002, 8, 2371–2399. [Google Scholar] [CrossRef] [PubMed]
- Savegnago, L.; Nogueira, C.W.; Fachinetto, R.; Rocha, J.B. Characterization of ATP and ADP hydrolysis activity in rat gastric mucosa. Cell Biol. Int. 2005, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kiffer-Moreira, T.; Fernandes Sampaio, M.E.; Alviano, D.S.; Axelband, F.; Cesar, G.V.; Cosentino-Gomes, D.; Rodrigues, M.L.; Nimrichter, L.; Vieyra, A.; Alviano, C.S.; et al. Biochemical characterization of an ecto-ATP diphosphohydrolase activity in Candida parapsilosis and its possible role in adenosine acquisition and pathogenesis. FEMS Yeast Res. 2010, 10, 735–746. [Google Scholar] [CrossRef]
- Salgado, A.I.; Cunha, R.A.; Ribeiro, J.A. Facilitation by P(2) receptor activation of acetylcholine release from rat motor nerve terminals: Interaction with presynaptic nicotinic receptors. Brain Res. 2000, 877, 245–250. [Google Scholar] [CrossRef]
- Fahim, M.A.; Holley, J.A.; Robbins, N. Topographic comparison of neuromuscular junctions in mouse slow and fast twitch muscles. Neuroscience 1984, 13, 227–235. [Google Scholar] [CrossRef]
- Larionov, I.; Larionov, A.L.; Nikolsky, E.E. Can the ampere forces be a factor of the ion channels’ lateral mobility? Mathematical modeling. Biol. Membr. 2018, 35, 3–15. [Google Scholar]
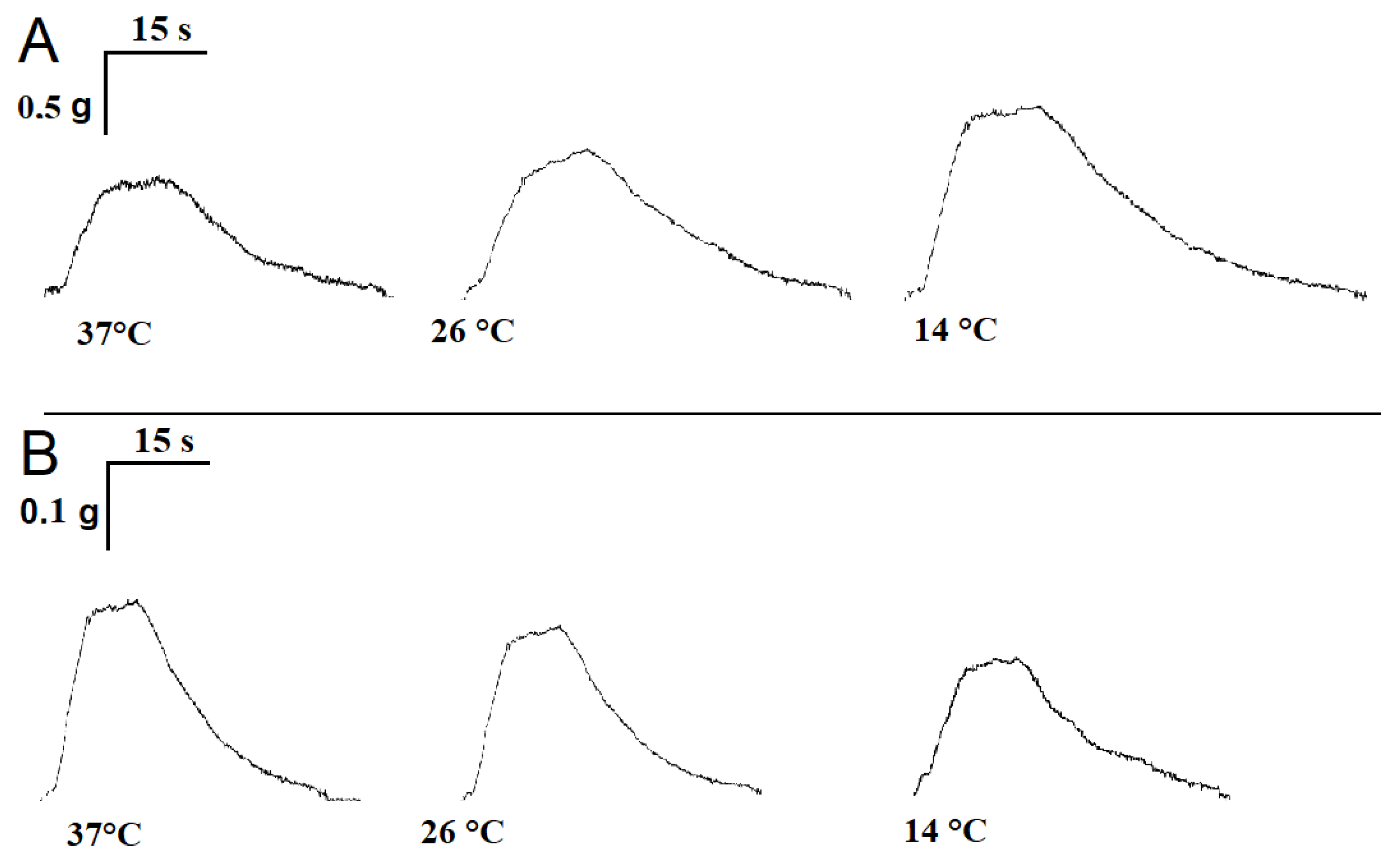

| Muscles | Substances | Temperature | ||
|---|---|---|---|---|
| 37 °C | 26 °C | 14 °C | ||
| m. soleus | Control | 100 | 112 ± 7.1 | 153 ± 5.9 * |
| ATP | 69 ± 8.0 # | 82 ± 7.7 # | 157 ± 9.5 * | |
| suramin + ATP | 103 ± 5.7 | 95 ± 6.4 | 107 ± 6.8 # | |
| m. EDL | Control | 100 | 79 ± 5.8 | 64 ± 7.2 * |
| ATP | 86 ± 5.5 # | 58 ± 7.9 # | 43 ± 6.2 *# | |
| suramin + ATP | 98 ± 7.1 | 97 ± 8.5 | 94 ± 9.1 # | |
| Muscles | Substances | 37 °C | 26 °C | 14 °C |
|---|---|---|---|---|
| m. soleus | Control | 12.5 | 10 | 7.5 |
| ATP | 12.5 | 7.5 | 5 | |
| suramin | 12.5 | 10 | 10 | |
| suramin + ATP | 12.5 | 10 | 7.5 | |
| m. EDL | Control | 17.5 | 17.5 | 15 |
| ATP | 17.5 | 17.5 | 15 | |
| suramin | 17.5 | 17.5 | 15 | |
| suramin + ATP | 17.5 | 17.5 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairullin, A.E.; Grishin, S.N.; Gabdrahmanov, A.I.; Ziganshin, A.U. Effects of ATP on Time Parameters of Contractility of Rats’ Slow and Fast Skeletal Muscles in Normal and Hypothermic Conditions. Muscles 2023, 2, 23-35. https://doi.org/10.3390/muscles2010003
Khairullin AE, Grishin SN, Gabdrahmanov AI, Ziganshin AU. Effects of ATP on Time Parameters of Contractility of Rats’ Slow and Fast Skeletal Muscles in Normal and Hypothermic Conditions. Muscles. 2023; 2(1):23-35. https://doi.org/10.3390/muscles2010003
Chicago/Turabian StyleKhairullin, Adel E., Sergey N. Grishin, Azat I. Gabdrahmanov, and Ayrat U. Ziganshin. 2023. "Effects of ATP on Time Parameters of Contractility of Rats’ Slow and Fast Skeletal Muscles in Normal and Hypothermic Conditions" Muscles 2, no. 1: 23-35. https://doi.org/10.3390/muscles2010003
APA StyleKhairullin, A. E., Grishin, S. N., Gabdrahmanov, A. I., & Ziganshin, A. U. (2023). Effects of ATP on Time Parameters of Contractility of Rats’ Slow and Fast Skeletal Muscles in Normal and Hypothermic Conditions. Muscles, 2(1), 23-35. https://doi.org/10.3390/muscles2010003






