Effects of Detraining on Muscle Strength and Hypertrophy Induced by Resistance Training: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Study Quality and Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Included Studies
3.2. Studies’ Characteristics
3.3. Quality and Risk of Bias Assessment
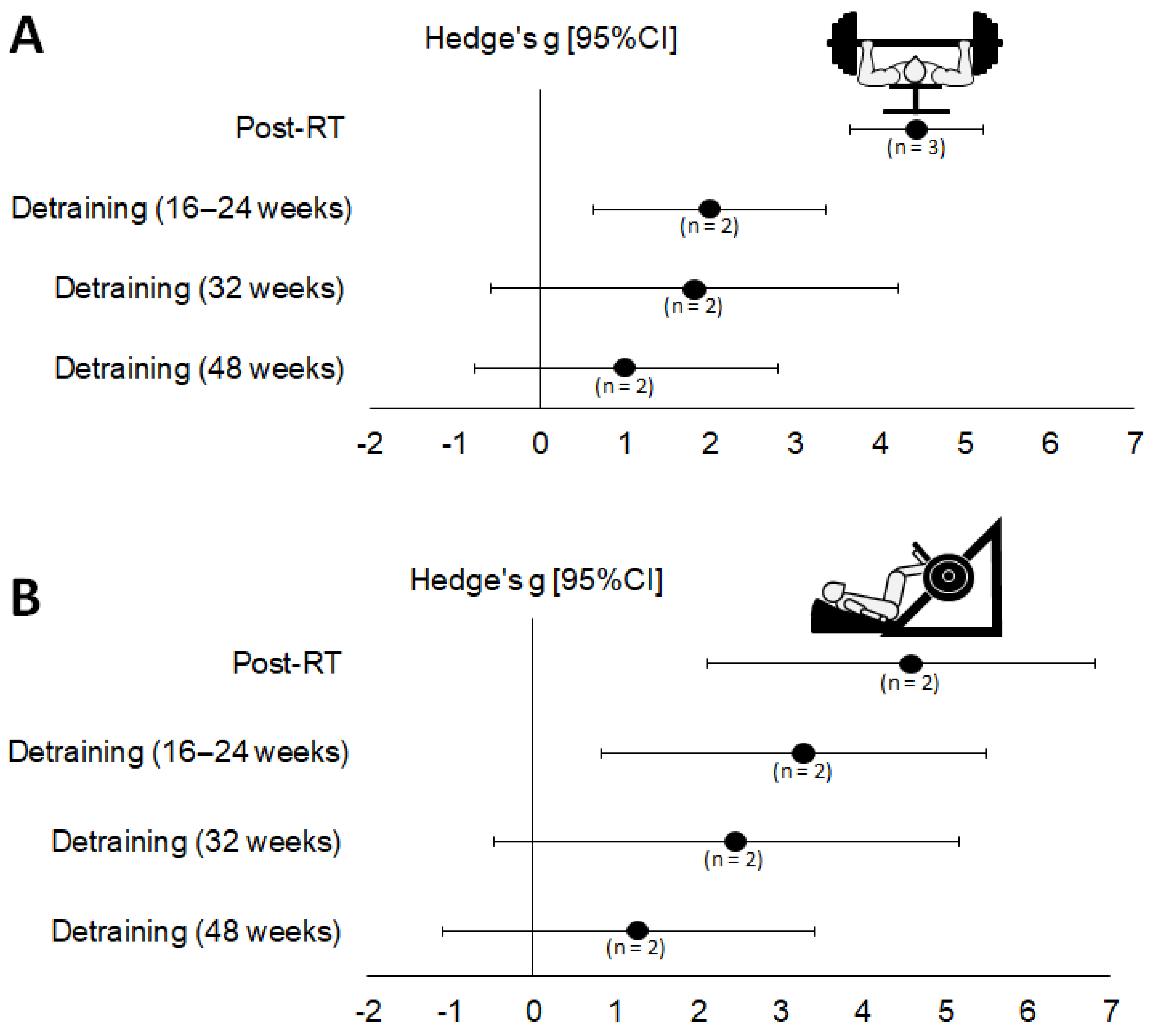
3.4. Meta-Analyses (1RM Strength)
3.5. Sensitivity Analyses and Publication Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Neural adaptation to resistance training: Changes in evoked V-wave and H-reflex responses. J. Appl. Physiol. 2002, 92, 2309–2318. [Google Scholar] [CrossRef]
- ACSM. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Alén, M.; Häkkinen, K. Physical health and fitness of an elite bodybuilder during 1 year of self-administration of testosterone and anabolic steroids: A case study. Int. J. Sports Med. 1985, 6, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Andersen, J.L.; Magnusson, S.P.; Aagaard, P. Neuromuscular adaptations to detraining following resistance training in previously untrained subjects. Eur. J. Appl. Physiol. 2005, 93, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Andersen, J.L.; Magnusson, S.P.; Suetta, C.; Madsen, J.L.; Christensen, L.R.; Aagaard, P. Changes in the human muscle force-velocity relationship in response to resistance training and subsequent detraining. J. Appl. Physiol. 2005, 99, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bell, Z.W.; Wong, V.; Spitz, R.W.; Chatakondi, R.N.; Viana, R.; Abe, T.; Loenneke, J.P. The contraction history of the muscle and strength change: Lessons learned from unilateral training models. Physiol. Meas. 2020, 41, 01TR01. [Google Scholar] [CrossRef]
- Blocquiaux, S.; Gorski, T.; Van Roie, E.; Ramaekers, M.; Van Thienen, R.; Nielens, H.; Delecluse, C.; De Bock, K.; Thomis, M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp. Gerontol. 2020, 133, 110860. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to meta-analysis. In Introduction to Meta-Analysis; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Brezhnev, Y.V.; Zaitsev, A.A.; Sazonov, S.V. To the analytical theory of the supercompensation phenomenon. Biophysics 2011, 56, 298–303. [Google Scholar] [CrossRef]
- Canepari, M.; Rossi, R.; Pellegrino, M.A.; Orrell, R.W.; Cobbold, M.; Harridge, S.; Bottinelli, R. Effects of resistance training on myosin function studied by the in vitro motility assay in young and older men. J. Appl. Physiol. 2005, 98, 2390–2395. [Google Scholar] [CrossRef]
- Carroll, T.J.; Selvanayagam, V.S.; Riek, S.; Semmler, J.G. Neural adaptations to strength training: Moving beyond transcranial magnetic stimulation and reflex studies. Acta Physiol. 2011, 202, 119–140. [Google Scholar] [CrossRef]
- Correa, C.S.; Baroni, B.M.; Radaelli, R.; Lanferdini, F.J.; Cunha, G.D.S.; Reischak-Oliveira, Á.; Vaz, M.A.; Pinto, R.S. Effects of strength training and detraining on knee extensor strength, muscle volume and muscle quality in elderly women. Age 2013, 35, 1899–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Counts, B.R.; Buckner, S.L.; Mouser, J.G.; Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Loenneke, J.P. Muscle growth: To infinity and beyond? Muscle Nerve 2017, 56, 1022–1030. [Google Scholar] [CrossRef]
- Dankel, S.J.; Bell, Z.W.; Spitz, R.W.; Wong, V.; Viana, R.B.; Chatakondi, R.N.; Buckner, S.L.; Jessee, M.B.; Mattocks, K.T.; Mouser, J.G.; et al. Assessing differential responders and mean changes in muscle size, strength, and the crossover effect to 2 distinct resistance training protocols. Appl. Physiol. Nutr. Metab. 2020, 45, 463–470. [Google Scholar] [CrossRef]
- Dankel, S.J.; Counts, B.R.; Barnett, B.E.; Buckner, S.L.; Abe, T.; Loenneke, J.P. Muscle adaptations following 21 consecutive days of strength test familiarization compared with traditional training. Muscle Nerve 2017, 56, 307–314. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Elliott, K.J. Effects of resistance training and detraining on muscle strength and blood lipid profiles in postmenopausal women. Br. J. Sports Med. 2002, 36, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatouros, I.G.; Kambas, A.; Katrabasas, I.; Nikolaidis, K.; Chatzinikolaou, A.; Leontsini, D.; Taxildaris, K. Strength training and detraining effects on muscular strength, anaerobic power, and mobility of inactive older men are intensity dependent. Br. J. Sports Med. 2005, 39, 776–780. [Google Scholar] [CrossRef] [Green Version]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke rehabilitation evidence-based review: Methodology. Top. Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef]
- Folland, J.P.; Williams, A.G. The adaptations to strength training: Morphological and neurological contributions to increased strength. Sports Med. 2007, 37, 145–168. [Google Scholar] [CrossRef]
- Graves, J.; Pollock, M.; Leggett, S.; Braith, R.; Carpenter, D.; Bishop, L. Effect of reduced training frequency on muscular strength. Int. J. Sports Med. 1988, 09, 316–319. [Google Scholar] [CrossRef]
- Griffin, L.; Cafarelli, E. Transcranial magnetic stimulation during resistance training of the tibialis anterior muscle. J. Electromyogr. Kinesiol. 2007, 17, 446–452. [Google Scholar] [CrossRef]
- Häkkinen, K.; Alen, M.; Kallinen, M.; Newton, R.U.; Kraemer, W.J. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur. J. Appl. Physiol. 2000, 83, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, K.; Alen, M.; Komi, P.V. Changes in isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol. Scand. 1985, 125, 573–585. [Google Scholar] [CrossRef]
- Häkkinen, K.; Komi, P. Electromyographic changes during strength training and detraining. Med. Sci. Sports Exerc. 1983, 15, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, K.; Komi, P.; Tesch, P. Effect of combined concentric and eccentric strength training and detraining on force-time, muscle fiber and metabolic characteristics of leg extensor muscles. Scand. J. Med. Sci. Sports 1981, 3, 50–58. [Google Scholar]
- Higgins, J.P.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Hortobágyi, T.; Houmard, J.; Stevenson, J.; Fraser, D.; Johns, R.; Israel, R. The effects of detraining on power athletes. Med. Sci. Sports Exerc. 1993, 25, 929–935. [Google Scholar] [PubMed]
- Houston, M.E.; Froese, E.A.; Valeriote, S.P.; Green, H.J.; Ranney, D.A. Muscle performance, morphology and metabolic capacity during strength training and detraining: A one leg model. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 51, 25–35. [Google Scholar] [CrossRef]
- Ivey, F.M.; Tracy, B.L.; Lemmer, J.T.; NessAiver, M.; Metter, E.J.; Fozard, J.L.; Hurley, B.F. Effects of strength training and detraining on muscle quality: Age and gender comparisons. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2000, 55, B152–B157. [Google Scholar] [CrossRef] [Green Version]
- Júnior, H.J.C.; Rodrigues, B.; de Oliveira Gonçalves, I.; Uchida, M.C. Effects of a short-term detraining period on muscle functionality and cognition of strength trained older women: A preliminary report. J. Exerc. Rehabil. 2017, 13, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Kalapotharakos, V.I.; Smilios, I.; Parlavatzas, A.; Tokmakidis, S.P. The effect of moderate resistance strength training and detraining on muscle strength and power in older men. J. Geriatr. Phys. Ther. 2007, 30, 109–113. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef]
- Krutki, P.; Mrówczyński, W.; Bączyk, M.; Łochyński, D.; Celichowski, J. Adaptations of motoneuron properties after weight-lifting training in rats. J. Appl. Physiol. 2017, 123, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, M.S.; Lin, L.L.C.; Yao, W.-J.; Ma, M.-C. Training and detraining effects of the resistance vs. endurance program on body composition, body size, and physical performance in young men. J. Strength Cond. Res. 2011, 25, 2246–2254. [Google Scholar] [CrossRef] [Green Version]
- Loenneke, J.P.; Dankel, S.J.; Bell, Z.W.; Buckner, S.L.; Mattocks, K.T.; Jessee, M.B.; Abe, T. Is muscle growth a mechanism for increasing strength? Med. Hypotheses 2019, 125, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lovell, D.I.; Cuneo, R.; Gass, G.C. The effect of strength training and short-term detraining on maximum force and the rate of force development of older men. Eur. J. Appl. Physiol. 2010, 109, 429–435. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattocks, K.T.; Buckner, S.L.; Jessee, M.B.; Dankel, S.J.; Mouser, J.G.; Loenneke, J.P. Practicing the test produces strength equivalent to higher volume training. Med. Sci. Sports Exerc. 2017, 49, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- McCarrick, M.J.; Kemp, J.G. The effect of strength training and reduced training on rotator cuff musculature. Clin. Biomech. 2000, 15, S42–S45. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods Jrsm. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- McMahon, G.; Morse, C.I.; Winwood, K.; Burden, A.; Onambélé, G.L. Circulating tumor necrosis factor alpha may modulate the short-term detraining induced muscle mass loss following prolonged resistance training. Front. Physiol. 2019, 10, 527. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Muscular characteristics of detraining in humans. Med. Sci. Sports Exerc. 2001, 33, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Roi, G.S.; Landoni, L.; Minetti, A.E.; Cerretelli, P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 59, 310–319. [Google Scholar] [CrossRef]
- Ogasawara, R.; Yasuda, T.; Ishii, N.; Abe, T. Comparison of muscle hypertrophy following 6-month of continuous and periodic strength training. Eur. J. Appl. Physiol. 2013, 113, 975–985. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Porter, M.M.; Nelson, M.E.; Singh, M.A.F.; Layne, J.E.; Morganti, C.M.; Trice, I.; Economos, C.D.; Roubenoff, R.; Evans, W.J. Effects of long-term resistance training and detraining on strength and physical activity in older women. J. Aging Phys. Act. 2002, 10, 260–270. [Google Scholar] [CrossRef]
- Rhea, M.R.; Alvar, B.A.; Burkett, L.N.; Ball, S.D. A meta-analysis to determine the dose response for strength development. Med. Sci. Sports Exerc. 2003, 35, 456–464. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Avelar, A.; Schoenfeld, B.J.; Ritti Dias, R.M.; Altimari, L.R.; Cyrino, E.S. Resistance training promotes increase in intracellular hydration in men and women. Eur. J. Sport Sci. 2014, 14, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.; Grgic, J. Evidence-based guidelines for resistance training volume to maximize muscle hypertrophy. Strength Cond. J. 2017, 40, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Shaver, L.G. Cross transfer effects of conditioning and deconditioning on muscular strength. Ergonomics 1975, 18, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shima, N.; Ishida, K.; Katayama, K.; Morotome, Y.; Sato, Y.; Miyamura, M. Cross education of muscular strength during unilateral resistance training and detraining. Eur. J. Appl. Physiol. 2002, 86, 287–294. [Google Scholar] [CrossRef]
- Smith, K.; Winegard, K.; Hicks, A.L.; McCartney, N. Two years of resistance training in older men and women: The effects of three years of detraining on the retention of dynamic strength. Can. J. Appl. Physiol. 2003, 28, 462–474. [Google Scholar] [CrossRef]
- Staron, R.S.; Leonardi, M.J.; Karapondo, D.L.; Malicky, E.S.; Falkel, J.E.; Hagerman, F.C.; Hikida, R.S. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J. Appl. Physiol. 1991, 70, 631–640. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taaffe, D.R.; Marcus, R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin. Physiol. 1997, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.T.; Henwood, T.R.; Nalls, M.A.; Walker, D.G.; Lang, T.F.; Harris, T.B. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology 2009, 55, 217–223. [Google Scholar] [CrossRef]
- Weir, J.P.; Housh, D.J.; Housh, T.J.; Weir, L.L. The effect of unilateral concentric weight training and detraining on joint angle specificity, cross-training, and the bilateral deficit. J. Orthop. Sports Phys. Ther. 1997, 25, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Wernbom, M.; Augustsson, J.; Thomee, R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007, 37, 225–264. [Google Scholar] [CrossRef]
- Westerblad, H.; Allen, D.G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J. Gen. Physiol. 1991, 98, 615–635. [Google Scholar] [CrossRef] [Green Version]


| Author (s) | Weeks | Days/Week | Exercises | Sets | Repetitions | Workload | Rest Interval | Supervision | Detraining Period |
|---|---|---|---|---|---|---|---|---|---|
| Andersen et al. (2005a) [27] | 12 | ~3× | Inclined leg press, hack squat isolated knee extension, and hamstring curl | 4× 4× 5× | 10–12RM 8–10RM 6–8RM | Adjusted based on RM range | NR | Yes | 12 weeks |
| Andersen et al. (2005b) [43] | 12 | ~3× | Inclined leg press, hack squat, isolated knee extension, and hamstring curl | 4× 4× 5× | 10–12RM 8–10RM 6–8RM | Adjusted based on RM range | NR | Yes | 12 weeks |
| Blocquiaux et al. (2020) [28] | 20 | 3× | Shoulder press, bent-over-row, abdominal crunches, bench press biceps curl, 45° leg press, 45° calf press, and leg extension | 2–3× | 8–15 (maximum effort in the last set) | ~65–80% of 1RM | NR | Yes | 12 weeks |
| Coelho Júnior et al. (2017) [45] | 22 | 2× | Squat on the chair, chest press, seated leg curl, frontal raise, calf raise, arm curl, triceps extension, and abdominal crunch | 3× | 8–10 | 5–6 out 10 in an adapted Borg scale (~70% of 1RM) | 1 min | Yes | 4 weeks |
| Elliott (2002) [30] | 8 | 3× | Leg press, bench press, knee extension, knee flexion, and lat pull-down | 3× | 8 | 80% of 1RM | 2 min | Yes | 8 weeks |
| Fatouros et al. (2005) [16] | 24 | 3× | Chest press, leg extension, shoulder press, leg curls, pull down, leg press, arm curls, and triceps extension Additional exercises: Abdominal crunches Low back extensions | LIST: 2–3× HIST: 2–3× 2–3× | 14–16RM 6–8RM 6–10 | 50–55% of 1RM 80–85% of 1RM | 3 min 6 min | NR | 16, 32, and 48 weeks |
| Graves et al. (1988) [31] | 10 | Group 1: 3× Group 2: 2× | Bilateral knee extensions | 1× | 7–10RM | Adjusted based on RM range | NR | Yes | 12 weeks |
| Häkkinen et al. (1985) [10] | 24 | 3× | Squats (concentric action) Squats (eccentric action) 3rd, 5th, and 6th months | NR | 1–10 3–5 | 70–100% of 1RM 100–120% of 1RM | NR | NR | 12 weeks |
| Häkkinen et al. (1981) [32] | 16 | 3× | Squats (concentric action) Squats (eccentric action) 3rd, 5th, and 6th months | NR | 1–6 1–2 (3–4 s) | 80–100% of 1RM (concentric action) 100–120% of 1RM (eccentric action) | NR | NR | 8 weeks |
| Houston et al. (1983) [44] | 10 | 4× | One leg knee extension and one leg press | 3× | 8–10RM | Adjusted based on RM range | NR | NR | 12 weeks |
| Kalapothara-kos et al. (2007) [32] | 10 | 3× | Leg extension, chest press, leg curl, latissimus pull down, arm curls, and triceps extension | 3× | 15 | 60% of 1RM | 2 min | NR | 6 weeks |
| Lo et al. (2011) [34] | 24 | 3× | Seated chest press, lat pull down, seated shoulder press, seated biceps curl, seated triceps extension, seated leg extension, lying leg curl, seated back extension, seated abdominal curl, and standing calf raise | 1× 2× | 10 4 | 75% of 1RM 90% of 1RM | NR | Yes | 24 weeks |
| Lovell et al. (2010) [35] | 16 | 3× | Inclined squat machine | 1× 3× 3× | 10 8 6–10 | 50% of 1RM 50% of 1RM 70–90% of 1RM | 2 min 2 min 2 min | NR | 4 weeks |
| McCarrick and Kemp (2000) [36] | 12 | 3× | Horizontal abduction, external rotation, scaption internal rotation, and external rotation | 3× | 8–12RM | Adjusted based on RM range | NR | NR | 12 weeks |
| McMahon et al. (2019) [37] | 8 | 3× | Back squat, leg press, leg extension, lunge, Bulgarian split squat, and Sampson chair | 3× 4× | 10 8 | 80% of 1RM 80% of 1RM (adjusted weight) | NR | Yes | 4 weeks |
| Porter et al. (2002) [38] | 1 year | 2× | Knee extension, lat pull-down, double-leg press, abdominal curl, and back extension | 3× | 8 | 80% of 1RM (adjusted weight) | NR | Yes | 1 year |
| Shaver (2007) [39] | 6 | 3× | Unilateral biceps curl | 3× | 10RM | 50%, 75%, and 100% of 10RM | 2 min | NR | 1, 4, 6 or 8 weeks |
| Shima et al. (2002) [40] | 6 | 4× | Unilateral plantar flexion | 3× | 10–12 | 70–75% of 1RM | 1–2 min | NR | 6 weeks |
| Smith et al. (2003) [41] | 2 years | 2× | Unilateral arm curl overhead, unilateral military press, bilateral supine bench press, bilateral triceps extensions, unilateral leg press, calf press, unilateral knee extensions, and unilateral dorsi- and plantar-flexion | 2–3× | 8–10 (upper) 10–12 (lower) | ≤80% of 1RM ≤80% of 1RM | NR | Yes | 3 years |
| Weir et al. (1997) [42] | 8 | 3× | Unilateral leg extension (concentric) | 3–5× | 6 | 80% of 1RM (adjusted weight) | NR | NR | 8 weeks |
| Study | Eligibility Criteria | Random Allocation | Concealed Allocation | Baseline Comparability | Blind Subjects | Blind Therapists | Blind Assessors | Adequate Follow-Up | Intention-to-Treat Analysis | Between-Group Comparisons | Point Estimates and Variability | PEDro Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al. (2005a) [27] | N | N | N | Y | N | N | N | N | N | N | Y | 2 |
| Andersen et al. (2005b) [43] | N | N | N | Y | N | N | N | Y | Y | N | Y | 4 |
| Blocquiaux et al. (2020) [28] | N | N | N | Y | N | N | Y | Y | N | Y | Y | 4 |
| Coelho Junior et al. (2017) [45] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Elliott (2002) [30] | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Fatouros et al. (2015) [16] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Graves et al. (1988) [31] | N | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Häkkinen et al. (1985) [10] | N | N | N | Y | N | N | N | Y | Y | N | Y | 4 |
| Häkkinen et al. (1981) [32] | N | N | N | Y | N | N | N | Y | Y | N | Y | 4 |
| Houston et al. (1983) [44] | N | N | N | Y | N | N | N | N | N | N | Y | 2 |
| Kalapotharakos et al. (2007) [33] | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Lo et al. (2011) [34] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Lovell et al. (2010) [35] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| McCarrick and Kemp (2000) [36] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| McMahon et al. (2019) [37] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Porter et al. (2002) [38] | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Shaver (2007) [39] | N | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Shima et al. (2002) [40] | N | Y | N | Y | N | N | N | Y | Y | N | Y | 5 |
| Smith et al. (2003) [41] | Y | N | N | Y | N | N | N | Y | Y | Y | Y | 5 |
| Weir et al. (1997) [42] | Y | N | N | Y | N | N | N | Y | Y | Y | Y | 5 |
| Period Analyzed and 1RM Strength Test Used | r = 0.5 | r = 0.7 | r = 0.9 | |||
|---|---|---|---|---|---|---|
| Hedges’ g (95% CI) | p | Hedges’ g (95% CI) | p | Hedges’ g (95% CI) | p | |
| Detraining period (16–24 weeks) | ||||||
| Chest press (n = 3) | ||||||
| Post-RT—Baseline | 3.57 [2.89; 4.24] | <0.001 | 4.43 [3.65; 5.22] | <0.001 | 6.68 [5.37; 8.00] | <0.001 |
| Detraining—Post-RT | −1.67 [−2.16; −1.19] | <0.001 | −2.10 [−2.62; −1.58] | <0.001 | −3.29 [−3.94; −2.64] | <0.001 |
| Detraining—Baseline | 1.60 [0.49; 2.72] | 0.005 | 1.99 [0.62; 3.36] | 0.004 | 2.98 [0.98; 4.98] | <0.001 |
| Leg press (n = 2) | ||||||
| Post-RT—Baseline | 3.64 [1.81; 5.47] | <0.001 | 4.47 [2.12; 6.82] | <0.001 | 6.35 [2.55; 10.15] | 0.001 |
| Detraining—Post-RT | −1.12 [−1.64; −0.61] | <0.001 | −1.43 [−2.05; −0.81] | <0.001 | −2.33 [−3.22; −1.43] | <0.001 |
| Detraining—Baseline | 2.56 [0.61; 4.51] | 0.010 | 3.16 [0.82; 5.50] | 0.008 | 4.60 [1.55; 7.66] | 0.003 |
| Detraining period (32 weeks) | ||||||
| Chest press (n = 2) | ||||||
| Post-RT—Baseline | 3.67 [2.88; 4.46] | <0.001 | 4.53 [3.48; 5.58] | <0.001 | 6.57 [4.62; 8.52] | <0.001 |
| Detraining—Post-RT | −2.29 [−2.90; −1.68] | <0.001 | −2.85 [−3.53; −2.16] | <0.001 | −4.28 [−5.15; −3.41] | <0.001 |
| Detraining—Baseline | 1.43 [−0.39; 3.25] | 0.124 | 1.81 [−0.59; 4.21] | 0.139 | 2.94 [−1.40; 7.28] | 0.184 |
| Leg press (n = 2) | ||||||
| Post-RT—Baseline | 3.64 [1.81; 5.47] | <0.001 | 4.47 [2.12; 6.82] | <0.001 | 6.35 [2.55; 10.15] | <0.001 |
| Detraining—Post-RT | −2.00 [−2.59; −1.41] | <0.001 | −2.52 [−3.15; −1.88] | <0.001 | −3.96 [−4.79; −3.14] | <0.001 |
| Detraining—Baseline | 1.85 [−0.39; 4.09] | 0.106 | 2.34 [−0.48; 5.16] | 0.104 | 3.73 [−0.65; 8.12] | 0.095 |
| Detraining period (48 weeks) | ||||||
| Chest press (n = 2) | ||||||
| Post-RT—Baseline | 3.67 [2.88; 4.46] | <0.001 | 4.53 [3.48; 5.58] | <0.001 | 6.57 [4.62; 8.52] | <0.001 |
| Detraining—Post-RT | −2.79 [−3.46; −2.12] | <0.001 | −3.51 [−4.28; −2.74] | <0.001 | −5.47 [−6.66; −4.29] | <0.001 |
| Detraining—Baseline | 0.80 [−0.61; 2.21] | 0.267 | 1.01 [−0.76; 2.79] | 0.263 | 1.57 [−1.22; 4.35] | 0.270 |
| Leg press (n = 2) | ||||||
| Post-RT—Baseline | 3.64 [1.81; 5.47] | <0.001 | 4.47 [2.12; 6.82] | <0.001 | 6.35 [2.55; 10.15] | <.0001 |
| Detraining—Post-RT | 2.34 [−2.97; −1.72] | <0.001 | −3.01 [−3.72; −2.31] | <0.001 | −5.12 [−6.11; −4.12] | <0.001 |
| Detraining—Baseline | 0.96 [−0.88; 2.80] | 0.308 | 1.16 [−1.09; 3.42] | 0.311 | 1.64 [−1.52; 4.79] | 0.309 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnação, I.G.A.; Viana, R.B.; Soares, S.R.S.; Freitas, E.D.S.; de Lira, C.A.B.; Ferreira-Junior, J.B. Effects of Detraining on Muscle Strength and Hypertrophy Induced by Resistance Training: A Systematic Review. Muscles 2022, 1, 1-15. https://doi.org/10.3390/muscles1010001
Encarnação IGA, Viana RB, Soares SRS, Freitas EDS, de Lira CAB, Ferreira-Junior JB. Effects of Detraining on Muscle Strength and Hypertrophy Induced by Resistance Training: A Systematic Review. Muscles. 2022; 1(1):1-15. https://doi.org/10.3390/muscles1010001
Chicago/Turabian StyleEncarnação, Irismar G. A., Ricardo B. Viana, Saulo R. S. Soares, Eduardo D. S. Freitas, Claudio A. B. de Lira, and João B. Ferreira-Junior. 2022. "Effects of Detraining on Muscle Strength and Hypertrophy Induced by Resistance Training: A Systematic Review" Muscles 1, no. 1: 1-15. https://doi.org/10.3390/muscles1010001
APA StyleEncarnação, I. G. A., Viana, R. B., Soares, S. R. S., Freitas, E. D. S., de Lira, C. A. B., & Ferreira-Junior, J. B. (2022). Effects of Detraining on Muscle Strength and Hypertrophy Induced by Resistance Training: A Systematic Review. Muscles, 1(1), 1-15. https://doi.org/10.3390/muscles1010001






