Enhanced CO2 Sequestration in Recycled Aggregates: Exploring Novel Capture-Promoting Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbonation Conditions
2.3. Characterization Methods
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (FTIR-ATR)
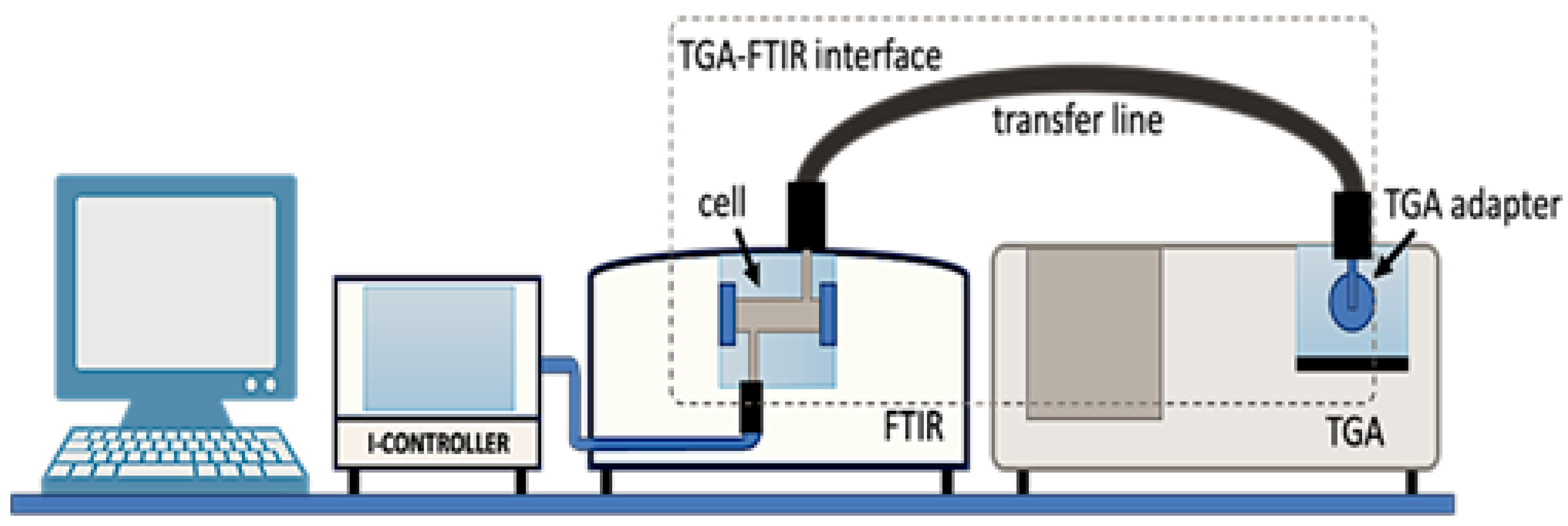
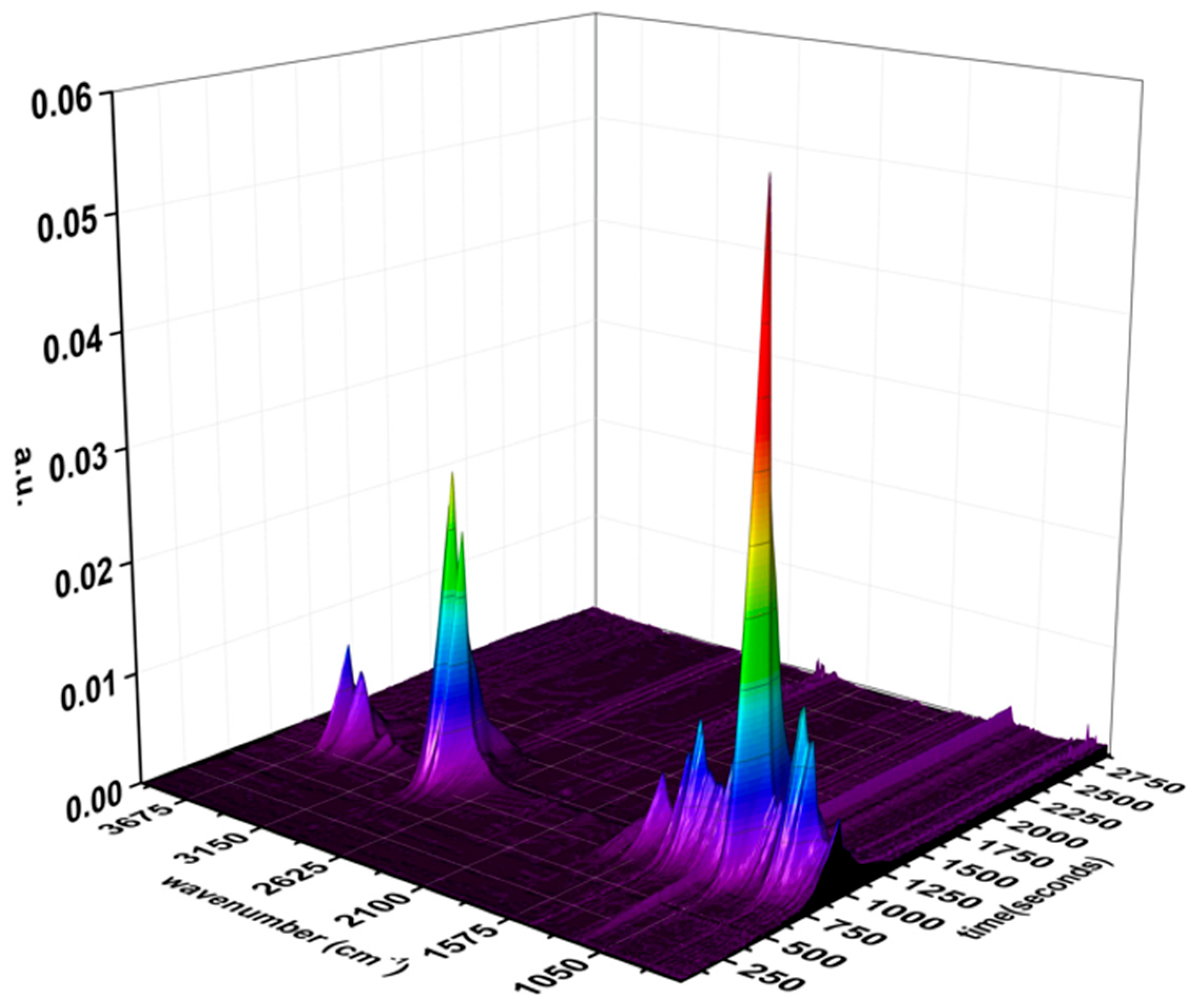
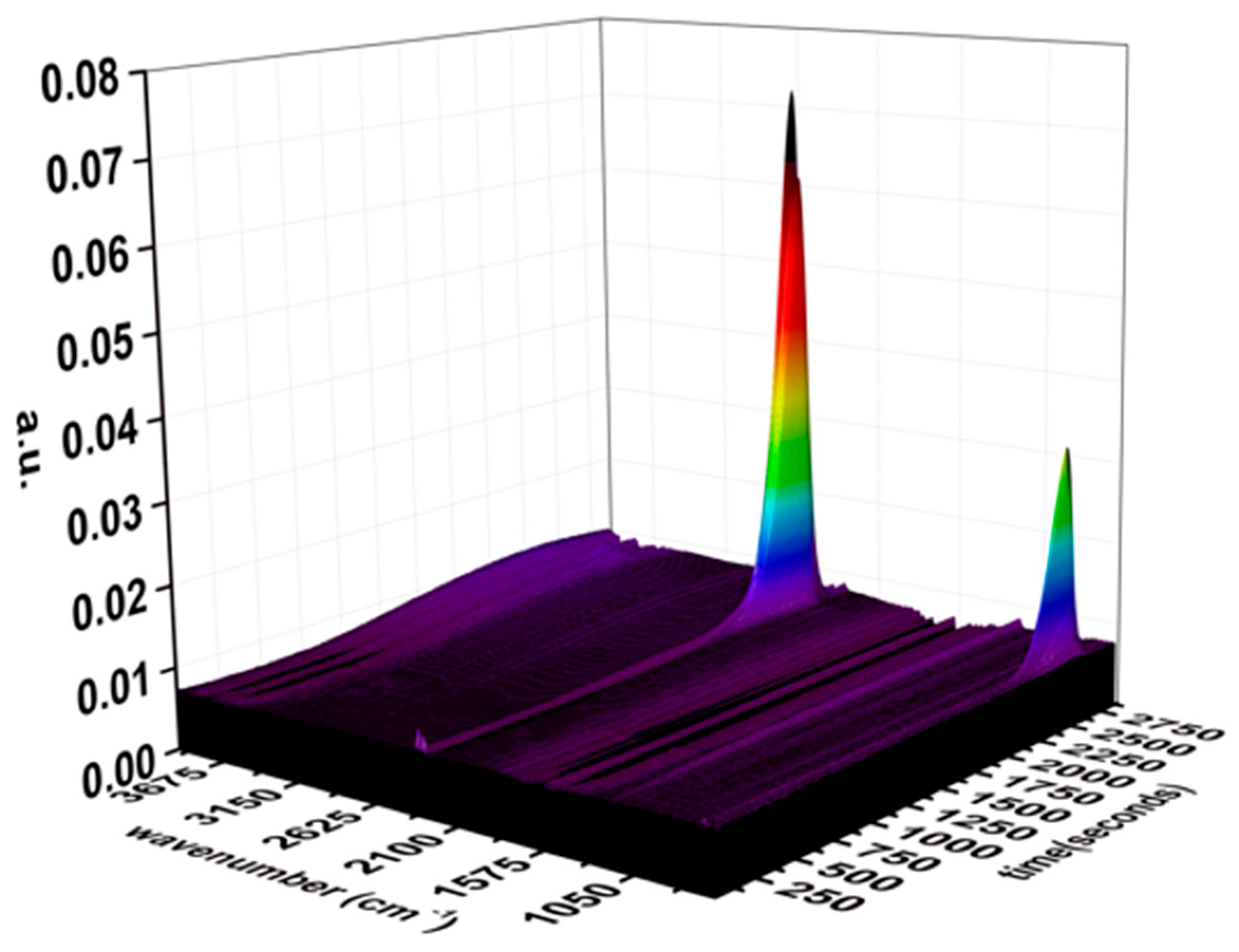
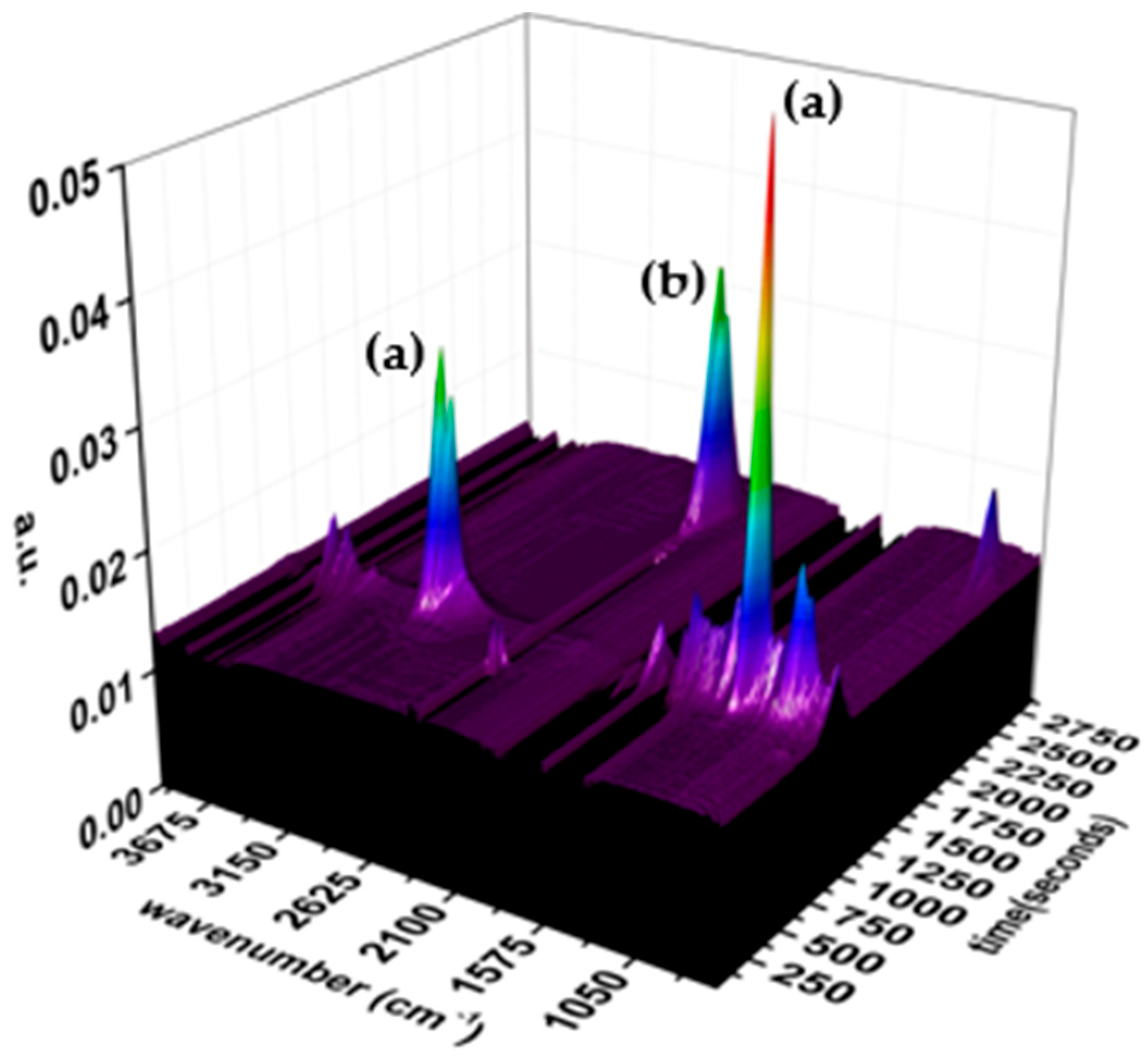
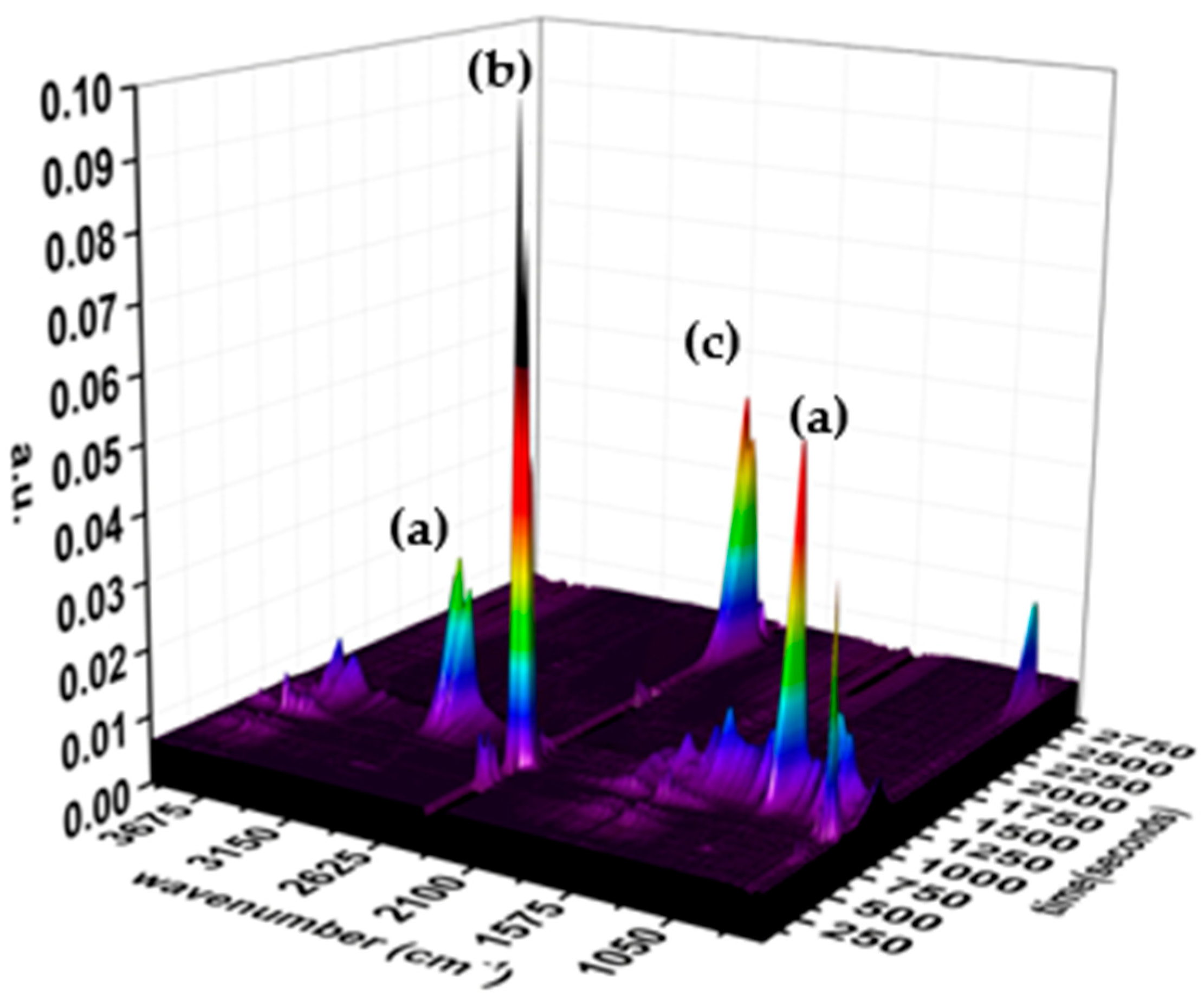
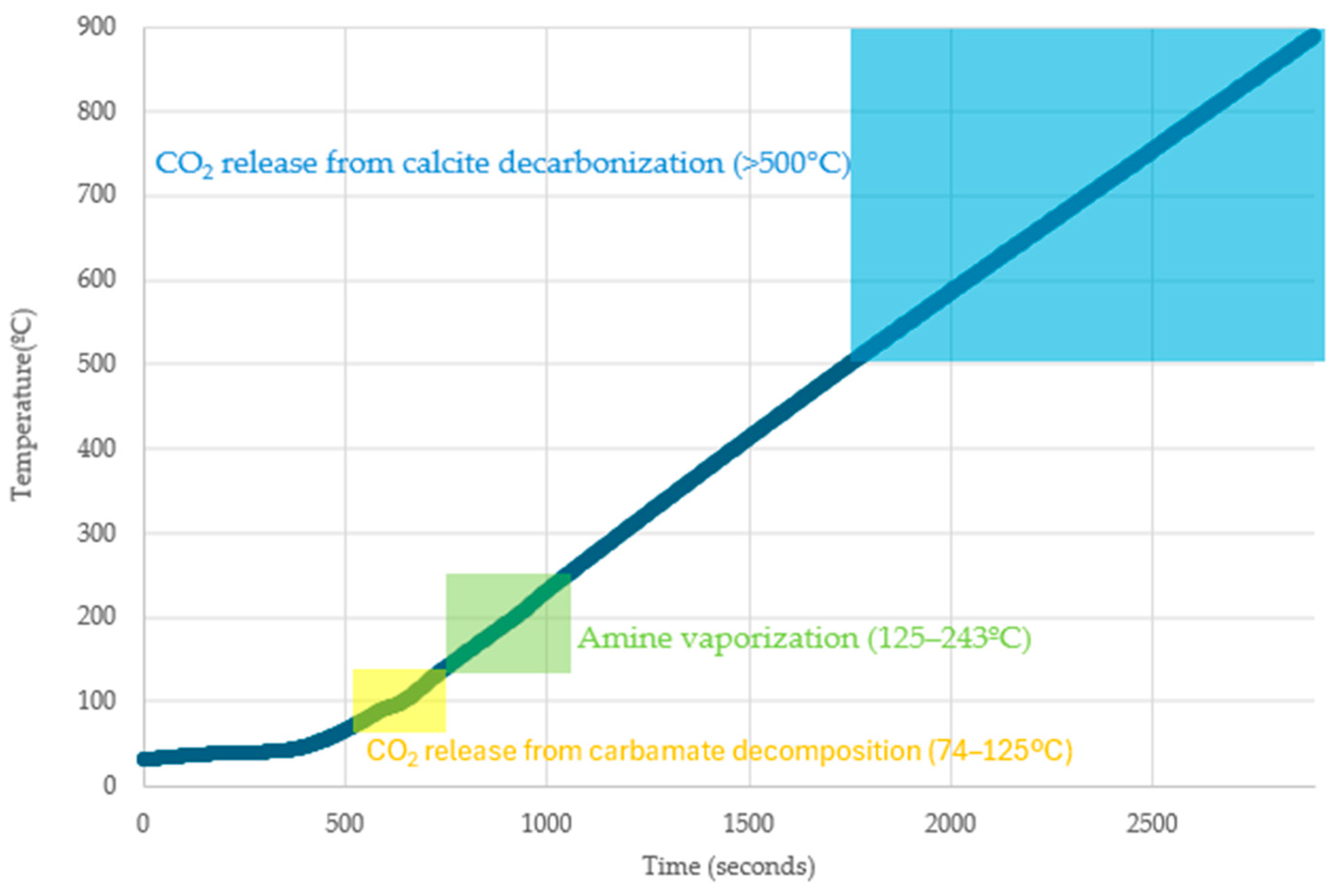
2.3.3. TG-FTIR
3. Results and Discussion
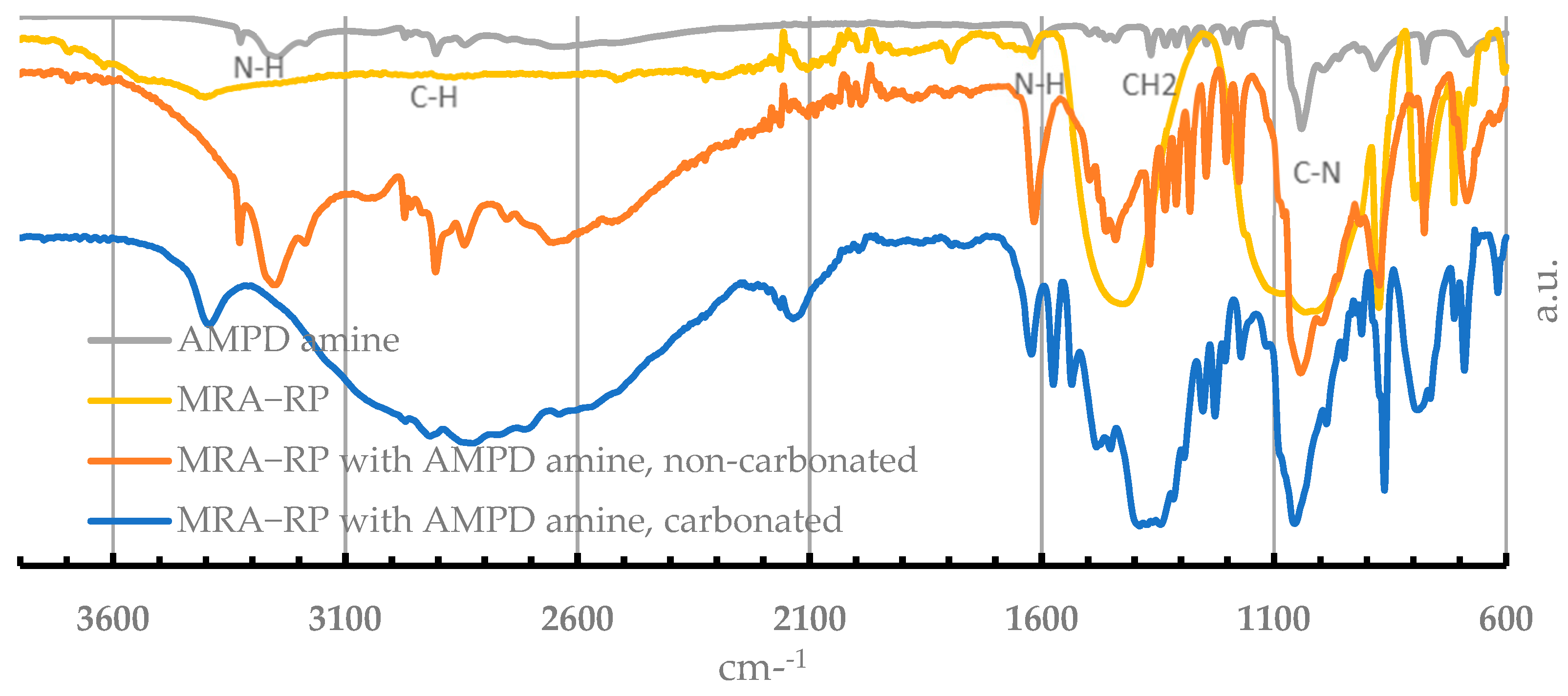
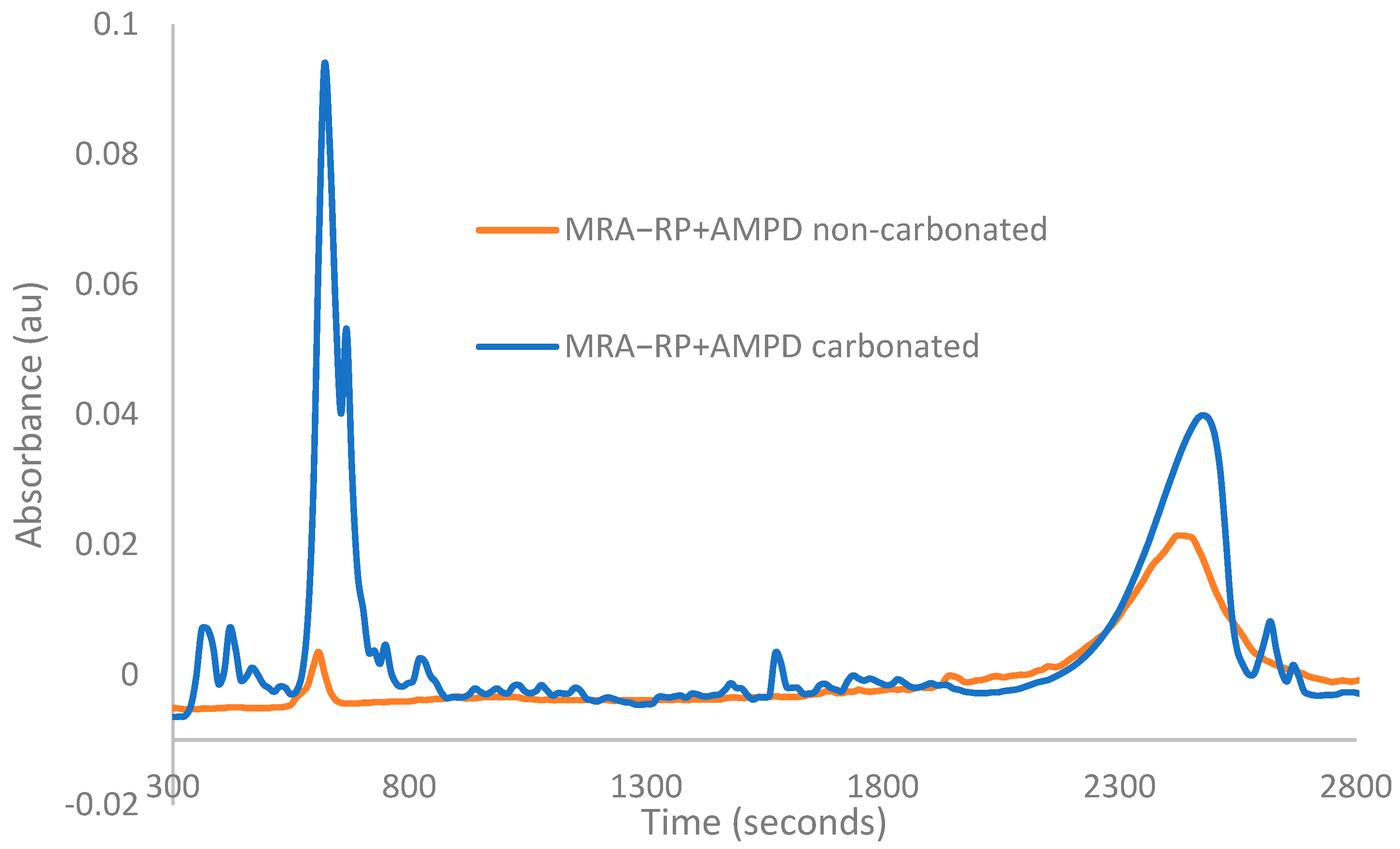
3.1. FTIR-ATR Characterization
- N-H stretching vibrations (3400–3200 cm−1)—this band represent the “free” stretching modes of the N-H bond.
- N-H bending (scissoring) vibration (1650–1580 cm−1).
- N-H wagging 909–666 cm−1, usually assigned to N-H wagging.
- C-N stretching vibrations band (1100–1000 cm−1)—C-N stretching coupled with the stretching of adjacent bonds in the molecule.
- 3387 cm−1—carbamate formation, a product of the reaction of the CO2 with the impregnated amine.
- 2128 cm−1—assigned to the N-H combinations of secondary ammonium ion (NH2+) bands.
- 1400–1300 cm−1—the absorption of the carbamate.
- 1574 cm−1—the asymmetric stretching mode of CO2 and symmetric deformation.
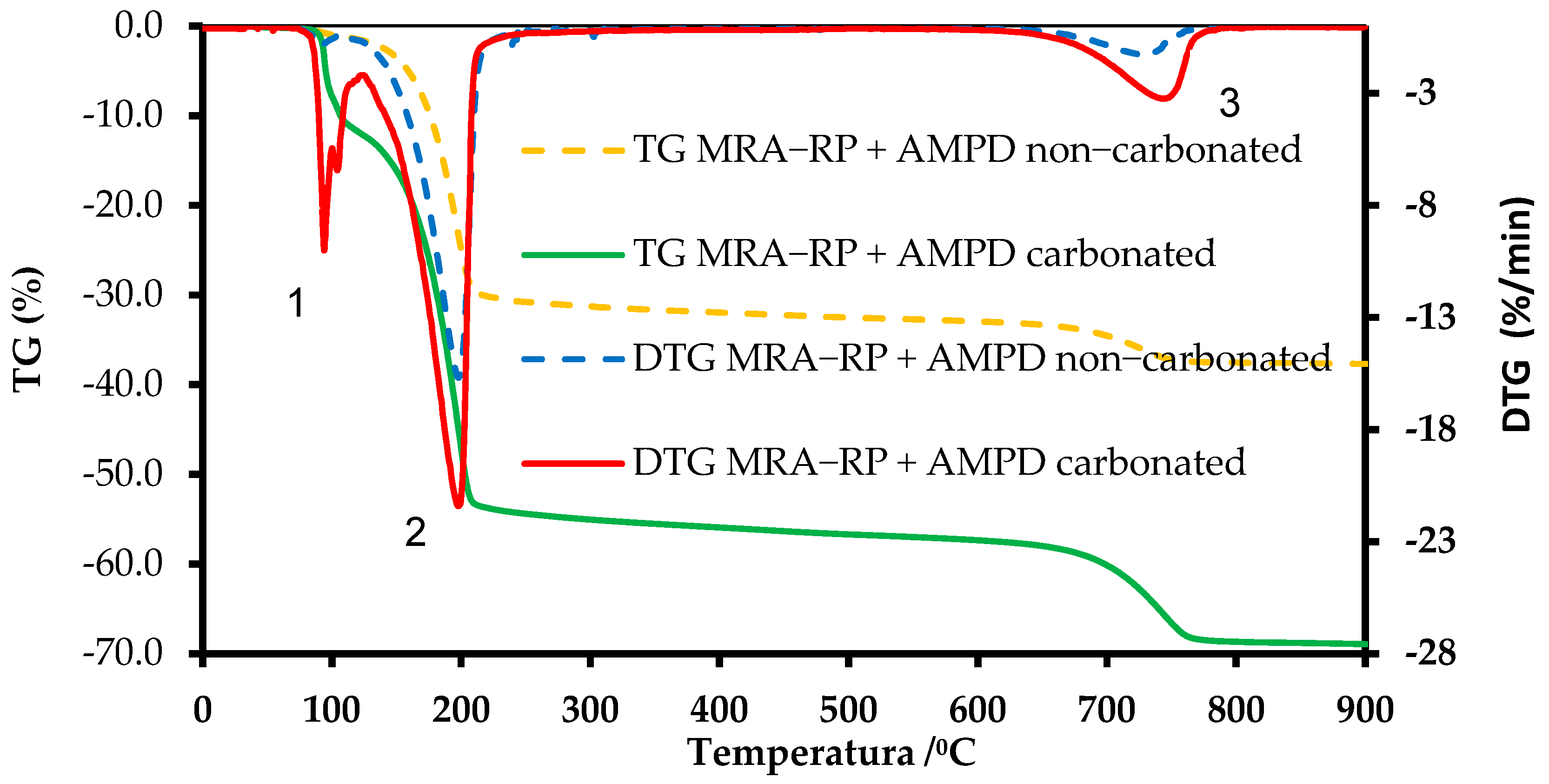
3.2. TGA
3.3. TG-FTIR Analysis
4. Conclusions
- It is possible to increase the value of an aggregate with a low potential for CO2 sequestration by using an amine as an additive.
- This study studies highlight the use of several techniques to quantify the capture of CO2 and amine impregnation.
- The use of TG-FTIR was crucial in evaluating and confirming the mass changes corresponding to CO2 uptake/release by AMPD.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDW | Construction and demolition waste |
| CCUS | Carbon Capture, Utilization and Storage |
| MRA | Mixed recycled aggregate |
| TGA | Thermogravimetric analysis |
| FTIR-ATR | Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy |
| AMPD | 2-amino-2-methyl-1,3-propanediol |
| RA | Recycled aggregate |
References
- Gomes, R.I.; Farinha, C.B.; Veiga, R.; de Brito, J.; Faria, P.; Bastos, D. CO2 sequestration by construction and demolition waste aggregates and effect on mortars and concrete performance—An overview. Renew. Sustain. Energy Rev. 2021, 152, 111668. [Google Scholar] [CrossRef]
- European Commission; IES Institute for Environment and Sustainability; European Commission; Joint Research Centre; JRC-IES. Supporting Environmentally Sound Decisions for Construction and Demolition (C&D) Waste Management; EUR 24918; JRC-IES: Ispra, Italy, 2011. [Google Scholar]
- Coelho, A.; De Brito, J. Economic viability analysis of a construction and demolition waste recycling plant in Portugal—Part I: Location, materials, technology and economic analysis. J. Clean. Prod. 2013, 39, 338–352. [Google Scholar] [CrossRef]
- Verian, K.P.; Ashraf, W.; Cao, Y. Properties of recycled concrete aggregate and their influence in new concrete production. Resour. Conserv. Recyc. 2018, 133, 30–49. [Google Scholar] [CrossRef]
- Bravo, M.; De Brito, J.; Pontes, J.; Evangelista, L. Mechanical performance of concrete made with aggregates from construction and demolition waste recycling plants. J. Clean. Prod. 2015, 99, 59–74. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Fan, Y.; Huang, X. An overview of study on recycled aggregate concrete in China (1996–2011). Constr. Build. Mater. 2012, 31, 364–383. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cem. Concr. Compos. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Sereng, M.; Djerbi, A.; Metalssi, O.O.; Dangla, P.; Torrenti, J.-M. Improvement of Recycled Aggregates Properties by Means of CO2 Uptake. Appl. Sci. 2021, 11, 6571. [Google Scholar] [CrossRef]
- Liang, C.; Li, B.; Guo, M.-Z.; Hou, S.; Wang, S.; Gao, Y.; Wang, X. Effects of early-age carbonation curing on the properties of cement-based materials: A review. J. Build. Eng. 2024, 84, 108495. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- CEMBUREAU. The Role of Cement in the 2050 Low Carbon Economy; The European Cement Association: Brussels, Belgium, 2013. [Google Scholar]
- IEA; CSI. Technology Roadmap: Low-Carbon Transition in the Cement Industry. International Energy Agency (IEA), Cement Sustainability Initiative (CSI). 2018. Available online: https://www.iea.org/reports/technology-roadmap-low-carbon-transition-in-the-cement-industry (accessed on 16 October 2020).
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon dioxide uptake by cement-based materials: A spanish case study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef]
- Cui, H.; Tang, W.; Liu, W.; Dong, Z.; Xing, F. Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr. Build. Mater. 2015, 93, 522–527. [Google Scholar] [CrossRef]
- Nogueira, R.; Silva, A.; Alexandre Bogas, J. Strategies for carbon capture in concrete production. In The Carbon Chain in Carbon Dioxide Industrial Utilization Technologies; CRC Press: Boca Raton, FL, USA, 2022; pp. 99–117. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.S.; Xie, Z. Influence of carbonated recycled concrete aggregate on properties of cement mortar. Constr. Build. Mater. 2015, 98, 1–7. [Google Scholar] [CrossRef]
- Yang, K.H.; Seo, E.A.; Tae, S.H. Carbonation and CO2 uptake of concrete. Environ. Impact Assess. Rev. 2014, 46, 43–52. [Google Scholar] [CrossRef]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Res. 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Li, L.; Poon, C.S.; Xiao, J.; Xuan, D. Effect of carbonated recycled coarse aggregate on the dynamic compressive behavior of recycled aggregate concrete. Constr. Build. Mater. 2017, 151, 52–62. [Google Scholar] [CrossRef]
- Hamdy, L.B.; Goel, C.; Rudd, J.A.; Barron, A.R.; Andreoli, E. The application of amine-based materials for carbon capture and utilisation: An overarching view. Mater. Adv. 2021, 2, 5843–5880. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Nwaoha, C.; Tontiwachwuthikul, P.; Benamor, A. CO2 capture from lime kiln using AMP-DA2MP amine solvent blend: A pilot plant study. J. Environ. Chem. Eng. 2018, 6, 7102–7110. [Google Scholar] [CrossRef]
- Ansaloni, L.; Hartono, A.; Awais, M.; Knuutila, H.K.; Deng, L. CO2 capture using highly viscous amine blends in non-porous membrane contactors. Chem. Eng. J. 2019, 359, 1581–1591. [Google Scholar] [CrossRef]
- Hairul, N.A.H.; Shariff, A.M.; Bustam, M.A. Mass transfer performance of 2-amino-2-methyl-1-propanol and piperazine promoted 2-amino-2-methyl-1-propanol blended solvent in high pressure CO2 absorption. Int. J. Greenh. Gas Control 2016, 49, 121–127. [Google Scholar] [CrossRef]
- Cormos, C.-C. Decarbonization options for cement production process: A techno-economic and environmental evaluation. Fuel 2022, 320, 123907. [Google Scholar] [CrossRef]
- Pérez-Calvo, J.-F.; Sutter, D.; Gazzani, M.; Mazzotti, M. Application of a Chilled Ammonia-based Process for CO2 Capture to Cement Plants. Energy Procedia 2017, 114, 6197–6205. [Google Scholar] [CrossRef]
- Dhanraj, D.; Biswas, P. Influence of Particles on Amine Losses During CO2 Capture: A Process Simulation Coupled Aerosol Dynamics Model. Int. J. Greenh. Gas Control 2020, 103, 103179. [Google Scholar] [CrossRef]
- Bastos, D.; Brazão Farinha, C.; Maia Pederneiras, C.; Veiga, R.; Bogas, J.A.; Infante Gomes, R.; Santos Silva, A. Pathway to Carbon Neutrality in the Cement Industry: CO2 Uptake by Recycled Aggregates from Construction and Demolition Waste. Appl. Sci. 2024, 14, 5224. [Google Scholar] [CrossRef]
- Gomes, R.I.; Bastos, D.; Farinha, C.B.; Pederneiras, C.M.; Veiga, R.; de Brito, J.; Faria, P.; Silva, A.S. Mortars with cdw recycled aggregates submitted to high levels of CO2. Infrastructures 2021, 6, 159. [Google Scholar] [CrossRef]
- Gomes, R.I.; Bastos, D.; Farinha, C.B.; Veiga, R.; de Brito, J.; Faria, P.; Silva, A.S. Carbonation Potential of Recycled Aggregates from Construction and Demolition Waste. In Concrete Structures: New Trends for Eco-Efficiency and Performance, Prceedings of the 18th fib Symposium, Lisbon, Portugal, 14–16 June 2021; FIB: Lausanne, Switzerland, 2021. [Google Scholar]
- EN 933-11; Tests for Geometrical Properties of Aggregates - Part 11: Classification Test for the Constituents of Coarse Recycled Aggregate. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- EN 13242; Aggregates for Unbound and Hydraulically Bound Materials for Use in Civil Engineering Work and Road Construction. Committee for Standardization (CEN): Brussels, Belgium, 2007.
- Rodig, O.R. Spectrometric Identification of Organic Compounds. J. Med. Chem. 1963, 6, 826–827. [Google Scholar] [CrossRef]
- Hahn, M.W.; Jelic, J.; Berger, E.; Reuter, K.; Jentys, A.; Lercher, J.A. Role of Amine Functionality for CO2 Chemisorption on Silica. J. Phys. Chem. B 2016, 120, 1988–1995. [Google Scholar] [CrossRef]
- Hahn, M.W.; Steib, M.; Jentys, A.; Lercher, J.A. Mechanism and kinetics of CO2 adsorption on surface bonded amines. J. Phys. Chem. C 2015, 119, 4126–4135. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Arenillas, A.; Rubiera, F.; Pis, J.J. Application of thermogravimetric analysis to the evaluation of aminated solid sorbents for CO2 capture. J. Therm. Anal. Calorim. 2008, 92, 601–606. [Google Scholar] [CrossRef]
- Bezerra, D.P.; da Silva, F.W.; de Moura, P.A.; Sousa, A.G.; Vieira, R.S.; Rodriguez-Castellon, E.; Azevedo, D.C. CO2 adsorption in amine-grafted zeolite 13X. Appl. Surf. Sci. 2014, 314, 314–321. [Google Scholar] [CrossRef]
- Danon, A.; Stair, P.C.; Weitz, E. FTIR study of CO2 adsorption on amine-grafted SBA-15: Elucidation of adsorbed species. J. Phys. Chem. C 2011, 115, 11540–11549. [Google Scholar] [CrossRef]
- Foo, G.S.; Lee, J.J.; Chen, C.H.; Hayes, S.E.; Sievers, C.; Jones, C.W. Elucidation of Surface Species through in Situ FTIR Spectroscopy of Carbon Dioxide Adsorption on Amine-Grafted SBA-15. ChemSusChem 2017, 10, 266–276. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Srikanth, C.S.; Chuang, S.S.C. In situ ATR and DRIFTS studies of the nature of adsorbed CO2 on tetraethylenepentamine films. ACS Appl. Mater. Interfaces 2014, 6, 13617–13626. [Google Scholar] [CrossRef]
- Khanna, R.K.; Moore, M.H. Carbamic acid: Molecular structure and IR spectra. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 1999, 55, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Rolle, F.; Sega, M. Use of FTIR spectroscopy for the measurement of CO2 carbon stable isotope ratios. In Proceedings of the 19th International Congress of Metrology, Paris, France, 24–26 September 2019; p. 05002. [Google Scholar]
- Schott, J.A.; Do-thanh, C.; Shan, W.; Puskar, N.G.; Dai, S.; Mahurin, S.M. Green Chemical Engineering FTIR investigation of the interfacial properties and mechanisms of CO2 sorption in porous ionic liquids. Green Chem. Eng. 2022, 2, 392–401. [Google Scholar] [CrossRef]
- Wang, C.; Chazallon, C.; Braymand, S.; Hornych, P. Thermogravimetric analysis (TGA) for characterization of self-cementation of recycled concrete aggregates in pavement. Thermochim. Acta 2024, 733, 179680. [Google Scholar] [CrossRef]
- Peeters, A.; Ameloot, R.; De Vos, D.E. Carbon dioxide as a reversible amine-protecting agent in selective Michael additions and acylations. Green Chem. 2013, 15, 1550. [Google Scholar] [CrossRef]
- Aboudi, J.; Vafaeezadeh, M. Efficient and reversible CO2 capture by amine functionalized-silica gel confined task-specific ionic liquid system. J. Adv. Res. 2015, 6, 571–577. [Google Scholar] [CrossRef]
- Hampe, E.M.; Rudkevich, D.M. Exploring reversible reactions between CO2 and amines. Tetrahedron 2003, 59, 9619–9625. [Google Scholar] [CrossRef]
- Ordonez-Loza, J.; Chejne, F.; Jameel, A.G.A.; Telalovic, S.; Arrieta, A.A.; Sarathy, S.M. An investigation into the pyrolysis and oxidation of bio-oil from sugarcane bagasse: Kinetics and evolved gases using TGA-FTIR. J. Environ. Chem. Eng. 2021, 9, 106144. [Google Scholar] [CrossRef]
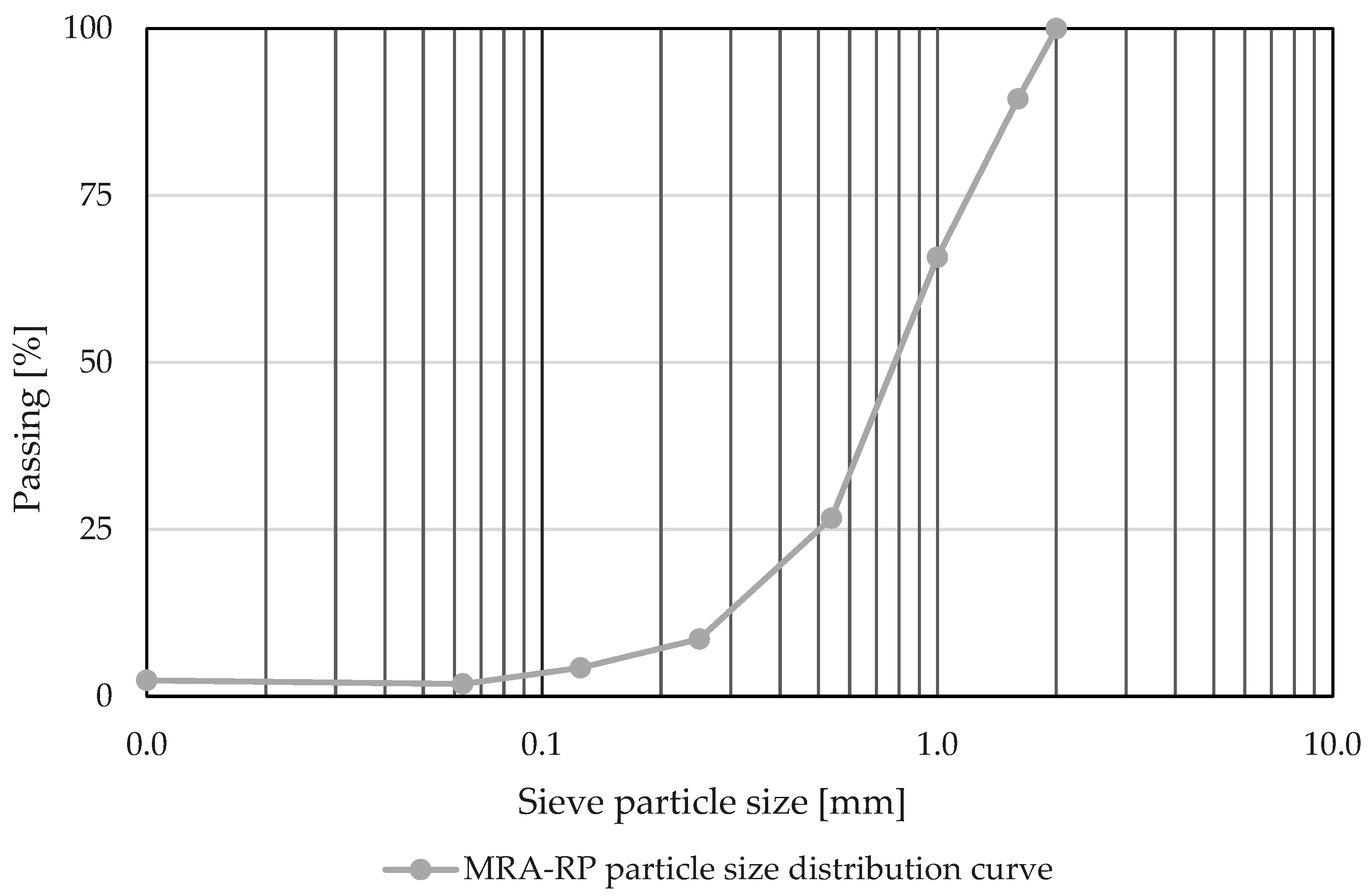
| Waste | Rc (%) | Ru (%) | Rb (%) | Ra (%) | Rg (%) | X (%) | Fl (%) | Classification According to EN 933-11 [33] and EN 13242 [34]* (as per Results Obtained in Laboratory) |
|---|---|---|---|---|---|---|---|---|
| MRA-RP | 42.5 | 27.5 | 21.7 | 9.6 | 0.2 | 0.8 | Fl5- | Rc declared Rcug70 Rb30- Ra10- Rg2- X1- Fl5- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, D.; Infante Gomes, R.; Gonçalves, D.; Brazão Farinha, C.; Pederneiras, C.M.; Veiga, R.; Santos Silva, A.; Bogas, J.A.; Galhano dos Santos, R. Enhanced CO2 Sequestration in Recycled Aggregates: Exploring Novel Capture-Promoting Additives. Waste 2025, 3, 17. https://doi.org/10.3390/waste3020017
Bastos D, Infante Gomes R, Gonçalves D, Brazão Farinha C, Pederneiras CM, Veiga R, Santos Silva A, Bogas JA, Galhano dos Santos R. Enhanced CO2 Sequestration in Recycled Aggregates: Exploring Novel Capture-Promoting Additives. Waste. 2025; 3(2):17. https://doi.org/10.3390/waste3020017
Chicago/Turabian StyleBastos, David, Ricardo Infante Gomes, Diogo Gonçalves, Catarina Brazão Farinha, Cinthia Maia Pederneiras, Rosário Veiga, António Santos Silva, José Alexandre Bogas, and Rui Galhano dos Santos. 2025. "Enhanced CO2 Sequestration in Recycled Aggregates: Exploring Novel Capture-Promoting Additives" Waste 3, no. 2: 17. https://doi.org/10.3390/waste3020017
APA StyleBastos, D., Infante Gomes, R., Gonçalves, D., Brazão Farinha, C., Pederneiras, C. M., Veiga, R., Santos Silva, A., Bogas, J. A., & Galhano dos Santos, R. (2025). Enhanced CO2 Sequestration in Recycled Aggregates: Exploring Novel Capture-Promoting Additives. Waste, 3(2), 17. https://doi.org/10.3390/waste3020017










