Cellulose-Based Biopolymers from Banana Pseudostem Waste: Innovations for Sustainable Bioplastics
Abstract
1. Introduction
Objectives
2. Synthesis of Key Literature on Bioplastics and Identified Gaps
2.1. Properties and Application of Bioplastics
2.2. Advantages and Disadvantages of Bioplastics from Natural Sources
3. Properties of Cellulose and Cellulose Derivatives for Packaging
3.1. Sources of Cellulose
3.2. Viability of Banana Pseudo Stem Waste for Cellulose Extraction
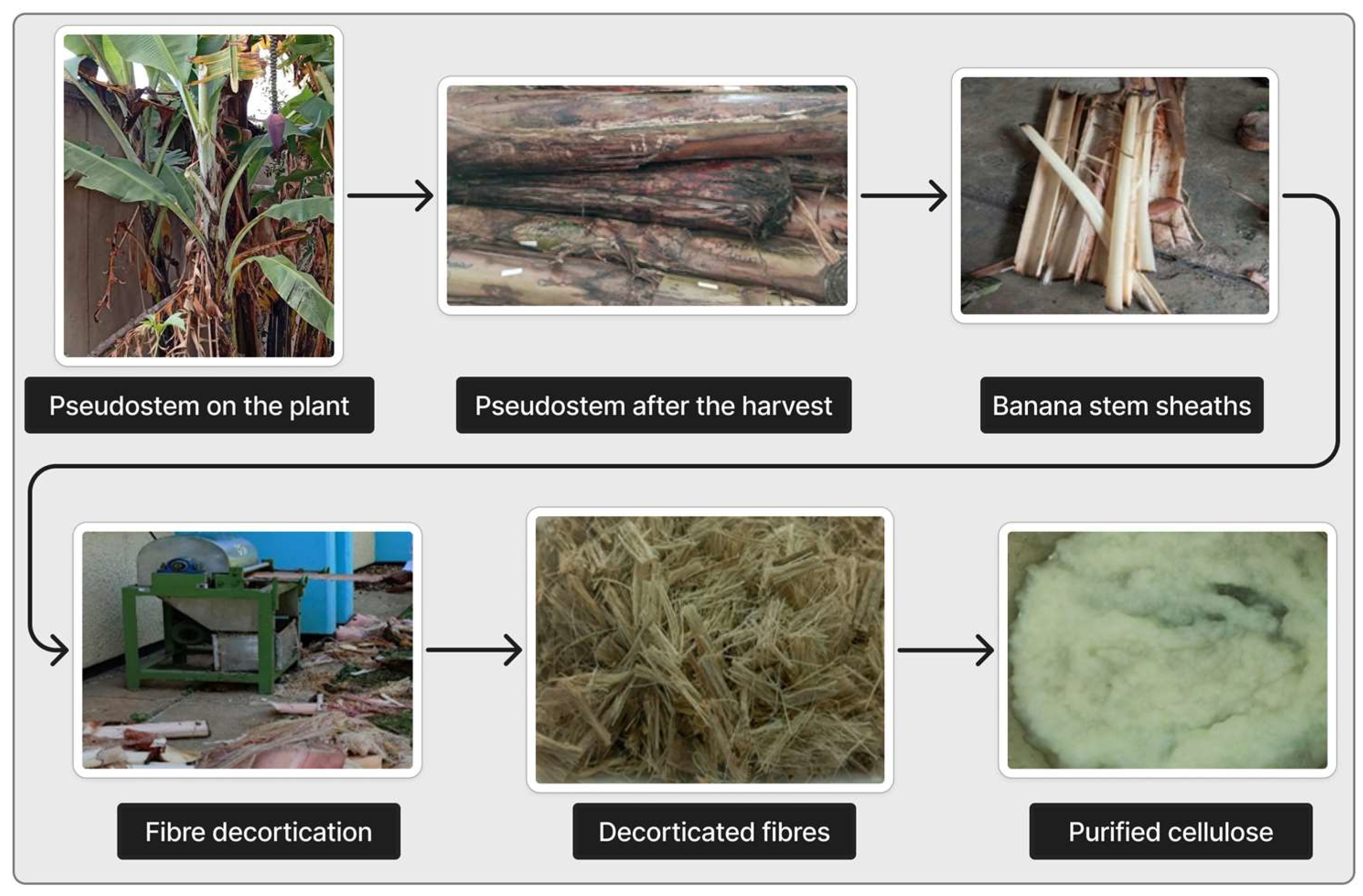
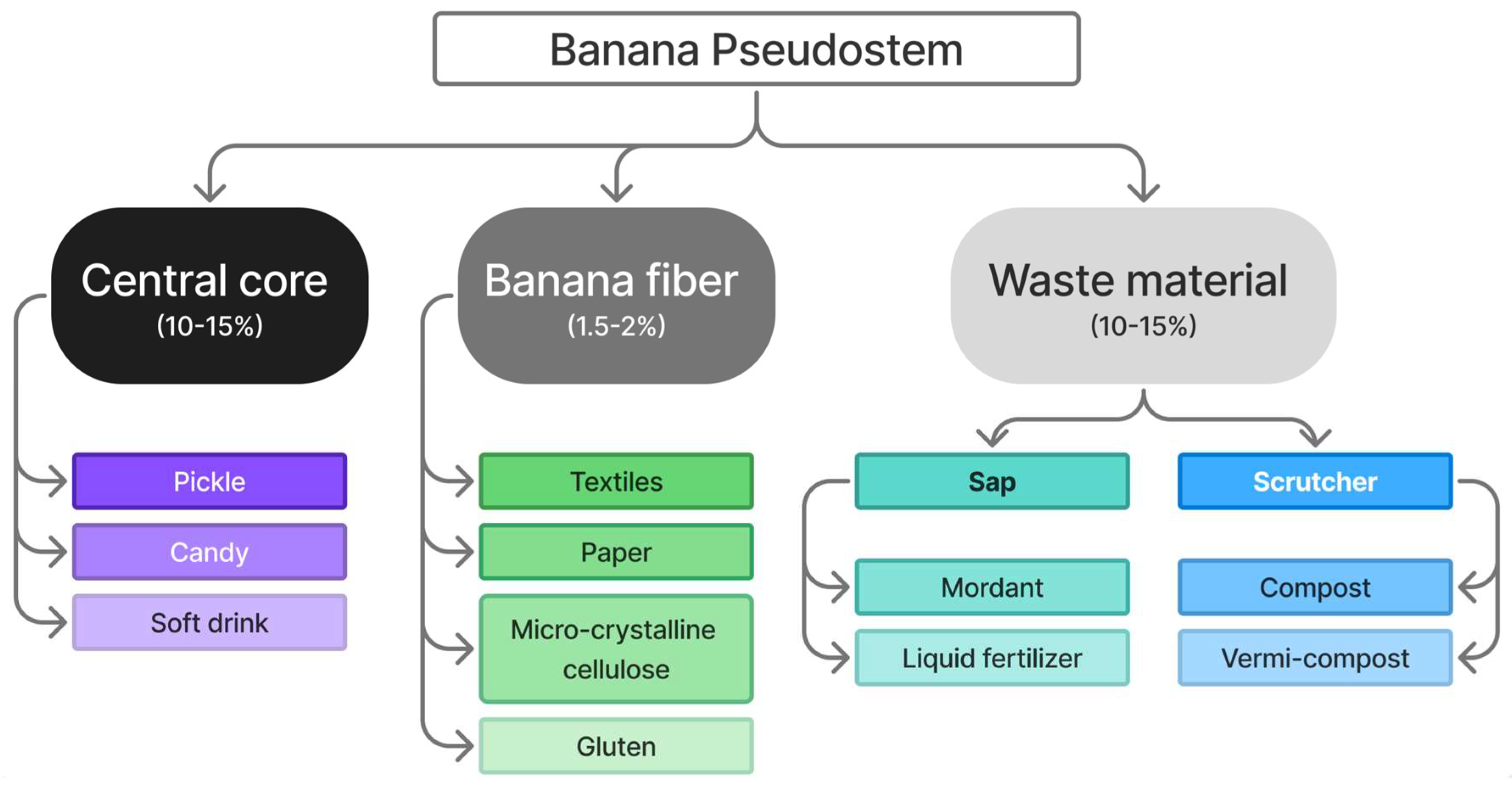
4. Isolation and Purification of Cellulose from Banana Pseudostem Waste
4.1. Chemical Composition of Banana Pseudostem
4.2. Methods of Cellulose Isolation and Purification
4.2.1. Degumming/Conditioning
4.2.2. Pretreatment
4.2.3. Hydrolysis/Pulping
4.2.4. Bleaching/Purification
5. Cellulose Modification for Bioplastic Films
5.1. Cellulose Acetylation
5.2. Cellulose Acetate (CA) Film Formation
6. Cellulose Characterization
6.1. Kappa Number
6.2. Degree of Polymerization (DP)
6.3. Color Measurement
6.4. Mechanical Properties
6.5. X-Ray Diffraction (XRD)
6.6. Thermal Stability Analysis
6.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
6.8. Scanning Electron Microscope (SEM) Analysis
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α | Alpha |
| β | Beta |
| γ | Gamma |
| NaOH | Sodium hydoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| C2H4O2 or CH3COOH - | Acetic acid |
| CH2O2 or HCOOH | Formic acid |
| Na2SiO3 | sodium silicate |
| FTIR | Fourier Transform Infrared |
| SEM | Scanning electron Microscope |
| TGA | Thermogravimetric analysis |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray diffraction |
References
- Nandi, S.; Guha, P.A. Review on Preparation and Properties of Cellulose Nanocrystal Incorporated Natural Biopolymer. J. Packag. Technol. Res. 2018, 2, 149–166. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef] [PubMed]
- Prathipa, R.; Silvakumar, C.; Shanmugasundaram, B. Biodegradable polymers for sustainable packaging applications. Int. J. Mech. Eng. Technol. 2018, 9, 293–303. [Google Scholar]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibres and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Statista. Plastic Production Worldwide in 2017 by Industrial Sector. 2024. Available online: https://www.statista.com/statistics/1134796/plastic-production-by-industrial-sector-worldwide/ (accessed on 20 May 2025).
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117. [Google Scholar] [CrossRef]
- Dokl, M.; Copot, A.; Krajnc, D.; Van Fan, Y.; Vujanović, A.; Aviso, K.B.; Čuček, L. Global projections of plastic use, end of life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050. Sustain. Prod. Consum. 2024, 51, 498–518. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Chozhavendhan, S.; Usha, P.; Sowmiya, G.; Rohini, G. A review on bioplastic production—A need to the society. Int. J. Pharm. Sci. Rev. Res. 2020, 62, 27–32. [Google Scholar]
- Bhat, M.S.; Lakshmi, M.A.; Singh, R.D. Bioplastic from waste: A short review. Biol. Forum-Int. J. 2021, 13, 638–644. [Google Scholar]
- Rahman, R.; Sood, M.; Gupta, N.; Bandral, J.D.; Hameed, F.; Ashraf, S. Bioplastics for food packaging: A review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2311–2321. [Google Scholar] [CrossRef]
- Shamsuddin, I.M.; Jafar, J.A.; Shawai, A.S.A.; Yusuf, S.; Lateefah, M.; Aminu, I. Bioplastics as Better Alternative to Petroplastics and Their Role in National Sustainability: A Review. Adv. Biosci. Bioeng. 2017, 5. [Google Scholar] [CrossRef]
- Hatzilyberis, K.; Tsakanika, L.A.; Lymperopoulou, T.; Georgiou, P.; Kiskira, K.; Tsopelas, F.; Ochsenkühn, K.M.; Ochsenkühn Petropoulou, M. Design of an advanced hydrometallurgy process for the intensified and optimized industrial recovery of scandium from bauxite residue. Chem. Eng. Process.-Process Intensif. 2020, 155, 108015. [Google Scholar] [CrossRef]
- Naveena, B.; Sharma, A. Review on properties of bioplastics for packaging applications and its advantages. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1428–1432. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V. An overview of bioplastic research on its relation to national policies. Sustainability 2021, 13, 7848. [Google Scholar] [CrossRef]
- Mikucioniene, D.; Mínguez García, D.; Repon, M.R.; Milašius, R.; Priniotakis, G.; Chronis, I.; Kiskira, K.; Hoge-boom, R.; Belda Anaya, R.; Díaz García, P. Understanding and addressing the water footprint in the textile sector: A review. AUTEX Res. J. 2024, 24, 20240004. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Campen, M.J. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, S.; Das, D. Enhancing Packaging Sustainability with Natural Fiber Reinforced Bio-Composites: An Outlook into the Future. E3S Web Conf. 2023, 436, 08016. [Google Scholar] [CrossRef]
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, M.C.D.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef]
- Krepsztul, J.W.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent Progress in Bi-odegradable Polymers and Nanocomposites Based Packaging Materials for Sustainable Environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Vinay, G.M.; Modi, R.B.; Prakasha, R. Banana Pseudostem: An Innovative and Sustainable Packaging Material: A Review. J. Packag. Technol. Res. 2024, 8, 95–107. [Google Scholar] [CrossRef]
- Chyerochana, N.; Huynh, Q.T.; Jaitham, U.; Phitsuwan, P.; Aryusuk, K.; Hongsibsong, S.; Chang, K.L. Sustaina-ble Production of High-Performance Bioplastics from Agricultural and Industrial Biomass Waste by Integrating Deep Eutectic Solvent (DES) Pretreatment and Acetylation Processes. ACS Omega 2025, 10, 10949–10961. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Development Update 2024. Available online: https://www.european-bioplastics.org/bioplastics-market-development-update-2024 (accessed on 31 May 2025).
- George, N.; Debroy, A.; Bhat, S.; Bindal, S.; Singh, S. Biowaste to Bioplastics: An Ecofriendly Approach for a Sustainable Future. J. Appl. Biotechnol. Rep. 2021, 8, 221–233. [Google Scholar] [CrossRef]
- Huang, S.; Dong, Q.; Che, S.; Li, R.; Tang, K.H.D. Bioplastics and Biodegradable Plastics: A Review of Recent Advances, Feasibility and Cleaner Production. Sci. Total Environ. 2025, 969, 178911. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable Packaging Application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Kiskira, K.; Lymperopoulou, T.; Lourentzatos, I.; Tsakanika, L.A.; Pavlopoulos, C.; Papadopoulou, K.; Ochsenkühn, K.M.; Tsopelas, F.; Chatzitheodoridis, E.; Lyberatos, G.; et al. Bioleaching of Scandium from Bauxite Residue Using Fungus Aspergillus niger. Waste Biomass Valorization 2023, 14, 3377–3390. [Google Scholar] [CrossRef]
- Tajeddin, B.; Arabkhedri, M. Polymers and Food Packaging. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–543. [Google Scholar] [CrossRef]
- Delucis, R.A.; Cademartori, P.H.G.; Fajardo, A.R.; Amico, S.C. Cellulose and Its Derivatives: Properties and Applications. In Polysaccharides; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 221–252. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Ligno-cellulosic Biomass. Waste Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Stergiou, M.; Panagiotatos, G.; Kiskira, K.; Priniotakis, G. Regenerated Cellulosic Fibers from Agricultural Waste. AIP Conf. Proc. 2022, 2430, 080006. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Asif, M.; Siddique, M.; Abbas, A.; Abubakar, A.M.; Pandit, G.K.; Selele, M.I. Production of Sustainable Bio-plastic Derived from Renewable Lignocellulosic Agricultural Biomass: A Comprehensive Review. Front. Water Environ. 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Pereira, A.L.; Nascimento, D.M.; Cordeiro, N.; D’Almeida, M.L.O.; Rosa, M.F.; Azeredo, H.M.C.; Feitosa, J.P.A. Banana (Musa sp. cv. Pacovan) Pseudostem Fibers Are Composed of Varying Lignocellulosic Composition throughout the Diameter. BioResources 2014, 9, 7749–7763. [Google Scholar] [CrossRef]
- Nascimento, R.E.; Carvalheira, M.; Crespo, J.G.; Neves, L.A. Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem. Clean Technol. 2023, 5, 1028–1043. [Google Scholar] [CrossRef]
- Biron, M. Genesis of Renewable Plastics and Integration in the Plastics Stream. In Industrial Applications of Renewable Plastics; William Andrew: Norwich, NY, USA, 2017; pp. 35–66. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef]
- Helanto, K.; Matikainen, L.; Talja, R.; Rojas, O.J. Biobased Polymers for Sustainable Packaging and Bio-Barriers: A Critical Review. Bioresources 2019, 14, 4902–4951. [Google Scholar] [CrossRef]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N.T. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Shahar, F.S.; Sultan, M.T.H.; Shah, A.U.M.; Safri, S.N.A.; MatYazik, M.H. Overview of Bio-plastic Introduction and Its Applications in Product Packaging. Coatings 2021, 11, 1423. [Google Scholar] [CrossRef]
- Bordón, P.; Paz, R.; Peñalva, C.; Vega, G.; Monzón, M.; García, L. Biodegradable Polymer Compounds Rein-forced with Banana Fibre for the Production of Protective Bags for Banana Fruits in the Context of Circular Economy. Agronomy 2021, 11, 242. [Google Scholar] [CrossRef]
- Ai, B.; Zheng, L.; Li, W.; Zheng, X.; Yang, Y.; Xiao, D.; Shi, J.; Sheng, Z. Biodegradable Cellulose Film Prepared from Banana Pseudo-Stem Using an Ionic Liquid for Mango Preservation. Front. Plant Sci. 2021, 12, 625878. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Raghunath, S.S.; Deepali, V.P.; Priyadharsini, V.; Vidhya, S.; Chithananthan, C.; Choudhary, S.; Krithika, S.; Keerthana, G. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, B.; Ma, M.G.; Wang, B. Review of Recent Development on Preparation, Properties, and Applications of Cellulose-Based Functional Materials. Int. J. Polym. Sci. 2018, 2018, 8973643. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Arteaga-Cardona, F.; de Anda Reyes, M.E.; Gervacio-Arciniega, J.J.; Salazar-Kuri, U. Magnetic Bioplastics Based on Isolated Cellulose from Cotton and Sugarcane Bagasse. Mater. Chem. Phys. 2019, 238, 121921. [Google Scholar] [CrossRef]
- Libog, L.; Aime, J.; Joseph, N.; Ndiwe, B.; Meva’a, L.; Ateba, A.; Laurent, L. Physico-Chemical and Thermal Characterization of the Banana Pseudo-Stem Fibers (BF). Eur. J. Exp. Biol. 2021, 9, 33–52. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Jayandra, P.S.; Luthra, R.P.; Roy, S. Characterization and Performance Analysis of Composite Bioplastics Syn-thesized Using Titanium Dioxide Nanoparticles with Corn Starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Med-ical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Kiskira, K.; Kalkanis, K.; Coelho, F.; Plakantonaki, S.; D’onofrio, C.; Psomopoulos, C.S.; Priniotakis, G.; Ioannidis, G.C. Life Cycle Assessment of Organic Solar Cells: Structure, Analytical Framework, and Future Product Concepts. Electronics 2025, 14, 2426. [Google Scholar] [CrossRef]
- Shah, M.; Rajhans, S.; Himanshu, A.; Pandya, A.; Mankad, A.U. Bioplastic for Future: A Review Then and Now. World J. Adv. Res. Rev. 2021, 9, 56–67. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Balart, R.; Garcia-Garcia, D.; Fombuena, V.; Quiles-Carrillo, L.; Arrieta, M.P. Biopolymers from Natural Resources. Polymers 2021, 13, 2532. [Google Scholar] [CrossRef]
- Hong, L.G.; Yuhana, N.Y.; Zawawi, E.Z.E. Review of Bioplastics as Food Packaging Materials. AIMS Mater. Sci. 2021, 8, 166–184. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)-PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Maraz, K.M.; Karmaker, N.; Meem, R.A.; Khan, R.A. Global Development of Biodegradable Packaging Materials from Bio-Based Raw Materials. J. Res. Updates Polym. Sci. 2019, 8, 66–84. [Google Scholar] [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.; Alves, L.; Medronho, B.; Ferreira, P.J.; Rasteiro, M.D.G. Eco-Friendly Methods for Extraction and Modification of Cellulose: An Overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Deriva-tives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14055602, Cellulose Gel. PubChem. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cellulose-gel (accessed on 23 June 2025).
- Gupta, U.S.; Dhamarikar, M.; Dharkar, A.; Chaturvedi, S.; Tiwari, S.; Namdeo, R. Surface Modification of Ba-nana Fiber: A Review. Mater. Today Proc. 2021, 43, 904–915. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Nulend, J.K. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Tajeddin, B. Cellulose-Based Polymers for Packaging Applications. In Lignocellulosic Polymer Composites: Processing, Characterization, and Properties; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 477–498. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing Cellulose Degree of Polymerization and Its Relevancy to Cellulosic Ethanol. Biofuels Bioprod. Biorefin. 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review on Analysis in Pretreatment of Lignocelluloses: Degree of Polymerization, Adsorption/Desorption, and Accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef]
- Syarif, M.A.; Fahma, F.; Sailah, I. Bioplastic Beads Composite Production Based on Cellulose Acetate-Starch Blend: A Literature Study. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012015. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Kiskira, K.; Zacharopoulos, N.; Belessi, V.; Sfyroera, E.; Priniotakis, G.; Athanasekou, C. Inves-tigating the Routes to Produce Cellulose Fibres from Agro-Waste: An Upcycling Process. Chem. Eng. 2024, 8, 112. [Google Scholar] [CrossRef]
- Arfin, T. Cellulose and Hydrogel Matrices for Environmental Applications. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of Biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Moura, I.G.; Sa, A.V.; Abreu, A.S.L.M.; Machando, A.V.A. Bioplastics from Agro-Wastes for Food Packaging Applications. In Food Packaging; Bioplastics and Biocomposites from Agro-Wastes; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of Biofilms on the Conversion of Cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Qasim, U.; Osman, A.I.; Al Muhtaseb, A.H.; Farrell, C.; Abri, M.A.; Ali, M.; Vo, D.V.N.; Jamil, F.; Rooney, D.W. Renewable Cellulosic Nanocomposites for Food Packaging to Avoid Fossil Fuel Plastic Pollution: A Review. Environ. Chem. Lett. 2021, 19, 613–641. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Stoica, M.; Bichescu, C.I.; Crețu, C.M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Balda, S.; Sharma, A.; Capalash, N.; Sharma, P. Banana Fibre: A Natural and Sustainable Bioresource for Eco-Friendly Applications. Clean Technol. Environ. Policy 2021, 23, 1389–1401. [Google Scholar] [CrossRef]
- Gumisiriza, R.; Hawumba, J.F.; Okure, M.; Hensel, O. Biomass Waste-to-Energy Valorisation Technologies: A Review Case for Banana Processing in Uganda. Biotechnol. Biofuels 2017, 10, 11. [Google Scholar] [CrossRef]
- Diarsa, M.; Gupte, A. Preparation, Characterization and Its Potential Applications in Isoniazid Drug Delivery of Porous Microcrystalline Cellulose from Banana Pseudostem Fibers. 3 Biotech 2021, 11, 334. [Google Scholar] [CrossRef]
- Khiari, R.; Belgacem, M.N. Potential for Using Multiscale Posidonia oceanica Waste: Current Status and Prospects in Material Science. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 447–471. [Google Scholar] [CrossRef]
- Subaigyo, A.; Chafidz, A. Banana Pseudo-Stem Fibre: Preparation, Characteristics, and Applications. In Ligno-cellulosic Polymer Composites; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Gomes, C.V.; Araújo, J.C.; Chaves, D.M.; Fangueiro, R.; Ferreira, D.P. Improving Textile Circular Economy through Banana Fibres from the Leaves Central Rib: Effect of Different Extraction Methods. Food Bioprod. Process. 2024, 146, 195–204. [Google Scholar] [CrossRef]
- Badanayak, P.; Jose, S.; Bose, G. Banana Pseudostem Fiber: A Critical Review on Fiber Extraction, Characteriza-tion, and Surface Modification. J. Nat. Fibers 2023, 20, 2168821. [Google Scholar] [CrossRef]
- Van, N.T.T.; Gaspillo, P.A.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Ha, H.K.P. Cellulose from the Banana Stem: Optimization of Extraction by Response Surface Methodology (RSM) and Characterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef]
- Bekraoui, N.; El Qoubaa, Z.; Chouiyakh, H.; Faqir, M.; Essadiqi, E. Banana Fiber Extraction and Surface Charac-terization of Hybrid Banana Reinforced Composite. J. Nat. Fibers 2022, 19, 12982–12995. [Google Scholar] [CrossRef]
- Quintana, E.; Valls, C.; Roncero, M.B. Dissolving-Grade Pulp: A Sustainable Source for Fiber Production. Wood Sci. Technol. 2024, 58, 23–85. [Google Scholar] [CrossRef]
- Ramadevi, P.; Sampathkumar, D.; Srinivasa, C.V.; Bennehalli, B. Effect of Alkali Treatment on Water Absorp-tion of Single Cellulosic Abaca Fiber. BioResources 2012, 7, 3515–3524. [Google Scholar] [CrossRef]
- Sango, T.; Yona, A.M.C.; Duchatel, L.; Marin, A.; Ndikontar, M.K.; Joly, N.; Lefebvre, J.M. Step-Wise Multi-Scale Deconstruction of Banana Pseudo-Stem (Musa acuminata) Biomass and Morpho-Mechanical Characterization of Extracted Long Fibres for Sustainable Applications. Ind. Crops Prod. 2018, 122, 657–668. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Zacharopoulos, N.; Christopoulos, M.; Kiskira, K.; Markou, G.; Tsakanika, L.A.; Priniotakis, G. Upcycling Industrial Peach Waste to Produce Dissolving Pulp. Environ. Sci. Pollut. Res. 2025, 32, 4636–4655. [Google Scholar] [CrossRef]
- Kumar, V.; Chakraborty, P.; Janghu, P.; Umesh, M.; Sarojini, S.; Pasrija, R.; Sivalingam, A.M. Potential of Bana-na Based Cellulose Materials for Advanced Applications: A Review on Properties and Technical Challenges. Carbohydr. Polym. Technol. Appl. 2023, 6, 100366. [Google Scholar]
- Iliyin, I.; Purwaningsih, H.; Irawadi, T.T. Isolation and Characterization of Cellulose from Banana Stems Using Microwave Heating. J. Kimia Valensi 2021, 6, 169–176. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, J.; Zhou, Y.; Li, R.; Zhou, W. Characterization of Cellulose from Banana Pseudo-Stem by Heterogeneous Liquefaction. Carbohydr. Polym. 2015, 134, 495–501. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Alwani, M.S.; Omar, A.M. Chemical composition, anatomy, lignin distribution, and cell wall structure of Malaysian plant waste fibers. BioResources 2006, 1, 220–232. [Google Scholar] [CrossRef]
- Bilba, K.; Arsene, M.-A.; Ouensanga, A. Study of Banana and Coconut Fibers: Botanical Composition, Thermal Degradation and Textural Observations. Bioresour. Technol. 2007, 98, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Samanta, K.K.; Chattopadhyay, S.K.; Reddy, E.N.; Mukhopadhyay, S.; Datta, S.; Hadge, G.B. Flame Resistant Cellulosic Substrate Using Banana Pseudostem Sap. Pol. J. Chem. Technol. 2015, 17, 33–39. [Google Scholar] [CrossRef]
- Hidayati, S.; Zuidar, A.S.; Satyajaya, W. Effect of Acetic Acid:Formic Acid Ratio on Characteristics of Pulp from Oil Palm Empty Fruit Bunches (OPEFB). ARPN J. Eng. Appl. Sci. 2017, 12, 3802–3807. [Google Scholar]
- Chopra, L.; Manikanika. Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Othman, J.A.S.; Ilyas, R.A.; Nordin, A.H.; Ngadi, N.; Alkbir, M.F.M.; Knight, V.F.; Norrrahim, M.N.F. Optimization of Delignification and Mercerization Processes for High-Purity Cellulose Extraction from Semantan Bamboo (Gigantochloa scortechinii) Using Response Surface Modelling. Carbohydr. Polym. Technol. Appl. 2025, 10, 100784. [Google Scholar] [CrossRef]
- Merais, M.S.; Khairuddin, N.; Salehudin, M.H.; Mobin Siddique, M.B.; Lepun, P.; Chuong, W.S. Preparation and Characterization of Cellulose Nanofibers from Banana Pseudostem by Acid Hydrolysis: Physico-Chemical and Thermal Properties. Membranes 2022, 12, 451. [Google Scholar] [CrossRef]
- Benítez, A.N.; Monzón, M.D.; Angulo, I.; Ortega, Z.; Hernández, P.M.; Marrero, M.D. Treatment of Banana Fiber for Use in the Reinforcement of Polymeric Matrices. Measurement 2013, 46, 1065–1073. [Google Scholar] [CrossRef]
- Reddy, M.I.; Sethuramalingam, P.; Sahu, R.K. Isolation of Microcrystalline Cellulose from Musa paradisiaca (Banana) Plant Leaves: Physicochemical, Thermal, Morphological, and Mechanical Characterization for Light-weight Polymer Composite Applications. J. Polym. Res. 2024, 31, 114. [Google Scholar] [CrossRef]
- Gong, L.; Kumar, A.; Chunxiao, Y.P.; Kumar, T.V.; Newbold, J.; Clark, W.; Jiang, Y.; Kumar, S.; Kumar, G.V. Sustainable Utilization of Fruit and Vegetable Waste Bioresources for Bioplastics Production. Crit. Rev. Biotechnol. 2024, 44, 236–254. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Ornaghi Jr, H.L.; Arantes, V.; Cioffi, M.O.H. Effect of Chemical Treatment of Pineapple Crown Fiber in the Production, Chemical Composition, Crystalline Structure, Thermal Stability and Thermal Deg-radation Kinetic Properties of Cellulosic Materials. Carbohydr. Res. 2021, 499, 108227. [Google Scholar] [CrossRef]
- Hidayati, S.; Suroso, E.; Satyajaya, W.; Iryani, D.A. Chemistry and Structure Characterization of Bamboo Pulp with Formacell Pulping. IOP Conf. Ser. Mater. Sci. Eng. 2019, 532, 012024. [Google Scholar] [CrossRef]
- Kamoga, O.; Lwako, M.; Byaruhanga, J.K.; Kirabira, J.B. A Review on Pulp Manufacture from Non-Wood Plant Materials. Int. J. Chem. Eng. Appl. 2013, 4, 144–148. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Fernandes, L.L.; Santos, L.B.U.; Ornaghi Jr, H.L.; Cioffi, M.O.H. Different Sequential Chemical Treatments Used to Obtain Bleached Cellulose from Orange Bagasse. J. Nat. Fibers 2022, 19, 12849–12861. [Google Scholar] [CrossRef]
- Ardila, A.A.N.; Arriola Villaseñor, E.; González, E.E.V.; Guerrero, H.E.G.; Hernández Maldonado, J.A.; Gutiérrez-Pineda, E.; Villa, C.C. Enhanced Cellulose Extraction from Banana Pseudostem Waste: A Comparative Analysis Using Chemical Methods Assisted by Conventional and Focused Ultrasound. Polymers 2024, 16, 2785. [Google Scholar] [CrossRef]
- Vardhini, K.V.; Murugan, R.; Surjit, R. Effect of Alkali and Enzymatic Treatments of Banana Fibre on Properties of Banana/Polypropylene Composites. J. Ind. Text. 2017, 47, 1849–1864. [Google Scholar] [CrossRef]
- Samathoti, P.; Ramya, B.S.; Dogiparthi, L.K.; Rani, G.U.; Susmitha, A.; Devi, B.N.; Reddy, V.S.; Vidyavathi, M.; Mallikarjuna, B.P. Methods of Extraction of Cellulose from Bio Waste of Banana Plant and Applications: A Review. Asian J. Pharm. 2023, 17, 659–669. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; Hoop, C.D.; Hu, T.X.; Qi, J.Q.; Shupe, T.F. Isolation and Characterization of Cellulose Nan-ofibers from Bamboo Using Microwave Liquefaction Combined with Chemical Treatment and Ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.A.N.; Jai, J. Response Surface Methodology for Optimization of Cellulose Extraction from Ba-nana Stem Using NaOH-EDTA for Pulp and Papermaking. Heliyon 2022, 8, e09114. [Google Scholar] [CrossRef] [PubMed]
- Samara, F.S.; Novia, N.; Melwita, E. Enzymatic Hydrolysis of Cellulose Banana Stem (Alkaline Micro-wave-Assisted Pre-Treatment). J. Integr. Adv. Eng. 2024, 4, 21–30. [Google Scholar] [CrossRef]
- Meng, F.; Wang, G.; Du, X.; Wang, Z.; Xu, S.; Zhang, Y. Extraction and Characterization of Cellulose Nano-fibers and Nanocrystals from Liquefied Banana Pseudo-Stem Residue. Compos. Part B Eng. 2019, 160, 341–347. [Google Scholar] [CrossRef]
- Nigam, S.; Das, A.K.; Matkawala, F.; Patidar, M.K. An Insight Overview of Bioplastics Produced from Cellulose Extracted from Plant Material, Its Applications and Degradation. Environ. Sustain. 2022, 5, 423–441. [Google Scholar] [CrossRef]
- Plakantonaki, S. Converting Industrial Peach Waste into Sustainable Fibres via Physicochemical Processes towards Circular Economy. Ph.D. Dissertation, University of West Attica, Aigaleo, Greece, 2024. [Google Scholar]
- Tufan, M.Z.; Özel, C. Cellulose and Its Derivatives as Biodegradable Materials: A Review. J. Sci. Rep. A 2024, 59, 87–104. [Google Scholar] [CrossRef]
- Nigam, S.; Das, A.K.; Patidar, M.K. Synthesis, Characterization and Biodegradation of Bioplastic Films Pro-duced from Parthenium hysterophorus by Incorporating a Plasticizer (PEG600). Environ. Challenges 2021, 5, 100280. [Google Scholar] [CrossRef]
- Khaleed, G.; Sharanagat, V.S.; Upadhyay, S.; Desai, S.; Kumar, K.; Dhiman, A.; Suhag, R. Sustainable Ap-proach Toward Biodegradable Packaging Through Naturally Derived Biopolymers: An Overview. J. Packag. Technol. Res. 2024, 9, 19–46. [Google Scholar] [CrossRef]
- Bourtsalas, A.C.; Papadatos, P.E.; Kiskira, K.; Kalkanis, K.; Psomopoulos, C.S. Ecodesign for Industrial Furnaces and Ovens: A Review of the Current Environmental Legislation. Sustainability 2023, 15, 9436. [Google Scholar] [CrossRef]
- Ali, Z.; Talpur, F.N.; Afridi, H.I.; Ahmed, F.; Brohi, N.A.; Abbasi, H. Analytical Approaches and Advancement in the Analysis of Natural and Synthetic Fiber: A Comprehensive Review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 326, 125164. [Google Scholar] [CrossRef]
- Kiruthika, A.V.; Veluraja, K. Experimental Studies on the Physico-Chemical Properties of Banana Fibre from Various Varieties. Fibers Polym. 2009, 10, 193–199. [Google Scholar] [CrossRef]
- International Standards Organisation. Pulps—Determination of Kappa Number; Edition 3, 2015. Available online: https://standards.iteh.ai/catalog/standards/sist/cd48da24-6cf1-46a0-b630-168a2ba96566/iso-302-2015 (accessed on 10 September 2025).
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Das, M.; Chakraborty, D. Influence of Alkali Treatment on the Fine Structure and Morphology of Bamboo Fi-bers. J. Appl. Polym. Sci. 2006, 102, 5050–5056. [Google Scholar] [CrossRef]
- Abrha, H.; Cabrera, J.; Dai, Y.; Irfan, M.; Toma, A.; Jiao, S.; Liu, X. Bio-Based Plastics Production, Impact and End of Life: A Literature Review and Content Analysis. Sustainability 2022, 14, 4855. [Google Scholar] [CrossRef]
- Kalkanis, K.; Kiskira, K.; Papageorgas, P.; Kaminaris, S.D.; Piromalis, D.; Banis, G.; Mpelesis, D.; Batagiannis, A. Advanced manufacturing design of an emergency mechanical ventilator via 3D printing—Effective crisis response. Sustainability 2023, 15, 2857. [Google Scholar] [CrossRef]
- Alevizos, V.; Gerolimos, N.; Edralin, S.; Xu, C.; Simasiku, A.; Priniotakis, G.; Papakostas, G.A.; Yue, Z. Systematic Review on Sustainable Design Thinking through Biomimetic Approach. In Proceedings of the 2025 International Conference on Artificial Intelligence in Information and Communication (ICAIIC), Fukuoka, Japan, 18–21 February 2025; pp. 219–223. [Google Scholar] [CrossRef]
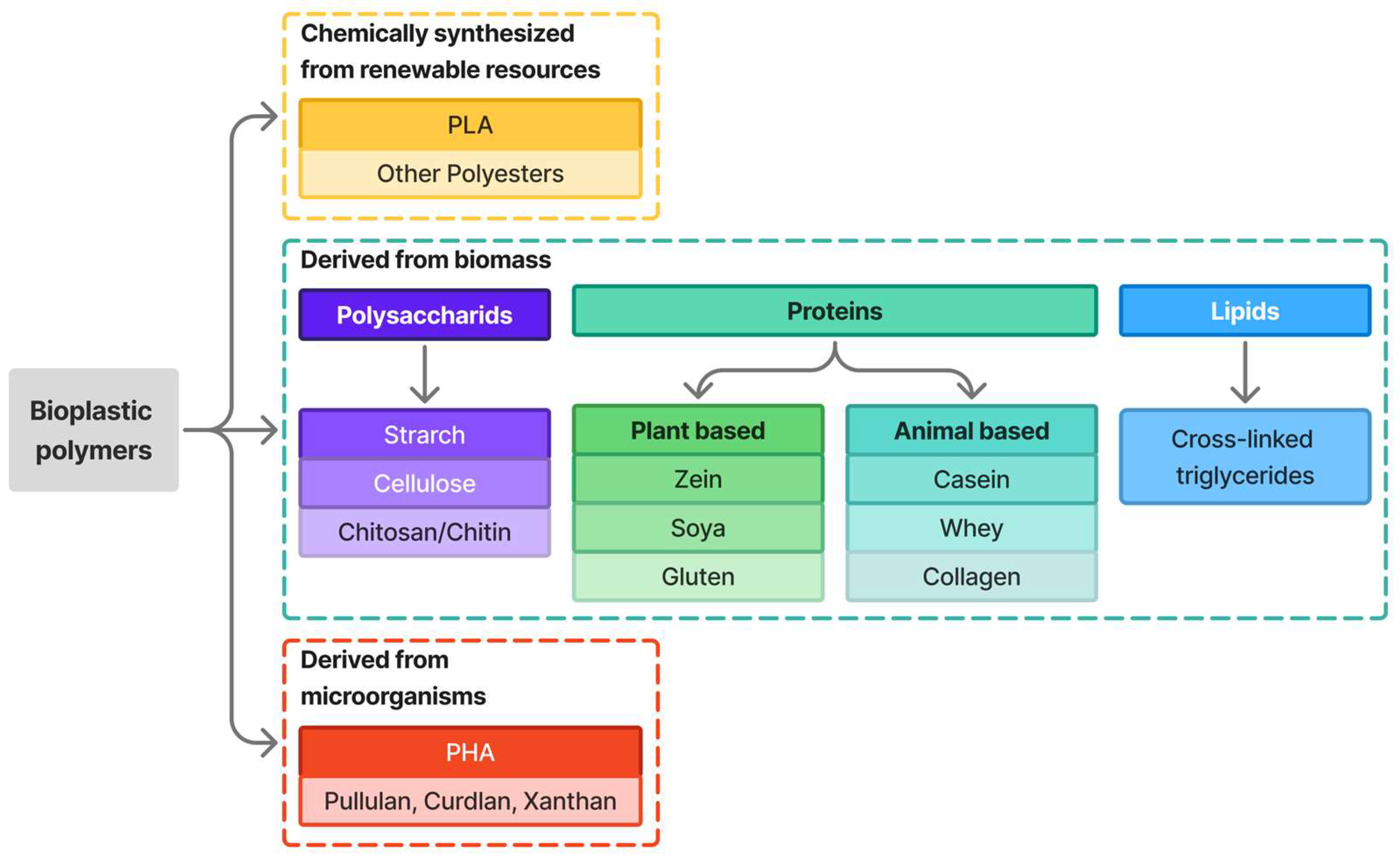


| Conventional Synthetic Plastics | Bioplastics |
|---|---|
| Derived from fossil fuels and petrochemicals | Derived from renewable, natural resources |
| Non-renewable source | Renewable source |
| Generally non-biodegradable | Mostly biodegradable |
| Persist in environment as microplastics | Degrade in 3–6 months under industrial composting |
| High environmental impact, greenhouse gases | Environmentally friendly, lower ecological impact, carbon-neutral potential |
| Used in packaging, construction, textiles | Used in biodegradable packaging, edible films, biomedical tools |
| Category | Bio Based | Petroleum Based |
|---|---|---|
| Biodegradable | Starch, Cellulose, polyhydroxyalkanoates, Polylactic acid | Polycaprolactone (PCL), Polybutylene succinate, Polybutylene adipate Terephthalate (PAT) |
| Non-biodegradable | Bio-polyethylene, Biopolypropylene | PVC, Polypropylene, Polyethylene |
| Type of Bioplastic | Primary Feedstock |
|---|---|
| Thermoplastic starch (TPS) | Starch [44,56] |
| Plastarch material (PSM) | Starch [8,55] |
| Starch/polycaprolactone (or polyvinyl acetate) mix | Starch/ petroleum [25,44] |
| Polylactic Acid (PLA) | Starch sugars [6,25,33] |
| Polyhydroxyalkanoates (PHA) | Starch sugars [6,25,33] |
| Cellulose acetate | Wood, cotton or hemp cellulose [8,30,57] |
| Lignin | Wood [38,42] |
| Bio Polyethylene | Sugarcane-derived bioethanol [6,33] |
| Bio Polyurethane | Soya beans [6,25,33] |
| BioPBS (Polybutylene succinate) | Sugar cane, corn [6,8,25] |
| Biopolymer | Advantages | Disadvantages |
|---|---|---|
| Natural biopolymers | Biologically renewable, biodegradable, biocompatible, non-toxic, bio adhesive material, bifunctional. | Less stable, low melting point, high surface tension, structurally more complex, poor barrier properties, weak mechanical properties, high cost of production, functionality |
| Synthetic polymers | Biocompatibility, higher reproducibility, better mechanical, and chemical stability | Toxic, non-biodegradable, expensive synthesis procedure. |
| Strengths | Limitation |
|---|---|
| Renewable: Derived from renewable resources, reducing reliance on fossil fuels. | Non-waterproof: Moisture-resistant but not waterproof; unsuitable for freezing. |
| Biodegradable: Reduces environmental waste. | Durability: Less durable than conventional plastics. |
| Lightweight: Reduces transportation and distribution carbon emissions. | Water-intensive production: Requires large volumes of water. |
| Strong: Provides adequate protection despite being lightweight. | Limited food preservation: Does not extend shelf life alone; needs additional barrier materials. |
| Temperature-resistant: Suitable for transporting pre-prepared foods. | Non-cold resistant: Not suitable for freezer storage. |
| Versatile: Can be molded and customized for various applications. | Specialized recycling: Requires specialized facilities for effective recycling. |
| Low-cost: Byproduct of paper industry; abundant raw materials reduce cost compared to other eco-friendly plastics. | High cost: More expensive than synthetic plastics due to raw materials, production, and equipment. |
| Lower carbon footprint: Uses less energy, emits fewer greenhouse gases, and reduces fossil fuel dependence. | Thermal limitations: Inadequate thermal resistance. |
| Source/Reference | Banana Species or Origin | Processing/Extraction Method | Cellulose % | Hemicellulose % | Lignin % | Extractives % | Ash Content % | Moisture Content % |
|---|---|---|---|---|---|---|---|---|
| [93] | Musa acuminata (China) | Alkali treatment (5% NaOH) + bleaching with NaClO2 | 63.20 | 18.6 | 5.10 | 1.4 | 1.02 | 10.00 |
| [94] | Musa sapientum (Malaysia) | Soxhlet extraction + alkaline pulping (NaOH) | 31.27 | 14.98 | 15.07 | 4.46 | 8.65 | 9.74 |
| [82] | Musa paradisiaca (India) | Mechanical + alkali extraction (NaOH 10%) | 63.9 | 1.3 | 18.6 | 10.6 | 1.5 | - |
| [95] | Musa textilis (Guadeloupe, Caribbean) | Water retting + NaOH + bleaching (NaClO2) | 31.26 | 14.98 | 15.07 | 4.45 | 8.64 | 9.74 |
| [96] | 57 | 10.33 | 15.55 | - | - | 20.23 | ||
| Average | 49.33 | 12.04 | 13.88 | 5.23 | 4.95 | 12.43 |
| Method | Description |
|---|---|
| Alkaline Hydrolysis | Treats biomass with alkaline solutions (e.g., NaOH) to remove lignin and hemicellulose, yielding purified cellulose. |
| Acid Hydrolysis | Uses acids (e.g., H2SO4) to hydrolyze hemicellulose into sugars, leaving cellulose and lignin intact; employs dilute acids at 160–220 °C, breaking glycosidic bonds and improving sugar conversion. |
| Steam Explosion | Applies high-pressure steam followed by rapid decompression to disrupt lignocellulose and enhance enzymatic hydrolysis. |
| Organosolv Fractionation | Treats biomass with organic solvents (ethanol, acetone) under acidic (acetic/formic) or alkaline conditions to dissolve lignin and hemicellulose, leaving cellulose as residue. |
| Mechanical Methods | Mechanically processes biomass (milling, grinding) to reduce particle size and facilitate chemical or enzymatic extraction. |
| Ionic Liquid Pretreatment | Uses ionic liquids (molten salts) to dissolve lignin and disrupt lignocellulosic structure, easing further processing. |
| Deep Eutectic Solvents | Employs low-toxicity, biodegradable solvents for versatile lignocellulose pretreatment. |
| Method/Process | Main Extraction Conditions | Cellulose Yield (%) | Crystallinity % | Lignin/Hemicellulose Removal | Key Observations | References |
|---|---|---|---|---|---|---|
| Alkali treatment (NaOH 10–20%) | Soaking in NaOH 10–20%, ambient T to 90 °C, followed by washing and drying | (qualitative increase) | ↑ up to 15% NaOH | Lignin ↓ and hemicellulose ↓ until 15%; no further removal at 20% | 15% NaOH → optimum crystallinity, tenacity, and thermal stability; >15% NaOH → degradation and darker color | [78] |
| Mild acidic treatment after alkali degumming | Neutralization with dilute acid (e.g., acetic or HCl) after Na2CO3/NaOH degumming, 30–60 min boil | 60–65 | ≈62–65 | Removes residual lignin/hemicellulose; prevents cellulose degradation | Improves fiber purity, brightness, and surface smoothness; stabilizes structure and crystallinity | [84] |
| Ultrasound-Assisted Alkaline and Peroxide Extraction (NaOH/H2O2) | NaOH 25–30% or H2O2 8%; 30–40 °C; 15–60 min; 200–300 W ultrasound | 13–33 (global yield); up to 99.5 (purity) | 20–79.6 | Lignin ≈ 0%; Hemicellulose ↓ 2–14% | Ultrasound greatly enhanced extraction efficiency and crystallinity vs. conventional alkaline pulping. | [108] |
| Optimized Alkaline Extraction (NaOH) | 11 g NaOH L−1; 150 min; 90 °C; optimized by response surface methodology (RSM) | ↑ (~20) | ↑ (~60–70) | Lignin −40%; Hemicellulose −50% | Optimization in-creased α-cellulose by ~20%, improved crystallinity and fiber structure. Demonstrated balance between delignification efficiency and cellulose preservation | [109] |
| Microcrystalline Cellulose via Alkaline + Acid Hydrolysis | Sequential NaOH delignification (10%) → H2SO4 hydrolysis (40%, 60 min, 80 °C) | 90.44 | 72.3 | Complete removal of lignin and hemicellulose confirmed by FTIR | Produced fine-grade MCC with uniform particle size (~50 μm), good thermal stability, and crystallinity enhancement suitable for composite packaging films. | [80] |
| Microwave-Assisted Alkaline + Bleaching + Acid Hydrolysis | Banana stem and peel residues; NaOH 1–4% (10–30 min, 60–80 °C); H2O2/NaOCl bleaching (2 × 3 h, 80 °C); 1% H2SO4 acid hydrolysis (80 °C, 1 h); microwave heating optional | ≈65 | ≈65 | Lignin ≈ 85%, Hemicellulose ≈ 75% removed | Review synthesized multiple experimental studies; highlighted microwave-assisted extraction as efficient, low-chemical, and scalable route for cellulose/nanocellulose recovery from banana waste. | [110] |
| Microwave-assisted liquefaction → alkali delignification → peroxide bleaching | Liquefaction: glycerol:methanol (2:1) + 1.75% H2SO4, MW 3 min; Delignification: 4% NaOH, MW 3 min; Bleaching: 5% H2O2, MW 2 × 4 min; microwave power 450–800 W; total process ≈14 min | 86.43 (cellulose content in recovered pulp at 800 W) | 56.8 | Lignin: FTIR 1519 cm−1 peak absent; Hemicellulose: reduced after NaOH/H2O2 steps | Microwave heating cut total time from ~4 h 30 min (conventional) to ~14 min; higher power increased cellulose content; cellulose type I confirmed (XRD/FTIR); rough, fibrillated surface (SEM) | [92] |
| Microwave liquefaction + chemical purification + ultrasonication (bamboo) | Microwave liquefaction for 7 min; chemical purification with mild alkali + bleaching using reduced chemical dosage; ultrasonic nanofibrillation for fiber separation | ≈80–85 (estimated cellulose in purified residues) | ≈ 65–70 | Nearly complete lignin removal (>90%); effective hemicellulose dissolution | Combined microwave and chemical treatment removed non-cellulosic compounds rapidly with low reagent use; ultrasonication produced nanosized fibrils (elementary + aggregated bundles); cellulose nanofibers showed high thermal stability (TGA) and were proposed for biomaterial reinforcement | [111] |
| Alkaline-acid hydrolysis/Enzymatic hydrolysis/TEMPO oxidation (banana pseudostem) | Three PS particle sizes tested (≤180 µm, ≥2000 µm, unsieved). • Alkaline-acid hydrolysis: 5% NaOH 1 h @ 90 °C → 5% NaOH + 16% H2O2 @ 55 °C → 60% H2SO4 45 min RT. • Enzymatic: 17.5% NaOH 15 h RT → 4% H2O2 + 2% NaOH @ 90 °C 3 h → cellulase (Optimash VR) @ 50 °C 42 h. • TEMPO oxidation: 5% KOH 16 h RT → 1% NaClO2 @ 70 °C 1 h → TEMPO/NaBr/NaOCl 3 h RT. | 3.40 ± 0.11 (alk-acid); 14.58 ± 0.30 (enzymatic); 25.25 ± 0.08 (TEMPO) | 13.5 (alk-acid); 68.98 (enzymatic); ≈55 (TEMPO) | Alk-acid → incomplete removal of lignin/hemicellulose (dark extract); TEMPO → high cellulose yield but oxidative degradation; enzymatic → most efficient delignification with high crystallinity and thermal stability. | Enzymatic hydrolysis produced cellulose with highest crystallinity (68.98%) and thermal stability (~250 °C). TEMPO gave highest yield (25%) but oxidized cellulose with low stability. Fine particles favored better interaction and cellulose extraction. | [37] |
| Microwave-assisted alkaline-peroxide treatment (banana pseudostem) | Central Composite Design optimization: NaOH (1–4%), H2O2 (10–20%), time (10–30 min), T = 100 ± 5 °C. Optimal: 2.9% NaOH, 15.1% H2O2, 21.3 min. | 82.14 | 67.1 | Hemicellulose 17.9%, lignin 2.2% (removed >80%) | Optimal condition maximized cellulose yield and crystallinity; microwave heating reduced reaction time and reagent use. Confirmed cellulose I structure and smooth fibrillar surface (SEM, FTIR, XRD). | [85] |
| NaOH-EDTA pulping optimized by RSM (banana stem) | Central Composite Design used to optimize NaOH (14–20%), EDTA (5–10%), 30 min at 100 ± 5 °C. Optimum: 17.7% NaOH, 10% EDTA, liquid-to-solid ratio 10 mL/g. | 86.3 ± 1.1 | - | Lignin removal ≈ 58.1 ± 1.5%; pulp yield ≈ 62.7 ± 1.2%. | EDTA esterified to cellulose (FTIR 1738 cm−1, C=O stretch), stabilizing reducing ends and minimizing alkaline hydrolysis. Degree of polymerization = 2140 (vs. 1907 without EDTA). Demonstrated efficient delignification at low temperature with high yield and polymerization, suitable for papermaking or bioplastics. | [112] |
| Alkaline microwave-assisted pre-treatment + enzymatic hydrolysis (banana stem) | 5% KOH (1:10 w/v) at 80 °C for 30 min → microwave 300 W for 10 min → neutralization → enzymatic hydrolysis with Aspergillus niger cellulase (1:1–1:10 substrate:enzyme, 50 °C, 200 rpm, 5–45 h). | 77.55 (after treatment) | - | Lignin ↓ from 14.9 → 1.46%; hemicellulose ↓ from 13.1 → 8.56%. | Combined KOH and microwave pretreatment significantly increased cellulose accessibility and hydrolysis efficiency; maximum reducing sugar = 1.3 mg mL−1 at 1:1 enzyme:substrate for 45 h. Demonstrated synergistic delignification and enhanced bioethanol potential. | [113] |
| Ternary Deep Eutectic Solvent (ChCl-EG-Oxalic acid) pretreatment + NaOH/H2O2 purification | Biomass (sugarcane bagasse, wood pulp waste, boxboard waste) treated in DES (1:2:0.8 molar ratio) at 130 °C for 2 h → alkaline (5% NaOH) + 30% H2O2 at 90 °C for 2 h. | SCB 72.86, wood pulp 43.82, boxboard 38.81 | - | Lignin ↓ to 5–8%, hemicellulose ↓ to 3–4% (≈80–90% removal) | DES pretreatment (ChCl-EG-OA) highly effective in lignin/hemicellulose removal, enhancing cellulose purity and accessibility. Acetylation produced cellulose acetate films with high mechanical strength (boxboard film 11.23 MPa tensile strength, 3.14% elongation). Demonstrated efficient, low-toxicity extraction applicable to mixed-source bioplastics. | [23] |
| Ester Bases | Cellulose Ester | Reagent |
|---|---|---|
| Organic esters | Cellulose acetate | Acetic acid and acetic anhydride |
| Cellulose triacetate | Acetic acid and acetic anhydride | |
| Cellulose propionate | Propanoic acid | |
| Cellulose acetate propionate | Acetic acid and propanoic acid | |
| Cellulose acetate butyrate | Acetic acid and butyric acid | |
| Cellulose Xanthate Xanthic acid | Xanthic acid | |
| Inorganic esters | Nitrocellulose (cellulose nitrate) ➝ Cell-OH + HNO3 Cell-O-NO2 + H2O | Nitric acid or another powerful nitrating agent |
| Cellulose sulfate ➝ Cell-OH + H2SO4 Cell-O-SO3H + H2O | Sulfuric acid or another powerful sulfuring agent | |
| Cellulose phosphate ➝ Cell-OH + H2PO4 Cell-O-PO3H + H2O | Phosphoric or another powerful phosphoring agent |
| Material | Tensile Strength (MPa) | Elongation at Break (%) | Water Vapor Permeability (×10−11 g m/m2 s Pa) | Reference |
|---|---|---|---|---|
| Cellulose acetate (banana pseudostem) | 50–70 | 5–12 | 2.5 | [22,23] |
| PLA | 55–65 | 6–10 | 3.2 | [26] |
| PHA | 45–60 | 10–20 | 3.0 | [6] |
| Starch-based film | 25–35 | 5–8 | 5.5 | [8] |
| PET (conventional) | 65–75 | 20–50 | 2.0 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waithaka, A.; Plakantonaki, S.; Kiskira, K.; Mburu, A.W.; Chronis, I.; Zakynthinos, G.; Githaiga, J.; Priniotakis, G. Cellulose-Based Biopolymers from Banana Pseudostem Waste: Innovations for Sustainable Bioplastics. Waste 2025, 3, 37. https://doi.org/10.3390/waste3040037
Waithaka A, Plakantonaki S, Kiskira K, Mburu AW, Chronis I, Zakynthinos G, Githaiga J, Priniotakis G. Cellulose-Based Biopolymers from Banana Pseudostem Waste: Innovations for Sustainable Bioplastics. Waste. 2025; 3(4):37. https://doi.org/10.3390/waste3040037
Chicago/Turabian StyleWaithaka, Alice, Sofia Plakantonaki, Kyriaki Kiskira, Ann W. Mburu, Ioannis Chronis, Georgios Zakynthinos, John Githaiga, and Georgios Priniotakis. 2025. "Cellulose-Based Biopolymers from Banana Pseudostem Waste: Innovations for Sustainable Bioplastics" Waste 3, no. 4: 37. https://doi.org/10.3390/waste3040037
APA StyleWaithaka, A., Plakantonaki, S., Kiskira, K., Mburu, A. W., Chronis, I., Zakynthinos, G., Githaiga, J., & Priniotakis, G. (2025). Cellulose-Based Biopolymers from Banana Pseudostem Waste: Innovations for Sustainable Bioplastics. Waste, 3(4), 37. https://doi.org/10.3390/waste3040037









