Abstract
Plastic materials are widely used for packaging due to their versatility and availability. Global production, mainly from petrochemicals, is estimated at 380 million tons, increasing annually by 4%. Packaging plastics have the shortest lifespan and contribute significantly to environmental pollution. Current production, use, and disposal of these plastics harm the environment, hu-mans, and ecosystems. Microplastics, (plastics particles ranging from 1 µm to 5 mm) formed through degradation, accumulate in ecosystems and the human body, including the brain. Bioplastics and biodegradable polymers from biological sources are a sustainable alternative; however, most production still relies on food crops, raising concerns about food security and sustainability. Utilizing organic wastes reduces production costs, lessens pressure on food systems, and supports waste management efforts. Cellulose, an abundant natural polymer, offers strong potential due to biodegradability, availability, and mechanical properties. This review explores extracting cellulose from banana pseudostem waste for packaging, high-lighting extraction and conversion methods and characterization via FTIR, TGA, SEM, XRD, and mechanical testing. FTIR confirmed the effective removal of lignin and hemicellulose, XRD revealed increased crystallinity corresponding to Type I cellulose, SEM showed a roughened fiber surface after alkaline treatment, and TGA indicated high thermal stability up to 250 °C. The goal is eco-friendly packaging by promoting agrowaste use. Further research should improve performance and scalability of cellulose-based bioplastics to meet industry needs and compete effectively with conventional plastics.
1. Introduction
Packaging is commercially defined as an encasement of a product designed to protect it from the external environment, prevent pilferage, provide statutory and commercial information, and attract consumers [1]. It separates products from external settings and serves purposes of containment, protection, convenience, and communication [2]. Packaging materials range from paper, glass, metals, wood, plastics, and composites. Among these, plastics are most commonly used due to their versatility and availability [3]. The global demand for plastics has driven widespread production and consumption [4], with production estimated to rise by 4% to reach 445.25 million tonnes by 2025 and 902–1124 million tonnes by 2050 [5]. Packaging represents the largest share of plastic use (31%), followed by construction (16.7%) and transportation (13.5%) [6]. Since 1950, about 6300 million tonnes of plastic waste has been generated, projected to rise from 353 Mt in 2019 to 1014 Mt by 2060 [7], constituting approximately 12% of global solid waste [8].
Plastics are long-chain man-made polymers that are lightweight, durable, and inexpensive [9]. They are classified as natural, semi-artificial, synthetic, thermoplastic, thermoset, and bio-plastics [10]. Conventional plastics, derived from petrochemicals, include polyvinylchloride (PVC), polypropylene (PP), polyethylene terephthalate (PET), polyethylene (PE), polyamide (PA) and polystyrene (PS), widely used in packaging due to high tear and tensile strength, gas barrier properties, heat sealability, and low cost [11,12]. Their inertness, durability, and moldability are desirable for packaging, yet these same properties contribute to environmental accumulation [13,14].
The production, use, and disposal of petroleum-based plastics are unsustainable. Packaging products often have the shortest working life of all industrial plastics, with single-use items lasting minutes before disposal [6]. This leads to landfill accumulation, environmental hazards, wildlife ingestion, entanglement, and chemical leaching [15]. In oceans, microplastics (<1–5 mm), formed by abrasion and UV degradation, enter the food chain and can be found in water, air, fish, salt, and even the human brain, which may contain up to 4800 mg of microplastics per gram, increasing by 50% between 2016 and 2024 [16,17]. Plastic pollution also economically impacts tourism, fishing, and shipping, while surging single-use plastics strain waste management systems [6].
The environmental burden of petrochemical plastics and fossil fuel depletion has driven interest in sustainable alternatives such as biocomposites for packaging [15,18,19]. Bioplastics, derived from renewable resources, integrate into natural cycles and reduce environmental footprints compared to petroleum-based polymers [20,21,22,23]. Differences between conventional plastics and bio-plastics are summarized in Table 1. This review emphasizes cellulose-based bioplastics, particularly those derived from banana pseudostem, as promising sustainable packaging materials within this broader context.

Table 1.
Distinction between conventional plastics and bioplastics.
Bioplastics currently represent about 1.5% of the ~414 million tonnes of plastics produced annually, with global production at 2.47 million metric tons in 2024 and projected to reach 5.73 million tonnes by 2029 [24,25]. Bioplastics have lower carbon footprints, reduce fossil fuel dependence, and can be recycled [26]. They are produced from proteins, polysaccharides, lipids, cellulose, and starch derivatives [27,28]. Cellulose, the most abundant natural polymer (~10 tons annually), is widely used in bioplastics [29,30]. First-generation feedstocks like sugarcane, corn, potato, wheat, and cassava are common but compete with food supply and arable land [31,32]. Lignocellulosic feed-stocks from agricultural residues, woody biomass, and invasive crops offer sustainable alternatives, containing 41–52% cellulose, 25–27% hemicellulose, 18–25% lignin, and 0.5–10% extractives and in-organics [33,34]. Cellulose can constitute up to 50 wt.% of lignocellulosic biomass [35].
Among various lignocellulosic feedstocks, banana pseudostem is a particularly promising and abundantly available residue from banana cultivation. Each hectare of banana plantation generates about 220 tons of pseudostem waste annually, which is often discarded after fruit harvest [36]. This biomass contains 60–74% cellulose, 12–18% hemicellulose, and 5–10% lignin, depending on cultivar and extraction method [37]. Valorization of this agro-waste not only mitigates environmental disposal issues but also provides a sustainable feedstock for high-quality cellulose suitable for bioplastic development. Therefore, this review emphasizes the extraction, characterization, and potential applications of cellulose derived from banana pseudostem for sustainable bioplastic production. Cellulose-based biopolymers are valued for transparency, gloss, colorability, low electrostatic buildup, mechanical and chemical balance, and resistance to oils and hydrocarbons [38]. However, weak hydrogen bonds reduce strength and flexibility, and cellulose is water-sensitive with poor thermal stability. These issues can be mitigated by blending with other polysaccharides like pectin and chitosan [33].
This review discusses the production techniques, properties, and packaging applications of cellulose-based biopolymers, emphasizing banana pseudostem waste conversion. Improper disposal of banana pseudostem generates greenhouse gases, yet the material is abundant and suitable for bioplastic films, promoting sustainable development and climate mitigation. Literature on cellulose-based bioplastics from banana pseudostem was analyzed to provide insights into cellulose pulp extraction and bioplastic synthesis.
Objectives
The main objective is to provide information on valorizing agricultural waste as eco-friendly packaging materials. Specific objectives include: (1) analyzing sources and properties of cellulose from lignocellulose waste, (2) identifying methods and optimal conditions for cellulose isolation from banana pseudostem and biopolymer synthesis, and (3) highlighting research gaps to inform future cellulose-based bioplastics development.
2. Synthesis of Key Literature on Bioplastics and Identified Gaps
This section presents a synthesis of reviewed research on bioplastics. A comprehensive summary of recent studies on bioplastics, including key findings, research gaps, and recommendations, is provided in Supplementary Material, Table S1 [6,8,12,25,27,29,33,39,40,41,42,43,44,45,46,47,48,49].
Three common fiber extraction techniques (manual, mechanical, and chemical) result in high waste and low yield. Mechanical methods cause fiber breakage, and chemical methods can be costly and destructive [50]. Microwave-assisted alkali pre-treatment may optimize extraction time [1]. Sophisticated characterization techniques are often expensive, affecting final bioplastic costs [51].
Bioplastics face challenges such as high production cost, poor mechanical and barrier properties, and low transparency when reinforced with fibers like banana pseudostem [14,44]. Research should focus on improving functionality through property optimization and blending cellulose with polysaccharides like pectin and chitosan to enhance flexibility, stability, and transparency [25]. Refining delignification methods can increase cellulose yield and purity, supporting scalable, high-quality bioplastic production [52,53]. End-of-life (EOL) assessment, life-cycle analysis (LCA), and land use change (LUC) studies remain limited and should be expanded to confirm sustainability and eco-friendliness [41,54]. In summary, the synthesis of recent studies indicates that future research should prioritize: (1) valorization of banana pseudostem waste as a high-cellulose renewable feedstock for bioplastic production, supporting circular-economy principles; (2) optimization of mechanical, thermal, and barrier properties through advanced blending and modification strategies to improve competitiveness with conventional plastics; (3) development of cost-effective and scalable extraction and processing techniques to enhance cellulose purity and reduce environmental impact; and (4) comprehensive sustainability assessment using LCA and EOL evaluations to validate environmental benefits. Collectively, these focal points capture the major research directions emerging from the literature and form the conceptual foundation for the subsequent sections of this review. 3. Types and classification of bioplastics.
This section classifies major types of bioplastics relevant to cellulose-based systems and their significance for sustainable packaging. Bioplastics are broadly grouped into three types: (1) biodegradable and bio-based, (2) biodegradable and petroleum-based, and (3) non-biodegradable and petroleum-based. Similarly, [55] categorized bioplastics with examples in each type, as shown in Table 2.

Table 2.
Types of Bioplastics [55].
Not all biodegradable plastics are bio-based; for example, PAT and PCL from fossil fuels are biodegradable, while not all bio-based materials are biodegradable [25]. Bio-based plastics are wholly or partly derived from renewable carbon sources like plant matter or biomass, whereas partially bio-based (hybrid) plastics combine renewable and fossil-based carbon [39,42]. Based on biodegradability and bio-based content, bioplastics are classified as: (1) drop-in bioplastics (bio-based or partly bio-based but non-biodegradable), (2) bio-based non-drop-in bioplastics, and (3) fossil-based non-drop-in bioplastics. Non-drop-ins are further grouped by origin into (i) vegetable- or animal-derived polysaccharides and proteins, (ii) microorganism-based polymers, and (iii) other polymers [39]; [38]. Drop-in bioplastics constitute a major sector of global bioplastics production. Biodegradable materials degrade biologically during composting into CO2, water, inorganic compounds, and biomass without leaving toxic residues [39]. Other classification systems based on origin (Figure 1) divide bioplastics into: natural polymers from biomass (e.g., starch- or cellulose-based), chemically synthesized bioplastics from renewable sources (e.g., PLA, bio-PE, bio-nylons, bio-polyurethanes), and synthetic polymers from microbial fermentation (e.g., PHAs) [8,11,25,29,39]. Among these bioplastic families, cellulose-based materials, particularly those extracted from banana pseudostem, represent a promising group with competitive mechanical and barrier properties compared with other bio-based polymers.
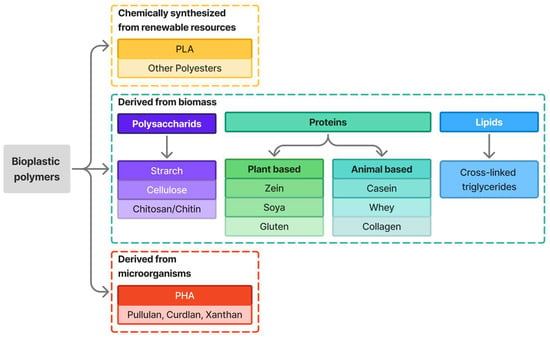
Figure 1.
Classification of bioplastics based on origin [11].
Bioplastics from agro-polymers are made from natural polymers like polysaccharides (starch, cellulose, pectins, hemicellulose) and proteins (casein, zein, gluten, gelatin) [8]. Further classification by natural feedstock is shown in Table 3.

Table 3.
Examples of bioplastics and their natural source.
2.1. Properties and Application of Bioplastics
Bioplastics’ properties vary by packaging application, spanning food, consumer electronics, cosmetics, vehicles, agriculture, toys, textiles, and other growing markets [43]. Desirable properties for food packaging include mechanical, optical, thermal, antimicrobial, eco-friendly, and barrier capabilities [58]. In particular, cellulose-based bioplastics, such as those derived from banana pseudostem fibers, combine high transparency and stiffness with renewable origin and compostability, making them strong candidates for sustainable packaging films. Mechanical and thermal properties protect products during storage, while transparency allows easy identification [14]. Gas barrier properties help maintain internal package composition, extending shelf life, and antimicrobial properties prevent contamination [58]. Nevertheless, cellulose-based materials often require chemical modification or blending to overcome intrinsic brittleness and moisture sensitivity, areas actively studied to enhance packaging performance. Biodegradability reduces carbon emissions during production and disposal. However, [14] noted limitations in mechanical and barrier properties and high production costs, highlighting the need for research to optimize performance and functionality.
2.2. Advantages and Disadvantages of Bioplastics from Natural Sources
Biopolymers from renewable resources possess biodegradability and compatibility advantages that drive their growing use in packaging [12,43]. Despite annual growth in bioplastic use, full replacement of conventional plastics faces challenges. Some biopolymers have high water vapor and oxygen permeability, fragility, low thermal stability, poor mechanical properties, low processability, difficult heat sealability, brittleness, low melt strength, and poor impact resistance. High production costs and disposal/recycling issues further hinder use [8,25]. Improper disposal may cause soil and water pollution and contribute to global warming. Biodegradation can take years under suboptimal conditions of temperature, microorganisms, and humidity [12]. Table 4 lists advantages and disadvantages of natural biopolymers versus synthetic polymers.

Table 4.
Advantages and disadvantages of natural and synthetic polymers. Source: [52].
Despite limitations, biopolymers are widely used in packaging, driving research to improve functionality and develop viable industrial production processes. Bioplastics in nanoparticles or bio-nanocomposites enhance barrier properties, helping meet demand for biodegradable plastics with required material performance [59].
3. Properties of Cellulose and Cellulose Derivatives for Packaging
This section discusses cellulose structure and its main properties in bioplastics production. According to [39], starch, cellulose, pectin, and animal or plant proteins such as casein and gluten are common feedstocks for agro-polymer-based bioplastics. Literature indicates that starch and cellulose are the most widely used renewable resources for biopolymer and biodegradable plastics. A study by [60] found that starch-based food packaging contains 32% starch, 27% cellulose, 10% chitosan, and 31% other materials. Weber et al. [29] reported that commercially used biobased food packaging materials are largely cellulose-based, partly because cellulose does not compete with the food industry, unlike starch [61]. Cellulose is extensively used in packaging due to its unique structure and properties [62] and is one of the most abundant naturally occurring organic materials, with an estimated annual production of 10 tons [29].
Cellulose, with the chemical formula (C6H10O5)n and molecular structure shown in Figure 2 [63], exhibits hierarchical structural organization from nanoscale to macroscopic fibril aggregates, fibrils, nanocrystallites, and disordered domains [64,65]. Each glucose residue contains three hydroxyl groups, and the degree of polymerization (DP) varies by source and extraction process. Trache et al. and Poulose et al. reported DP ranges of 10,000–15,000 [19,50], whereas [61] reported 1400–4750, and Tajeddin reported up to 650 [66]. Hallac and Ragauskas [67] found DP values between 925 and 5500 from native wood and non-wood cellulose. Longer chains with more hydrogen bonds are less hydrolyzable, while shorter chains are more enzymatically reactive [67]. Insoluble cellulose has a DP of 100–20,000, and soluble cellulose has 2–12, with chains <6 being soluble and 6–12 slightly soluble [68]. Cellulose is categorized into α (DP 600–1500, insoluble), β (DP 15–90, soluble), and γ (DP <15, soluble) based on DP and solubility [69]. In bioplastics, a moderate DP (~500–800) balances mechanical strength and processability [70].
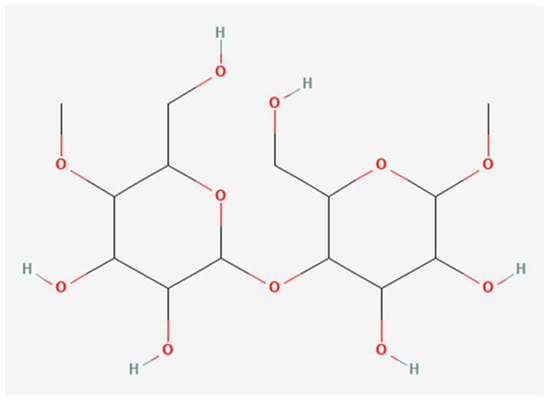
Figure 2.
Molecular structure of cellulose. Red color: Oxygen atoms (present in hydroxyl and ether groups). Source: [63].
Cellulose is also classified by source: wood-based (WC), plant-based (PC), bacteria-based (BC), algae-based, and tunicate-based, with PC and WC most common [64]. Due to its infusibility and insolubility, cellulose is converted into derivatives to improve processability [29]. Derivatization depends on source, maturity, pretreatment, processing, and reaction conditions. Common derivatives include ethylcellulose, hydroxypropyl cellulose (HPC), carboxymethyl cellulose (CMC), cellulose acetate (CA), methyl cellulose (MC), hydroxyethyl cellulose (HEC), cellulose nitrate, hydroxyethyl cellulose propionate, and hydroxypropyl methyl cellulose (HPMC) [27,30,44,45,71]. These derivatives have applications in pharmaceuticals, biomedical, food, automotive, and other industries [72]. Cellulose acetate, a tough, clear, flexible plastic with chemical resistance, is most widely used [25].
To reduce high commercial CMC costs [8,27], sugarcane bagasse has been used as raw material for plastic films. Extracted CMC shows strong barrier properties, good mechanical performance, degradability, non-toxicity, and availability. Economical extraction methods are needed for continuous supply [73]. Moura et al. emphasized environmentally friendly extraction to overcome difficulties in cellulose modification due to limited solvent availability. Study by [48] explored cotton and sugarcane bagasse cellulose for magnetic bioplastics in optical and magnetic applications. Cocoa pod husk and bagasse were used to produce moisture-resistant cellulose-based food packaging with 25% fiber and 75% cellulose [33]. Regenerated cellulose (RC) is obtained by dissolving insoluble cellulose and recovering it using various methods [57].
Cellulose exists as a mixture with hemicellulose and lignin (Figure 3) [25]. It resists strong alkali (17.5 wt.%) but is hydrolyzed by acids to water-soluble sugars and resists oxidation. Hemicellulose is hydrophilic, soluble in alkali, and acid-hydrolyzable, forming the supportive matrix for cellulose microfibrils. Lignin resists acids, dissolves in hot alkali, oxidizes easily, and condenses with phenol. Native cellulose is not plastic but can be modified into plastic via various techniques [25].
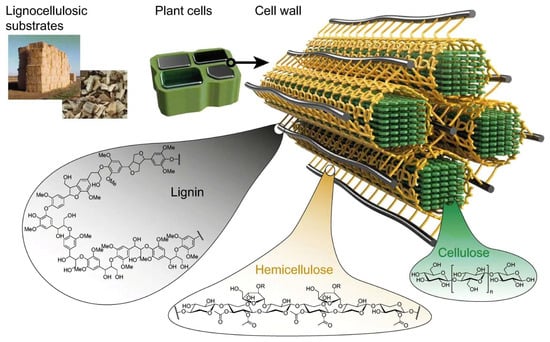
Figure 3.
Lignocellulosic material complex [74]. Published under CC BY 4.0 http://creativecommons.org/licenses/by/4.0/ (accessed on 15 August 2025). No changes were made.
Plant-derived cellulose is a suitable alternative to petroleum feedstocks due to mechanical, physicochemical, and biological properties, along with eco-friendliness. Desirable cellulose properties include biocompatibility, biodegradability, barrier resistance, mechanical strength, thermal stability, low weight, water absorbance, porosity, antimicrobial activity, crystallinity, solubility, and sensory qualities [1,75]. Cellulose-based packaging does not negatively affect pharmaceuticals and is safe for human use, and it can incorporate natural antimicrobial and antioxidant agents for food packaging [5]. Its strong hydrophilicity and low solubility in water and organic solvents limit some applications [76]. Table 5 summarizes cellulose strengths and limitations in packaging.

Table 5.
Strengths and limitations of cellulose in packaging [77].
Solutions to overcome cellulose limitations include incorporation of resins, waxes, reinforcing agents (clay, nanocellulose), surfactant coatings, blending with other biopolymers (gelatin, zein), and chemical modifications (acylation, esterification, grafting, silylation) [77]. Citric acid has been used to reduce water permeability [37], and hydrophobic compounds such as fatty acids can improve moisture barriers [55]. Plasticizers like glycerol enhance mechanical properties [41].
Nanocellulose offers improved characteristics, including high mechanical stability and transparency, with films from fibers 15–20 nm thick soluble in water. Biocomposites with nanofibers increase moisture resistance due to more hydrophobic compounds [8]. Nanocellulose fibers are produced via acid hydrolysis or mechanical grinding. Natural fibers or inorganic fillers further improve barrier, thermal, optical, and mechanical properties. With suitable techniques and materials, bioplastics can complement conventional plastics and offer multiple benefits [43].
3.1. Sources of Cellulose
Cellulose is the most abundant natural organic compound on Earth, with an estimated annual production of 180 billion tons, and is the main polysaccharide in plant cell walls [8]. It is primarily obtained from woody and non-woody residues, tunicates, bacteria, and algae [19]. Lignocellulosic fibers from sources such as sugarcane bagasse, rice straw, flax, kenaf, hemp, and forest wood are widely used for composite reinforcement.
Readily available non-woody cellulose sources include agricultural residues, factory and food wastes, water plants, grasses, rice and wheat bran, sugarcane bagasse, cereal husks, corn kernels, bacterial cellulose, and fruit and vegetable peels [62]. These renewable fibers are valued in bioplastic production for their low cost, low density, adequate strength, recyclability, and biodegradability [66]. For example, cellulose fibers from sugarcane, apple, and orange juice residues have been used to produce non-toxic, naturally biodegradable films [44,78].
However, reliance on 1st generation feedstocks can threaten food security due to high land demand and may contribute to eutrophication, ecotoxicity, and water consumption issues [70]. These challenges can be mitigated by using lignocellulosic agricultural biomass, which expands bio-based packaging production while minimizing climate impacts [27,58]. Agricultural residues, rich in cellulose, represent cost-effective renewable feedstocks for bioplastics [25].
Cellulose derived from such biomass improves barrier performance, mechanical and thermal stability, and processability of bioplastic films [8]. Utilizing lignocellulosic residues also supports circular-economy goals by reducing open-field burning and environmental pollution [70]. Among these residues, banana pseudostem offers particularly high cellulose content and low lignin levels, making it an efficient raw material for extraction and subsequent bioplastic synthesis. The choice of feedstock depends on desired film properties, resource availability, and economic viability [61]. Although several lignocellulosic sources have been explored, many agricultural wastes remain under-investigated [25].
3.2. Viability of Banana Pseudo Stem Waste for Cellulose Extraction
Natural fibers such as sisal, abaca, banana, kenaf, hemp, and jute have been tested as reinforcements in plastics. Among these, banana fibers (BF) from the pseudostem exhibit high specific tensile strength, greater than sisal, hemp, or jute, and comparable only to glass fibers [44]. Using banana pseudostem for biobased plastics aligns with the concept of waste valorization, defined by [79] as converting waste into useful products such as chemicals, materials, or fuels. This relies on the idea that residues still contain untapped polymeric substances that can be converted into valuable forms.
Banana is the second most important fruit crop globally, grown in over 130 countries [80], producing 116.78 million tonnes per year [37]. Each plant produces a single banana bunch, with a rhizomatous underground stem from which leaves arise, forming the pseudostem. Figure 4 shows the morphological parts of the banana plant, including vegetative structures (leaf sheath, pseudostem, midrib, leaf) and reproductive structures (inflorescence and fruit).
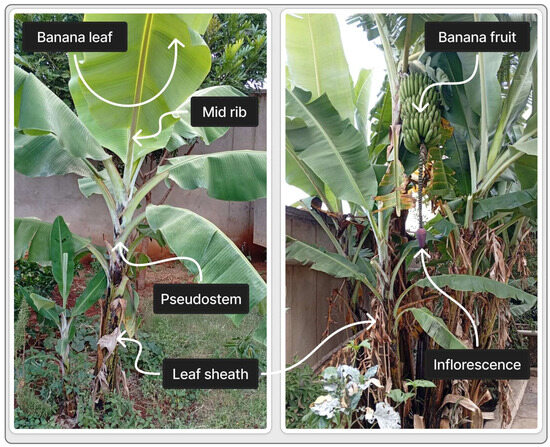
Figure 4.
Morphological parts of the banana plant (left: key vegetative structures, right: reproductive structures).
The banana stem is an abundant but underutilized cellulose source. Pseudostems and leaves, comprising about 88% of the plant’s weight, are typically discarded [45]. Each harvested banana bunch generates one pseudostem waste, which contains high lignocellulosic content, making it suitable for extracting cellulose and other value-added chemicals. Figure 5 illustrates the process: harvesting the pseudostem, separating outer sheaths, mechanically decorticating fibers, and processing them to obtain purified cellulose. Besides bioplastics, banana pseudostem has multiple applications, as shown in Figure 6, with percentages indicating the proportion of each component in the total biomass. The waste material fraction shown in the diagram refers to sap and scutcher residue generated during fiber extraction, which can be further utilized for products such as mordants, liquid fertilizers, compost, and vermicompost.
Thus, utilizing banana plants and their residues offers a sustainable way to increase cellulose derivative production and add value to agricultural waste [81].
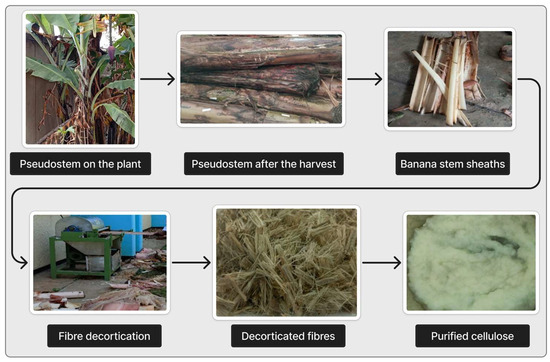
Figure 5.
Stepwise process for cellulose extraction from banana pseudostem.
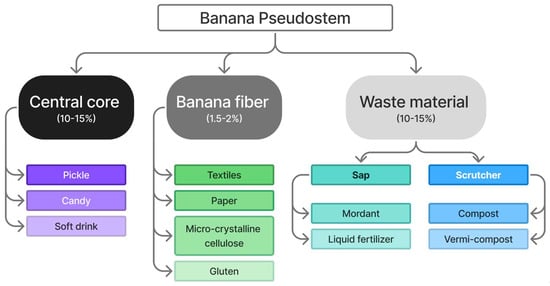
Figure 6.
Application pathways of the various components of the banana pseudostem [82].
4. Isolation and Purification of Cellulose from Banana Pseudostem Waste
Extraction and purification of cellulose from banana pseudostem involve a sequence of interrelated operations designed to separate cellulose from non-cellulosic components such as lignin, hemicellulose, and extractives. The process generally includes degumming or conditioning, pretreatment, pulping or hydrolysis, bleaching, and final purification. Each step aims to enhance cellulose purity, crystallinity, and functionality for use in bioplastics and packaging applications.
Cellulose applications in packaging can be grouped into three main areas. First, cellulose can be extracted from plants and used directly in composites. Fiber extraction can be performed mechanically, chemically, biologically, or using a combination of these methods [83]. Second, cellulosic bioplastics such as cellulose acetate can be produced, representing prime examples of biopolymers from renewable resources. Third, cellulose can be processed into coating materials and edible or non-edible films [29].
Although procedures vary, standard cellulose isolation involves fiber extraction or stem preparation, drying and sizing, pretreatment/delignification, bleaching, and hydrolysis. Yang at al. [4] proposed a four-step method: (1) sample preparation, (2) removal of extractives, (3) preparation of holocellulose (removal of lignin), and (4) preparation of α-cellulose (removal of hemicellulose). Lignocellulosic sources require elimination of non-cellulosic components, such as fats, tannins, resin, rosin, free sugars, flavonoids, terpenoids, terpenes, fatty acids, and waxes, followed by delignification and bleaching to obtain high-quality cellulose [82].
For banana pseudostem, cellulose extraction begins within five days after fruit harvesting. Fibers are obtained from the sheaths either manually or mechanically. Manual extraction uses sharp tools such as knives, blades, or broken crockery, producing high-quality fibers with minimal debris but is labor-intensive and low-yield [84]. Mechanical extraction is preferred. Alternatively, banana stems can be washed, chopped, dried, and ground to reduce size, though this yields lower cellulose compared to decorticated fibers [85]. The outer and middle parts of the pseudostem provide higher quantity and quality fibers, while the inner core yields fewer and weaker fibers due to brittleness, making peeling difficult [82,86].
4.1. Chemical Composition of Banana Pseudostem
Determining the composition of lignocellulosic biomass is essential for optimizing cellulose extraction, as varying amounts of cellulose, hemicellulose, and lignin in different sources affect extraction efficiency. Understanding biomass composition also guides the selection of pretreatment methods, improving yield and sustainability [53].
Banana PS composition varies with plant species, cultivation conditions, and extraction methods. The biomass contains cellulose, hemicellulose, lignin, and extractives. Hemicellulose in the cell wall, part of cellulose and holocellulose, dissolves and degrades easily in acid or alkaline solutions [87]. Hemicellulose content is determined as the difference between holocellulose and α-cellulose [49,88,89]. α-Cellulose represents the high-molecular-weight, crystalline, insoluble fraction of true cellulose [90].
Extractives are inorganic and organic substances removable by solvents, contributing to ash content. They include oils, fats, fatty acids, waxes, terpenes, terpenoids, flavonoids, rosin, resin, tannins, and free sugars, comprising 2–10% of biomass, partly soluble in water and organic solvents, and can challenge pulping if not removed [1].
Reported cellulose content in banana pseudostem varies: [78] reported 43.6% cellulose, 14% hemicellulose, 11% lignin, and 31.4% other substances (pectin, waxes). Reference [63] recorded 60–65% cellulose, 6–19% hemicellulose, 5–10% lignin, 3–5% pectin, 1–3% ash, and 3–6% extractives. Reference [91] found 81.8% total cellulose, 18–19% hemicellulose, 11% lignin, 41.9% residual gum, and 3–5% pectin. Cellulose content ranges from 32.4 to 64.0- wt% depending on recovery method [37], with 74.37% reported by [43,92]. Table 6 presents average chemical composition values of banana pseudostem from various studies.

Table 6.
Chemical composition of banana pseudo-stem from different studies. Values vary depending on banana species, cultivation region, and extraction method (data modified from [82]).
4.2. Methods of Cellulose Isolation and Purification
Common extraction methods and conversion processes include acid or alkaline hydrolysis, oxidation, ball milling, organosolv, deep eutectic solvents, steam explosion, enzymatic pretreatment, ultrasonication, extrusion, microwave-assisted treatment, etherification, esterification, or combinations thereof [46]. Extractives are removed via solvent extraction (ethanol-benzene, acetone-alcohol, ethanol-water, toluene-ethanol, etc.) [1]. Lignin and hemicellulose are removed through hydrolysis and bleaching, using agents such as sodium hypochlorite and hydrogen peroxide [82]. Table 7 summarizes commonly used methods.

Table 7.
Methods/techniques for cellulose isolation [53].
Organosolv processing is considered a sustainable alternative to conventional pulping for non-wood feedstocks due to low investment cost, non-polluting solvents, high pulp yield, and strong fiber quality [97]. The choice of isolation process depends on the target polymer, feedstock, and desired properties [61]. Factors affecting extraction include material type, solvent choice, temperature, pressure, duration, agitation, pH, particle size, and reagent concentration [98]. Statistical optimization approaches such as Central Composite Design, Box-Behnken, and Plackett-Burman designs have been applied to improve efficiency [90,99].
For banana fibers, alkaline and acid hydrolysis methods follow the sequence of sample collection, drying, delignification, bleaching, and hydrolysis [80].
4.2.1. Degumming/Conditioning
The decorticating process produces both fibers and non-fibrous materials known as gums (30–35%) [82]. Degumming removes these impurities by washing, chopping, and refluxing with ethanol and deionized water to remove extractives, thereby increasing the available surface area for further processing [53].
4.2.2. Pretreatment
Pretreatment aims to produce cellulose with high purity and reduced crystallinity by solubilizing lignin and hemicellulose, breaking down structural complexities, and removing impurities [53]. Alkali pretreatment removes hemicellulose, lignin, and other substances such as waxes [100]. During this step, cellulose undergoes structural alteration and increased crystallinity [19].
Both mild (soap, sodium carbonate) and strong (NaOH) alkalis are used to enhance solubility and thermal stability [78,101]. Hot alkali treatments have been used by [86] and by [7], who applied 20 mL of 5% NaOH per gram of biomass at 90 °C for 1 h, followed by water washing for neutralization. Similar techniques were used by [82,85,89,102]. Green pretreatments such as organosolv, enzymatic, deep eutectic solvents, or microwave-assisted systems provide sustainable alternatives [99,101].
4.2.3. Hydrolysis/Pulping
Hydrolysis is the main mechanism for converting polymers into monomers or intermediates, facilitating lignin removal [103]. Hydrolysis techniques include alkali, acid, enzymatic, organosolv, or combinations of these. The process involves cooking lignocellulosic material in an alkali solution under controlled temperature, pressure, and time. Chemicals such as potassium permanganate, benzoyl chloride, and stearic acid have also been used [78], with common alkalis including sodium hydroxide, ammonia, sodium carbonate, and calcium hydroxide [53].
Hot caustic extraction (HCE) has long been used to produce dissolving pulps, while cold caustic extraction (CCE) is applied when high-purity cellulose is required, such as for cellulose acetate [87,90]. Alkali treatment ionizes hydroxyl groups on cellulose to alkoxide, reducing surface hydroxyls, increasing surface roughness and hydrophobicity, which enhances adhesion with polymer matrices [19]. This method efficiently removes amorphous/non-cellulosic components [104].
Acid pretreatment is also critical for cellulose isolation, dissolving hemicellulose and lignin while leaving cellulose intact. Both mineral acids (HCl, H3PO4, H2SO4) and organic acids (acetic, citric, formic) are used [53]. Formic and acetic acids effectively break lignin α-ether bonds at low to moderate temperatures and pressures, with formic acid acting as a chelating agent for metal ions such as Ca, Mg, and Fe [23,70]. Formic acid selectively removes hemicellulose while minimizing cellulose dissolution [105]. Ethylenediaminetetraacetic Acid (EDTA) has also been applied to prevent cellulose loss.
The goal of pulping is to obtain high-quality dissolving-grade pulp with clear specifications, including α-cellulose content, alkali solubility, degree of DP, molecular weight distribution, accessibility, and reactivity [87]. High-purity cellulose (>90%), low lignin and extractives (<4%), and minimal hemicellulose (<2.8% for cellulose acetate) are essential. DP is influenced by both biomass source and extraction method.
Pulping techniques are classified as mechanical, thermal, semi-chemical, or fully chemical. Chemical pulping methods include Kraft, sulfite, soda, and organosolv, which degrade lignin and hemicellulose into water-soluble molecules without depolymerizing cellulose fibers. Hybrid techniques such as thermo-mechanical pulping (TMP) and chemical thermo-mechanical pulping (CTMP) combine chemical, thermal, and mechanical treatments for cellulose extraction [106].
4.2.4. Bleaching/Purification
The bleaching process enhances brightness and purity by removing residual lignin and hemicelluloses, modifying reactivity, and adjusting cellulose viscosity and molecular weight distribution [87]. It is the final step in obtaining pure bleached cellulose and typically uses oxidizing agents such as hydrogen peroxide (H2O2), sodium chlorite, and ozone. These agents oxidize and dissolve remaining lignin and hemicellulose [107].
H2O2 improves delignification by eliminating lignin and chromophores, producing purer cellulose. The process is carried out with an alkali to generate perhydroxyl anions and free radicals, which mediate the brightening reaction [90,107]. Anti-foaming agents such as sodium silicate or silicone prevent foaming during bleaching. Other agents, like chlorine, have been widely used, especially in the paper industry, but produce harmful chlorinated byproducts. H2O2, in contrast, decomposes into water and oxygen, leaving no toxic residues. Its efficacy depends on parameters such as pH, temperature, concentration, pulp-to-solution ratio, and raw material type [70]. Table 8 highlights some of the procedures that have been applied to extract and purify cellulose from banana pseudostem.

Table 8.
Cellulose extraction and purification process (↑: increase, ↓: decrease).
Banana pseudostem cellulose extraction has also applied NaOH with EDTA, yielding pulp with higher polymerization than without EDTA [53,112]. Organosolv processes have been studied for producing dissolving grade pulp (DGP) but are not yet industrially applied [87]. Combined bleaching and liquefaction methods, using polyethylene glycol 400 (PEG 400), glycerol, and sulfuric acid as a catalyst, have also been reported [45,114]. Organosolv pretreatment effectively solubilizes hemicellulose, lignin, and inorganic compounds, produces high-quality lignin as a value-added product, and allows solvent recovery, reducing water pollution [111]. However, industrial use is limited due to volatile, flammable solvents and high-pressure operation [87].
5. Cellulose Modification for Bioplastic Films
Bioplastics from cellulose are mainly derived from cellulose esters and derivatives [114]. Since cellulose is insoluble in water and most organic solvents and is not inherently plastic, it typically requires modification. This can be performed through surface alterations or chemical derivatization to improve processability and mechanical properties for bioplastic use [34]. In derivatization, cellulose is chemically converted into soluble intermediates such as cellulose acetate, while in non-derivatization, cellulose is directly dissolved by breaking hydrogen bonds [115,116].
5.1. Cellulose Acetylation
Cellulose acetylation is a specific type of esterification in which the hydroxyl groups of cellulose react with acetic anhydride or acetic acid, typically in the presence of a catalyst such as sulfuric acid, potassium acetate, or pyridine, to form cellulose acetate (CA). Other esterification reactions involving nitric, sulfuric, or phosphoric acids yield nitrate, sulfate, or phosphate esters of cellulose, respectively, which differ in structure and industrial use.
Cellulose acetate is produced by acetylating cellulose under either a homogeneous process, which employs glacial acetic acid, sulfuric acid, and acetic anhydride, or a heterogeneous process, where cellulose reacts with acetic anhydride and sulfuric acid in the presence of non-diluents [117,118]. Among cellulose esters, acetates, butyrates, and propionates are widely applied in textiles, plastics, photographic films, and electronics [23,25].
Table 9 summarizes common organic and inorganic cellulose ester derivatives.

Table 9.
Examples of cellulose ester derivatives: [66].
The solubility of cellulose acetate in water depends on the degree of substitution (DS), but soluble in solvents such as acetone. It is hydrolytically stable, non-toxic, heat-resistant, decomposable, and characterized by excellent film-forming ability along with high chemical and mechanical stability [2]. Two main types are distinguished: cellulose diacetate, with a DS between 2 and 2.5, and cellulose triacetate, with a DS greater than 2.8 [23]. The DS and acetyl concentration significantly influence the thermal behavior, hydrophobicity, transparency, processability, and solubility of the resulting material [117]. As DS increases, the glass transition temperature decreases, while water vapor permeability (WVP) is reduced because the hydrophilic hydroxyl groups of cellulose are replaced by hydrophobic acetyl groups, thus improving hydrophobicity and barrier properties [115]. However, excessive cellulose during acetylation can lead to agglomeration of cellulose granules within the polymeric matrix, thereby lowering both mechanical strength and barrier performance [8].
Other cellulose derivatives, such as cellulose carbamate are obtained through the reaction of cellulose with urea. In addition, a non-derivative dissolution process using N-methylmorpholine N-oxide (NMMO) has been reported. Unlike derivatization, this approach employs a green solvent that is 99% recyclable, eliminates hazardous additives, and does not produce intermediate derivatives [90].
5.2. Cellulose Acetate (CA) Film Formation
CA-based plastic films are produced by dissolving CA flakes in acetone to form a viscous dope, which is then cast into films [114]. The incorporation of plasticizers such as glycerol improves tensile strength and elongation while reducing the glass transition temperature of the films [66]. The influence of compatibilizers, plasticizers, and other additives on the thermoplastic behavior of cellulose materials is therefore critical. To achieve homogeneous film structures and prevent phase separation, emulsifiers such as gelatin may be incorporated into the film-forming solution [118]. Cellulose-based biopolymer films can be prepared through casting, extrusion, and electrohydrodynamic methods. Among these, solution casting is most widely employed at laboratory and pilot scales, whereas extrusion, via blown film, cast-film, and reactive extrusion techniques, represents a more scalable and cost-effective option for industrial production [119,120].
6. Cellulose Characterization
Characterization of both raw materials and finished products is essential for guiding processing and determining suitable applications. The performance of cellulose in textiles, composites, and bio-based materials is strongly influenced by its complex composition, making the determination of cellulose, hemicellulose, lignin, and pectin content critical for optimizing material properties [121]. The physico-chemical properties of treated Fibers and cellulose are assessed in terms of mechanical strength, thermal performance, and physical characteristics, each playing a distinct role in defining cellulose’s value as an industrial raw material [122]. Fibers are evaluated for their chemical composition as well as physical, morphological, structural, and thermal properties. The removal of lignin and hemicellulose after NaOH treatment is typically confirmed using scanning electron microscopy (SEM), FTIR spectroscopy, and crystallinity analysis, while thermal stability testing indicates that alkali treatment improves stability by up to 15% NaOH concentration [78]. In addition, TAPPI standards are commonly applied to determine the proportions of α-cellulose, β-cellulose, and γ-cellulose in pulp.
6.1. Kappa Number
The kappa number serves as an indicator of residual lignin content in pulp, providing critical insight into the efficiency of the bleaching process. A high kappa number reflects greater lignin content, whereas a low value indicates more effective lignin removal. The kappa number is determined by titration, following established standards, through the difference between the initial volume of permanganate blank solution and the final volume of potassium permanganate remaining after oxidation of lignin. It is expressed as the milliliters of 0.02 mol/L potassium permanganate solution consumed per gram of oven-dry pulp [123]. The lignin content (%) is then calculated using the formula: lignin (%) = kappa number × 0.13.
6.2. Degree of Polymerization (DP)
Changes in cellulose degree of DP are monitored before and during pretreatment, with viscometry and gel-permeation chromatography (GPC) being the most commonly applied techniques. Viscometry is preferred due to its simplicity and speed, and measurements are typically performed in cupri-ethylenediamine solution in accordance with standards such as ISO 5351:2012 [67,70]. Cellulose isolation and derivatization processes did not significantly alter the native DP, which is critical for maintaining mechanical integrity [67].
6.3. Color Measurement
The color of pulp is evaluated to assess purification or bleaching efficiency, using the colorimetric method with a differential colorimeter (Minolta CR-300, Germany). The technique measures CIE coordinates L*, a*, and b*, where L* represents lightness (0 = black, 100 = white), a* indicates the red-green axis (positive toward red, negative toward green), and b* indicates the yellow-blue axis (positive toward yellow, negative toward blue) [90].
6.4. Mechanical Properties
Banana fibers exhibit strong mechanical properties, with elastic modulus 27–32 GN m−2, UTS 711–789 MN m−2, and elongation 2.5–3.7% for fibers 50–250 µm in diameter. Tensile strength rises from 726 to 906 MN m−2 as testing speed increases from 500 µm min−1 to 100 mm min−1 [63].
To demonstrate the quantitative potential of cellulose-based bioplastics, Table 10 compares key mechanical and barrier properties with those of widely used bioplastics and conventional plastics. Cellulose acetate films derived from banana pseudostem show tensile strengths between 50 and 70 MPa- and elongation at break of 5–12%, values comparable to PLA (55–65 MPa) and higher than starch films (25–35 MPa). Their water vapor permeability (2.5 × 10−11 g m/m2 s Pa) is lower than that of starch films, indicating improved moisture resistance and packaging performance.

Table 10.
Quantitative comparison of mechanical and barrier properties of cellulose-based bioplastics with other bioplastics and conventional plastics.
6.5. X-Ray Diffraction (XRD)
XRD is used on untreated and treated banana fibers to evaluate crystallinity index (CI), which indicates crystalline cellulose content. CI values range from 39 to 67% depending- on technique (infrared (IR) spectroscopy, solid-state 13C NMR, Raman). XRD patterns show both amorphous and crystalline regions, reflecting the lignocellulosic nature of banana fibers [83,124]. Specifically, cellulose from banana pseudostem exhibits the Type I cellulose polymorph, characterized by a parallel arrangement of cellulose chains linked by β-1,4-glycosidic bonds [85].
6.6. Thermal Stability Analysis
Thermal analysis assesses molecular characteristics of polymers. Thermal stability of extracts is evaluated via Thermogravimetric Analysis (TGA) or Differential Scanning Calorimetry (DSC) [122]. Thermogravimetric analysis showed high thermal stability, with major weight loss starting near 250 °C, consistent with cellulose degradation [37].
6.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
FTIR is a rapid, non-destructive method to analyze biomass in the mid-IR region, identifying functional groups and molecular fragments. It is used to assess cellulose, hemicellulose, and lignin in untreated and treated banana fibers, providing insight into fiber structure and extraction efficiency [125]. Characteristic absorption peaks at 3300, 2900, and 1000 cm−1 were associated with O-H stretching and β-1,4-glycosidic C-O-C vibrations typical of cellulose [37].
6.8. Scanning Electron Microscope (SEM) Analysis
SEM is used to examine the surface morphology of cellulose fibers. Raw, alkali-treated, and bleached banana fibers are analyzed, showing varying degrees of fibrillation depending on alkali concentration [126]. SEM micrographs revealed a rough, fragmented surface containing pores and cracks, contrasting with the smooth, intact morphology of untreated banana stem fibers [85].
7. Discussion
Although lignocellulosic fibers have been studied for bioplastics for decades, matrix-fiber interfacial compatibility remains unaddressed [4]. Future research should integrate economic, social, and technological assessments to align bio-based production with circular economy and climate policies [127,128].
According to [39], preparation of the pure cellulose bioplastics from biosources is difficult. This is due to the highly structured intermolecular hydrogen bonding network of the polysaccharide biopolymer, which cannot be melted or dissolved by standard processes such as thermoforming. As a result, cellulose is usually used in industrial applications in the form of derivatives, such as esters or ethers, from which cellulose is then regenerated. Nevertheless, in recent years, several other approaches including use of solvents, acids, mechanical processing such as compression molding and blending to obtain biocomposites using a polymeric matrix from a renewable source have been on trial. Characterization techniques of cellulose in reference to application for packaging have been reported variously. The unique hierarchical architecture of natural cellulose consisting of nanoscale fibrils and crystallites allows the extraction of the nano constituents by mechanical and chemical methods or through a combination of both of these techniques [47].
Nanocellulose, producible at industrial scale, is versatile for nanocomposites, biomedical products, adhesives, electronics, membranes, antimicrobial films, packaging, cosmetics, and more. It offers renewability, chemical inertness, stiffness, strength, low thermal expansion, low density, dimensional stability, and tunable surface chemistry.
Quantitative comparison of key properties indicates that cellulose films derived specifically from banana pseudostem exhibit tensile strengths of 40–70 MPa and elongation at break of 5–15%, values within the range of commercial bioplastics such as PLA and PHA. Their water-vapour permeability coefficients (2–4 × 10−11 g m m−2 s−1 Pa−1) further demonstrate competitive mechanical and barrier performance for sustainable packaging applications. These findings reinforce that banana pseudostem cellulose represents a viable second-generation feedstock for high-performance, biodegradable bioplastics.
It is imperative that research and development is intensified to optimize the performance characteristics of agro-based bioplastics in order to make them competitive with conventional plastics and enhance their functionality and acceptability. In particular, research should consider such factors as cost reduction, and recyclability of the end product, as well as improving mechanical and barrier properties.
Beyond material performance, post-utilization pathways of cellulose-based bioplastics derived from banana pseudostem are critical to achieving full circularity. After their service life, these materials can undergo bio-composting, returning organic carbon to the soil without generating persistent microplastic residues. Process residues such as lignin-rich fractions and unreacted biomass can also be valorized through anaerobic digestion for biogas generation or converted into solid biofuel pellets, contributing to renewable energy production and waste minimization. These integrated valorization routes not only enhance the sustainability of banana pseudostem utilization but also align with industrial circular economy models, nature-based solutions, and the United Nations Sustainable Development Goals (SDGs) for responsible production and climate action [129].
Development of an optimized production scale biomass extraction protocol based on laboratory scale parameters. This method considers not only the yield of the target compound(s), but also the chemical and energy costs of the process and the implications for the downstream processing and valorization of the solid residue. A range of different extraction technologies, solvents, and process conditions are considered. Optimization at the lab scale followed by validation at higher technology readiness levels (TRLs) will support industrial adoption and environmental sustainability. Ultimately, valorizing banana pseudostem within a circular bioeconomy framework can reduce agricultural waste, promote local value chains, and advance the transition toward sustainable material systems.
8. Conclusions
Current fossil-based plastics are unsustainable and harmful to health and the environment, prompting research into eco-friendly alternatives. Bioplastics, especially cellulose-based, offer biodegradability, compatibility, and functional benefits. Lignocellulosic agricultural wastes, like banana pseudostem, provide abundant, renewable cellulose (31–81.8%, avg. 49.33%) but require chemical modification, e.g., acetylation, for bioplastic use. Various isolation methods (mechanical, chemical, solvent-based, enzymatic, or combined) have been studied, with organosolv noted as sustainable, cost-effective, and suitable for small-scale operations. Selection depends on polymer type, feedstock, and desired properties. Research gaps include poor transparency, barrier, and mechanical properties, unstable thermal behavior, and limited end-of-life, life cycle, and land use assessments. Future work should focus on valorizing banana waste for cellulose extraction and optimizing cellulose-based bioplastics for packaging applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/waste3040037/s1, Table S1: Summary of key literature on bioplastics: Findings and research gaps.
Author Contributions
Conceptualization, A.W. and K.K.; methodology, A.W., S.P. and K.K.; validation, A.W.M., K.K. and I.C.; formal analysis, G.Z. and I.C.; investigation, A.W. and A.W.M.; resources, J.G.; data curation, G.Z. and J.G.; writing—original draft preparation, A.W.; writing—review and editing, S.P. and K.K.; visualization, S.P.; super-vision, J.G. and G.P.; project administration, J.G. and G.P.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| α | Alpha |
| β | Beta |
| γ | Gamma |
| NaOH | Sodium hydoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| C2H4O2 or CH3COOH - | Acetic acid |
| CH2O2 or HCOOH | Formic acid |
| Na2SiO3 | sodium silicate |
| FTIR | Fourier Transform Infrared |
| SEM | Scanning electron Microscope |
| TGA | Thermogravimetric analysis |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray diffraction |
References
- Nandi, S.; Guha, P.A. Review on Preparation and Properties of Cellulose Nanocrystal Incorporated Natural Biopolymer. J. Packag. Technol. Res. 2018, 2, 149–166. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef] [PubMed]
- Prathipa, R.; Silvakumar, C.; Shanmugasundaram, B. Biodegradable polymers for sustainable packaging applications. Int. J. Mech. Eng. Technol. 2018, 9, 293–303. [Google Scholar]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibres and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Statista. Plastic Production Worldwide in 2017 by Industrial Sector. 2024. Available online: https://www.statista.com/statistics/1134796/plastic-production-by-industrial-sector-worldwide/ (accessed on 20 May 2025).
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117. [Google Scholar] [CrossRef]
- Dokl, M.; Copot, A.; Krajnc, D.; Van Fan, Y.; Vujanović, A.; Aviso, K.B.; Čuček, L. Global projections of plastic use, end of life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050. Sustain. Prod. Consum. 2024, 51, 498–518. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Chozhavendhan, S.; Usha, P.; Sowmiya, G.; Rohini, G. A review on bioplastic production—A need to the society. Int. J. Pharm. Sci. Rev. Res. 2020, 62, 27–32. [Google Scholar]
- Bhat, M.S.; Lakshmi, M.A.; Singh, R.D. Bioplastic from waste: A short review. Biol. Forum-Int. J. 2021, 13, 638–644. [Google Scholar]
- Rahman, R.; Sood, M.; Gupta, N.; Bandral, J.D.; Hameed, F.; Ashraf, S. Bioplastics for food packaging: A review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2311–2321. [Google Scholar] [CrossRef]
- Shamsuddin, I.M.; Jafar, J.A.; Shawai, A.S.A.; Yusuf, S.; Lateefah, M.; Aminu, I. Bioplastics as Better Alternative to Petroplastics and Their Role in National Sustainability: A Review. Adv. Biosci. Bioeng. 2017, 5. [Google Scholar] [CrossRef]
- Hatzilyberis, K.; Tsakanika, L.A.; Lymperopoulou, T.; Georgiou, P.; Kiskira, K.; Tsopelas, F.; Ochsenkühn, K.M.; Ochsenkühn Petropoulou, M. Design of an advanced hydrometallurgy process for the intensified and optimized industrial recovery of scandium from bauxite residue. Chem. Eng. Process.-Process Intensif. 2020, 155, 108015. [Google Scholar] [CrossRef]
- Naveena, B.; Sharma, A. Review on properties of bioplastics for packaging applications and its advantages. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1428–1432. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V. An overview of bioplastic research on its relation to national policies. Sustainability 2021, 13, 7848. [Google Scholar] [CrossRef]
- Mikucioniene, D.; Mínguez García, D.; Repon, M.R.; Milašius, R.; Priniotakis, G.; Chronis, I.; Kiskira, K.; Hoge-boom, R.; Belda Anaya, R.; Díaz García, P. Understanding and addressing the water footprint in the textile sector: A review. AUTEX Res. J. 2024, 24, 20240004. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Campen, M.J. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, S.; Das, D. Enhancing Packaging Sustainability with Natural Fiber Reinforced Bio-Composites: An Outlook into the Future. E3S Web Conf. 2023, 436, 08016. [Google Scholar] [CrossRef]
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, M.C.D.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef]
- Krepsztul, J.W.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent Progress in Bi-odegradable Polymers and Nanocomposites Based Packaging Materials for Sustainable Environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Vinay, G.M.; Modi, R.B.; Prakasha, R. Banana Pseudostem: An Innovative and Sustainable Packaging Material: A Review. J. Packag. Technol. Res. 2024, 8, 95–107. [Google Scholar] [CrossRef]
- Chyerochana, N.; Huynh, Q.T.; Jaitham, U.; Phitsuwan, P.; Aryusuk, K.; Hongsibsong, S.; Chang, K.L. Sustaina-ble Production of High-Performance Bioplastics from Agricultural and Industrial Biomass Waste by Integrating Deep Eutectic Solvent (DES) Pretreatment and Acetylation Processes. ACS Omega 2025, 10, 10949–10961. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Development Update 2024. Available online: https://www.european-bioplastics.org/bioplastics-market-development-update-2024 (accessed on 31 May 2025).
- George, N.; Debroy, A.; Bhat, S.; Bindal, S.; Singh, S. Biowaste to Bioplastics: An Ecofriendly Approach for a Sustainable Future. J. Appl. Biotechnol. Rep. 2021, 8, 221–233. [Google Scholar] [CrossRef]
- Huang, S.; Dong, Q.; Che, S.; Li, R.; Tang, K.H.D. Bioplastics and Biodegradable Plastics: A Review of Recent Advances, Feasibility and Cleaner Production. Sci. Total Environ. 2025, 969, 178911. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable Packaging Application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Kiskira, K.; Lymperopoulou, T.; Lourentzatos, I.; Tsakanika, L.A.; Pavlopoulos, C.; Papadopoulou, K.; Ochsenkühn, K.M.; Tsopelas, F.; Chatzitheodoridis, E.; Lyberatos, G.; et al. Bioleaching of Scandium from Bauxite Residue Using Fungus Aspergillus niger. Waste Biomass Valorization 2023, 14, 3377–3390. [Google Scholar] [CrossRef]
- Tajeddin, B.; Arabkhedri, M. Polymers and Food Packaging. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–543. [Google Scholar] [CrossRef]
- Delucis, R.A.; Cademartori, P.H.G.; Fajardo, A.R.; Amico, S.C. Cellulose and Its Derivatives: Properties and Applications. In Polysaccharides; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 221–252. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Ligno-cellulosic Biomass. Waste Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Stergiou, M.; Panagiotatos, G.; Kiskira, K.; Priniotakis, G. Regenerated Cellulosic Fibers from Agricultural Waste. AIP Conf. Proc. 2022, 2430, 080006. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Asif, M.; Siddique, M.; Abbas, A.; Abubakar, A.M.; Pandit, G.K.; Selele, M.I. Production of Sustainable Bio-plastic Derived from Renewable Lignocellulosic Agricultural Biomass: A Comprehensive Review. Front. Water Environ. 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Pereira, A.L.; Nascimento, D.M.; Cordeiro, N.; D’Almeida, M.L.O.; Rosa, M.F.; Azeredo, H.M.C.; Feitosa, J.P.A. Banana (Musa sp. cv. Pacovan) Pseudostem Fibers Are Composed of Varying Lignocellulosic Composition throughout the Diameter. BioResources 2014, 9, 7749–7763. [Google Scholar] [CrossRef]
- Nascimento, R.E.; Carvalheira, M.; Crespo, J.G.; Neves, L.A. Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem. Clean Technol. 2023, 5, 1028–1043. [Google Scholar] [CrossRef]
- Biron, M. Genesis of Renewable Plastics and Integration in the Plastics Stream. In Industrial Applications of Renewable Plastics; William Andrew: Norwich, NY, USA, 2017; pp. 35–66. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef]
- Helanto, K.; Matikainen, L.; Talja, R.; Rojas, O.J. Biobased Polymers for Sustainable Packaging and Bio-Barriers: A Critical Review. Bioresources 2019, 14, 4902–4951. [Google Scholar] [CrossRef]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N.T. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Shahar, F.S.; Sultan, M.T.H.; Shah, A.U.M.; Safri, S.N.A.; MatYazik, M.H. Overview of Bio-plastic Introduction and Its Applications in Product Packaging. Coatings 2021, 11, 1423. [Google Scholar] [CrossRef]
- Bordón, P.; Paz, R.; Peñalva, C.; Vega, G.; Monzón, M.; García, L. Biodegradable Polymer Compounds Rein-forced with Banana Fibre for the Production of Protective Bags for Banana Fruits in the Context of Circular Economy. Agronomy 2021, 11, 242. [Google Scholar] [CrossRef]
- Ai, B.; Zheng, L.; Li, W.; Zheng, X.; Yang, Y.; Xiao, D.; Shi, J.; Sheng, Z. Biodegradable Cellulose Film Prepared from Banana Pseudo-Stem Using an Ionic Liquid for Mango Preservation. Front. Plant Sci. 2021, 12, 625878. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Raghunath, S.S.; Deepali, V.P.; Priyadharsini, V.; Vidhya, S.; Chithananthan, C.; Choudhary, S.; Krithika, S.; Keerthana, G. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, B.; Ma, M.G.; Wang, B. Review of Recent Development on Preparation, Properties, and Applications of Cellulose-Based Functional Materials. Int. J. Polym. Sci. 2018, 2018, 8973643. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Arteaga-Cardona, F.; de Anda Reyes, M.E.; Gervacio-Arciniega, J.J.; Salazar-Kuri, U. Magnetic Bioplastics Based on Isolated Cellulose from Cotton and Sugarcane Bagasse. Mater. Chem. Phys. 2019, 238, 121921. [Google Scholar] [CrossRef]
- Libog, L.; Aime, J.; Joseph, N.; Ndiwe, B.; Meva’a, L.; Ateba, A.; Laurent, L. Physico-Chemical and Thermal Characterization of the Banana Pseudo-Stem Fibers (BF). Eur. J. Exp. Biol. 2021, 9, 33–52. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Jayandra, P.S.; Luthra, R.P.; Roy, S. Characterization and Performance Analysis of Composite Bioplastics Syn-thesized Using Titanium Dioxide Nanoparticles with Corn Starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Med-ical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Kiskira, K.; Kalkanis, K.; Coelho, F.; Plakantonaki, S.; D’onofrio, C.; Psomopoulos, C.S.; Priniotakis, G.; Ioannidis, G.C. Life Cycle Assessment of Organic Solar Cells: Structure, Analytical Framework, and Future Product Concepts. Electronics 2025, 14, 2426. [Google Scholar] [CrossRef]
- Shah, M.; Rajhans, S.; Himanshu, A.; Pandya, A.; Mankad, A.U. Bioplastic for Future: A Review Then and Now. World J. Adv. Res. Rev. 2021, 9, 56–67. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Balart, R.; Garcia-Garcia, D.; Fombuena, V.; Quiles-Carrillo, L.; Arrieta, M.P. Biopolymers from Natural Resources. Polymers 2021, 13, 2532. [Google Scholar] [CrossRef]
- Hong, L.G.; Yuhana, N.Y.; Zawawi, E.Z.E. Review of Bioplastics as Food Packaging Materials. AIMS Mater. Sci. 2021, 8, 166–184. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)-PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Maraz, K.M.; Karmaker, N.; Meem, R.A.; Khan, R.A. Global Development of Biodegradable Packaging Materials from Bio-Based Raw Materials. J. Res. Updates Polym. Sci. 2019, 8, 66–84. [Google Scholar] [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.; Alves, L.; Medronho, B.; Ferreira, P.J.; Rasteiro, M.D.G. Eco-Friendly Methods for Extraction and Modification of Cellulose: An Overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Deriva-tives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14055602, Cellulose Gel. PubChem. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cellulose-gel (accessed on 23 June 2025).
- Gupta, U.S.; Dhamarikar, M.; Dharkar, A.; Chaturvedi, S.; Tiwari, S.; Namdeo, R. Surface Modification of Ba-nana Fiber: A Review. Mater. Today Proc. 2021, 43, 904–915. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Nulend, J.K. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Tajeddin, B. Cellulose-Based Polymers for Packaging Applications. In Lignocellulosic Polymer Composites: Processing, Characterization, and Properties; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 477–498. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing Cellulose Degree of Polymerization and Its Relevancy to Cellulosic Ethanol. Biofuels Bioprod. Biorefin. 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review on Analysis in Pretreatment of Lignocelluloses: Degree of Polymerization, Adsorption/Desorption, and Accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef]
- Syarif, M.A.; Fahma, F.; Sailah, I. Bioplastic Beads Composite Production Based on Cellulose Acetate-Starch Blend: A Literature Study. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012015. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Kiskira, K.; Zacharopoulos, N.; Belessi, V.; Sfyroera, E.; Priniotakis, G.; Athanasekou, C. Inves-tigating the Routes to Produce Cellulose Fibres from Agro-Waste: An Upcycling Process. Chem. Eng. 2024, 8, 112. [Google Scholar] [CrossRef]
- Arfin, T. Cellulose and Hydrogel Matrices for Environmental Applications. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of Biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Moura, I.G.; Sa, A.V.; Abreu, A.S.L.M.; Machando, A.V.A. Bioplastics from Agro-Wastes for Food Packaging Applications. In Food Packaging; Bioplastics and Biocomposites from Agro-Wastes; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of Biofilms on the Conversion of Cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Qasim, U.; Osman, A.I.; Al Muhtaseb, A.H.; Farrell, C.; Abri, M.A.; Ali, M.; Vo, D.V.N.; Jamil, F.; Rooney, D.W. Renewable Cellulosic Nanocomposites for Food Packaging to Avoid Fossil Fuel Plastic Pollution: A Review. Environ. Chem. Lett. 2021, 19, 613–641. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Stoica, M.; Bichescu, C.I.; Crețu, C.M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Balda, S.; Sharma, A.; Capalash, N.; Sharma, P. Banana Fibre: A Natural and Sustainable Bioresource for Eco-Friendly Applications. Clean Technol. Environ. Policy 2021, 23, 1389–1401. [Google Scholar] [CrossRef]
- Gumisiriza, R.; Hawumba, J.F.; Okure, M.; Hensel, O. Biomass Waste-to-Energy Valorisation Technologies: A Review Case for Banana Processing in Uganda. Biotechnol. Biofuels 2017, 10, 11. [Google Scholar] [CrossRef]
- Diarsa, M.; Gupte, A. Preparation, Characterization and Its Potential Applications in Isoniazid Drug Delivery of Porous Microcrystalline Cellulose from Banana Pseudostem Fibers. 3 Biotech 2021, 11, 334. [Google Scholar] [CrossRef]
- Khiari, R.; Belgacem, M.N. Potential for Using Multiscale Posidonia oceanica Waste: Current Status and Prospects in Material Science. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 447–471. [Google Scholar] [CrossRef]
- Subaigyo, A.; Chafidz, A. Banana Pseudo-Stem Fibre: Preparation, Characteristics, and Applications. In Ligno-cellulosic Polymer Composites; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Gomes, C.V.; Araújo, J.C.; Chaves, D.M.; Fangueiro, R.; Ferreira, D.P. Improving Textile Circular Economy through Banana Fibres from the Leaves Central Rib: Effect of Different Extraction Methods. Food Bioprod. Process. 2024, 146, 195–204. [Google Scholar] [CrossRef]
- Badanayak, P.; Jose, S.; Bose, G. Banana Pseudostem Fiber: A Critical Review on Fiber Extraction, Characteriza-tion, and Surface Modification. J. Nat. Fibers 2023, 20, 2168821. [Google Scholar] [CrossRef]
- Van, N.T.T.; Gaspillo, P.A.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Ha, H.K.P. Cellulose from the Banana Stem: Optimization of Extraction by Response Surface Methodology (RSM) and Characterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef]
- Bekraoui, N.; El Qoubaa, Z.; Chouiyakh, H.; Faqir, M.; Essadiqi, E. Banana Fiber Extraction and Surface Charac-terization of Hybrid Banana Reinforced Composite. J. Nat. Fibers 2022, 19, 12982–12995. [Google Scholar] [CrossRef]
- Quintana, E.; Valls, C.; Roncero, M.B. Dissolving-Grade Pulp: A Sustainable Source for Fiber Production. Wood Sci. Technol. 2024, 58, 23–85. [Google Scholar] [CrossRef]
- Ramadevi, P.; Sampathkumar, D.; Srinivasa, C.V.; Bennehalli, B. Effect of Alkali Treatment on Water Absorp-tion of Single Cellulosic Abaca Fiber. BioResources 2012, 7, 3515–3524. [Google Scholar] [CrossRef]
- Sango, T.; Yona, A.M.C.; Duchatel, L.; Marin, A.; Ndikontar, M.K.; Joly, N.; Lefebvre, J.M. Step-Wise Multi-Scale Deconstruction of Banana Pseudo-Stem (Musa acuminata) Biomass and Morpho-Mechanical Characterization of Extracted Long Fibres for Sustainable Applications. Ind. Crops Prod. 2018, 122, 657–668. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Zacharopoulos, N.; Christopoulos, M.; Kiskira, K.; Markou, G.; Tsakanika, L.A.; Priniotakis, G. Upcycling Industrial Peach Waste to Produce Dissolving Pulp. Environ. Sci. Pollut. Res. 2025, 32, 4636–4655. [Google Scholar] [CrossRef]
- Kumar, V.; Chakraborty, P.; Janghu, P.; Umesh, M.; Sarojini, S.; Pasrija, R.; Sivalingam, A.M. Potential of Bana-na Based Cellulose Materials for Advanced Applications: A Review on Properties and Technical Challenges. Carbohydr. Polym. Technol. Appl. 2023, 6, 100366. [Google Scholar]
- Iliyin, I.; Purwaningsih, H.; Irawadi, T.T. Isolation and Characterization of Cellulose from Banana Stems Using Microwave Heating. J. Kimia Valensi 2021, 6, 169–176. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, J.; Zhou, Y.; Li, R.; Zhou, W. Characterization of Cellulose from Banana Pseudo-Stem by Heterogeneous Liquefaction. Carbohydr. Polym. 2015, 134, 495–501. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Alwani, M.S.; Omar, A.M. Chemical composition, anatomy, lignin distribution, and cell wall structure of Malaysian plant waste fibers. BioResources 2006, 1, 220–232. [Google Scholar] [CrossRef]
- Bilba, K.; Arsene, M.-A.; Ouensanga, A. Study of Banana and Coconut Fibers: Botanical Composition, Thermal Degradation and Textural Observations. Bioresour. Technol. 2007, 98, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Samanta, K.K.; Chattopadhyay, S.K.; Reddy, E.N.; Mukhopadhyay, S.; Datta, S.; Hadge, G.B. Flame Resistant Cellulosic Substrate Using Banana Pseudostem Sap. Pol. J. Chem. Technol. 2015, 17, 33–39. [Google Scholar] [CrossRef]
- Hidayati, S.; Zuidar, A.S.; Satyajaya, W. Effect of Acetic Acid:Formic Acid Ratio on Characteristics of Pulp from Oil Palm Empty Fruit Bunches (OPEFB). ARPN J. Eng. Appl. Sci. 2017, 12, 3802–3807. [Google Scholar]
- Chopra, L.; Manikanika. Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Othman, J.A.S.; Ilyas, R.A.; Nordin, A.H.; Ngadi, N.; Alkbir, M.F.M.; Knight, V.F.; Norrrahim, M.N.F. Optimization of Delignification and Mercerization Processes for High-Purity Cellulose Extraction from Semantan Bamboo (Gigantochloa scortechinii) Using Response Surface Modelling. Carbohydr. Polym. Technol. Appl. 2025, 10, 100784. [Google Scholar] [CrossRef]
- Merais, M.S.; Khairuddin, N.; Salehudin, M.H.; Mobin Siddique, M.B.; Lepun, P.; Chuong, W.S. Preparation and Characterization of Cellulose Nanofibers from Banana Pseudostem by Acid Hydrolysis: Physico-Chemical and Thermal Properties. Membranes 2022, 12, 451. [Google Scholar] [CrossRef]
- Benítez, A.N.; Monzón, M.D.; Angulo, I.; Ortega, Z.; Hernández, P.M.; Marrero, M.D. Treatment of Banana Fiber for Use in the Reinforcement of Polymeric Matrices. Measurement 2013, 46, 1065–1073. [Google Scholar] [CrossRef]
- Reddy, M.I.; Sethuramalingam, P.; Sahu, R.K. Isolation of Microcrystalline Cellulose from Musa paradisiaca (Banana) Plant Leaves: Physicochemical, Thermal, Morphological, and Mechanical Characterization for Light-weight Polymer Composite Applications. J. Polym. Res. 2024, 31, 114. [Google Scholar] [CrossRef]
- Gong, L.; Kumar, A.; Chunxiao, Y.P.; Kumar, T.V.; Newbold, J.; Clark, W.; Jiang, Y.; Kumar, S.; Kumar, G.V. Sustainable Utilization of Fruit and Vegetable Waste Bioresources for Bioplastics Production. Crit. Rev. Biotechnol. 2024, 44, 236–254. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Ornaghi Jr, H.L.; Arantes, V.; Cioffi, M.O.H. Effect of Chemical Treatment of Pineapple Crown Fiber in the Production, Chemical Composition, Crystalline Structure, Thermal Stability and Thermal Deg-radation Kinetic Properties of Cellulosic Materials. Carbohydr. Res. 2021, 499, 108227. [Google Scholar] [CrossRef]
- Hidayati, S.; Suroso, E.; Satyajaya, W.; Iryani, D.A. Chemistry and Structure Characterization of Bamboo Pulp with Formacell Pulping. IOP Conf. Ser. Mater. Sci. Eng. 2019, 532, 012024. [Google Scholar] [CrossRef]
- Kamoga, O.; Lwako, M.; Byaruhanga, J.K.; Kirabira, J.B. A Review on Pulp Manufacture from Non-Wood Plant Materials. Int. J. Chem. Eng. Appl. 2013, 4, 144–148. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Fernandes, L.L.; Santos, L.B.U.; Ornaghi Jr, H.L.; Cioffi, M.O.H. Different Sequential Chemical Treatments Used to Obtain Bleached Cellulose from Orange Bagasse. J. Nat. Fibers 2022, 19, 12849–12861. [Google Scholar] [CrossRef]
- Ardila, A.A.N.; Arriola Villaseñor, E.; González, E.E.V.; Guerrero, H.E.G.; Hernández Maldonado, J.A.; Gutiérrez-Pineda, E.; Villa, C.C. Enhanced Cellulose Extraction from Banana Pseudostem Waste: A Comparative Analysis Using Chemical Methods Assisted by Conventional and Focused Ultrasound. Polymers 2024, 16, 2785. [Google Scholar] [CrossRef]
- Vardhini, K.V.; Murugan, R.; Surjit, R. Effect of Alkali and Enzymatic Treatments of Banana Fibre on Properties of Banana/Polypropylene Composites. J. Ind. Text. 2017, 47, 1849–1864. [Google Scholar] [CrossRef]
- Samathoti, P.; Ramya, B.S.; Dogiparthi, L.K.; Rani, G.U.; Susmitha, A.; Devi, B.N.; Reddy, V.S.; Vidyavathi, M.; Mallikarjuna, B.P. Methods of Extraction of Cellulose from Bio Waste of Banana Plant and Applications: A Review. Asian J. Pharm. 2023, 17, 659–669. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; Hoop, C.D.; Hu, T.X.; Qi, J.Q.; Shupe, T.F. Isolation and Characterization of Cellulose Nan-ofibers from Bamboo Using Microwave Liquefaction Combined with Chemical Treatment and Ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.A.N.; Jai, J. Response Surface Methodology for Optimization of Cellulose Extraction from Ba-nana Stem Using NaOH-EDTA for Pulp and Papermaking. Heliyon 2022, 8, e09114. [Google Scholar] [CrossRef] [PubMed]
- Samara, F.S.; Novia, N.; Melwita, E. Enzymatic Hydrolysis of Cellulose Banana Stem (Alkaline Micro-wave-Assisted Pre-Treatment). J. Integr. Adv. Eng. 2024, 4, 21–30. [Google Scholar] [CrossRef]
- Meng, F.; Wang, G.; Du, X.; Wang, Z.; Xu, S.; Zhang, Y. Extraction and Characterization of Cellulose Nano-fibers and Nanocrystals from Liquefied Banana Pseudo-Stem Residue. Compos. Part B Eng. 2019, 160, 341–347. [Google Scholar] [CrossRef]
- Nigam, S.; Das, A.K.; Matkawala, F.; Patidar, M.K. An Insight Overview of Bioplastics Produced from Cellulose Extracted from Plant Material, Its Applications and Degradation. Environ. Sustain. 2022, 5, 423–441. [Google Scholar] [CrossRef]
- Plakantonaki, S. Converting Industrial Peach Waste into Sustainable Fibres via Physicochemical Processes towards Circular Economy. Ph.D. Dissertation, University of West Attica, Aigaleo, Greece, 2024. [Google Scholar]
- Tufan, M.Z.; Özel, C. Cellulose and Its Derivatives as Biodegradable Materials: A Review. J. Sci. Rep. A 2024, 59, 87–104. [Google Scholar] [CrossRef]
- Nigam, S.; Das, A.K.; Patidar, M.K. Synthesis, Characterization and Biodegradation of Bioplastic Films Pro-duced from Parthenium hysterophorus by Incorporating a Plasticizer (PEG600). Environ. Challenges 2021, 5, 100280. [Google Scholar] [CrossRef]
- Khaleed, G.; Sharanagat, V.S.; Upadhyay, S.; Desai, S.; Kumar, K.; Dhiman, A.; Suhag, R. Sustainable Ap-proach Toward Biodegradable Packaging Through Naturally Derived Biopolymers: An Overview. J. Packag. Technol. Res. 2024, 9, 19–46. [Google Scholar] [CrossRef]
- Bourtsalas, A.C.; Papadatos, P.E.; Kiskira, K.; Kalkanis, K.; Psomopoulos, C.S. Ecodesign for Industrial Furnaces and Ovens: A Review of the Current Environmental Legislation. Sustainability 2023, 15, 9436. [Google Scholar] [CrossRef]
- Ali, Z.; Talpur, F.N.; Afridi, H.I.; Ahmed, F.; Brohi, N.A.; Abbasi, H. Analytical Approaches and Advancement in the Analysis of Natural and Synthetic Fiber: A Comprehensive Review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 326, 125164. [Google Scholar] [CrossRef]
- Kiruthika, A.V.; Veluraja, K. Experimental Studies on the Physico-Chemical Properties of Banana Fibre from Various Varieties. Fibers Polym. 2009, 10, 193–199. [Google Scholar] [CrossRef]
- International Standards Organisation. Pulps—Determination of Kappa Number; Edition 3, 2015. Available online: https://standards.iteh.ai/catalog/standards/sist/cd48da24-6cf1-46a0-b630-168a2ba96566/iso-302-2015 (accessed on 10 September 2025).
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Das, M.; Chakraborty, D. Influence of Alkali Treatment on the Fine Structure and Morphology of Bamboo Fi-bers. J. Appl. Polym. Sci. 2006, 102, 5050–5056. [Google Scholar] [CrossRef]
- Abrha, H.; Cabrera, J.; Dai, Y.; Irfan, M.; Toma, A.; Jiao, S.; Liu, X. Bio-Based Plastics Production, Impact and End of Life: A Literature Review and Content Analysis. Sustainability 2022, 14, 4855. [Google Scholar] [CrossRef]
- Kalkanis, K.; Kiskira, K.; Papageorgas, P.; Kaminaris, S.D.; Piromalis, D.; Banis, G.; Mpelesis, D.; Batagiannis, A. Advanced manufacturing design of an emergency mechanical ventilator via 3D printing—Effective crisis response. Sustainability 2023, 15, 2857. [Google Scholar] [CrossRef]
- Alevizos, V.; Gerolimos, N.; Edralin, S.; Xu, C.; Simasiku, A.; Priniotakis, G.; Papakostas, G.A.; Yue, Z. Systematic Review on Sustainable Design Thinking through Biomimetic Approach. In Proceedings of the 2025 International Conference on Artificial Intelligence in Information and Communication (ICAIIC), Fukuoka, Japan, 18–21 February 2025; pp. 219–223. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).