Effect of Orofacial Myofunctional Therapy with Auto-Monitoring on the Apnea–Hypopnea Index and Secondary Outcomes in Treatment-Naïve Patients with Mild to Moderate Obstructive Sleep Apnea (OMTaOSA): A Multicenter Randomized Controlled Trial Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Sample Size
2.3. Participant Recruitment and Eligibility Criteria
2.4. Baseline Final Inclusion
2.5. Randomization and Allocation
2.6. Measurements
2.7. Sleep Studies
2.8. The Auto-Monitoring OMT Intervention
- Tongue brushing: Brush the superior and lateral surfaces of the tongue while fixing the tongue in different positions of the mouth. Repeat each movement five times, three times a day.
- Tongue sliding: Place the tip of the tongue against the hard palate behind your upper front teeth. Slide the tongue backwards along the hard palate. Repeat 20 times, three times a day.
- Tongue suction: Suck the entire tongue upwards against your hard palate and press upwards with both short and long pressure intervals. Repeat 20 times short, 20 times long, three times a day. Short repetitions were defined as 1 s hold, with long intervals as 10 s.
- Tongue down: Force the back of the tongue down against the floor of the mouth for one second. Keep the tip of the tongue in contact with the lower front teeth. Repeat 20 times, three times a day.
- Elevate the soft palate and uvula whilst saying “ah” both intermittently (“a-a-a”) for one second and continuously (“aaa”) for five seconds. Repeat 20 times, three times a day.
- Balloon blow: Inhale through your nose and blow into a balloon with force. Do not take the balloon off your mouth and repeat blowing five times, three times a day. If it is not socially acceptable to blow into the balloon, blow air through pressed lips five times.
- Put your finger in the oral cavity against your cheek. Pull against your finger with the cheek muscles. Repeat 10 times on each side, three times a day. If it is not socially acceptable to put a finger in the mouth during daytime, please use a finger in the mornings and evenings.
- Air pump: Trap air in your cheek while alternating sides. Keep the lips closed. Repeat 10 times on each side, three times a day.
2.9. The Auto-Monitor Control Condition
2.10. Outcome Evaluation
- Change in the Epworth Sleepiness scale and PROMs in the European Sleep Questionnaire. The scale is a validated tool measuring sleepiness between 0 and 24. Higher values represent more sleepiness.
- Orofacial myofunctional therapy adherence measured by application registration, between 1 and 3 per day. Three exercises per day is the maximum score.
- Change in desaturation severity parameter measured by medical device through photoplethysmography obtained by self-applied PSG. Greater severity represents worse disease.
- Change in desaturation duration measured by medical device through photoplethysmography obtained by self-applied PSG. A longer duration represents worse disease.
- Change in objective sleep quality measured by self-applied PSG. Sleep quality is the ratio between the total sleep time and time in bed. A higher ratio is better.
- Change in desaturation severity parameter measured by photoplethysmography obtained by wearable Withings Scan Watch. Greater severity represents worse disease.
- Change in desaturation duration measured by photoplethysmography obtained by wearable Withings Scan Watch. A longer duration represents worse disease.
- Change in Stroop test measured by flexibility game in application. More correct answers is better.
- Change in reaction test measured by reaction game in application. A shorter reaction time is better.
- Change in memory test measured by memory game in application. Longer sequences memorized are better.
- Change in perception test measured by perception game in application. More correct answers is better.
- Change in general health status measured by a visual analogue scale.
- Changes in the Orofacial Myofunctional Evaluation with Scores measured by a scorer blinded for randomization. Range 37–103. A lower score represents greater dysfunction.
- Changes in tongue and cheek strength measured by the IOPI. A higher score represents greater strength.
- Changes in hyoid position as assessed by a lateral cephalogram.
- Changes in upper airway volume as assessed by cone beam computer tomography (sub-study).
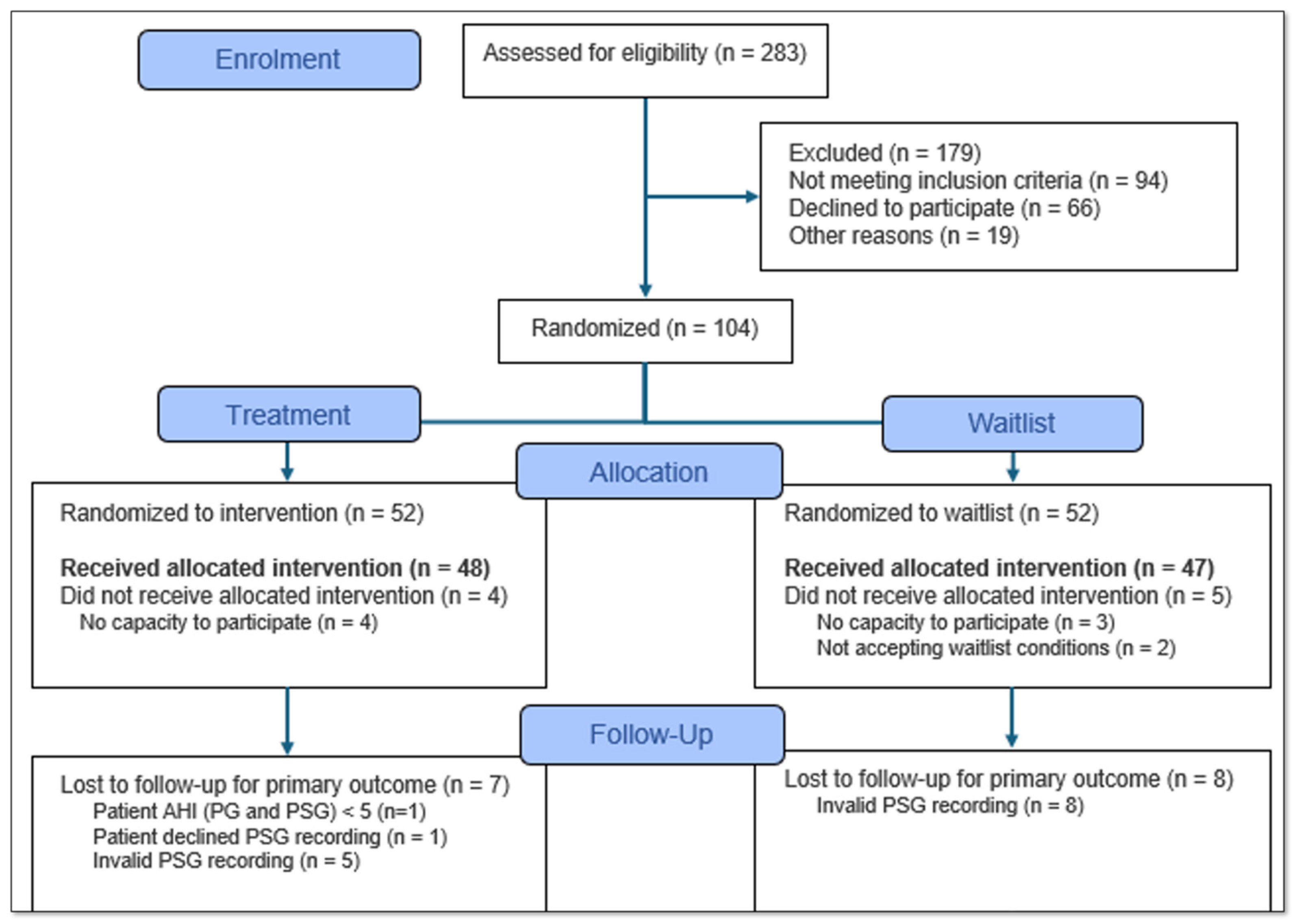
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OMT | Orofacial myofunctional therapy |
| OSA | Obstructive sleep apnea |
| AHI | Apnea–hypopnea index |
| PAP | Positive airway pressure |
| Mhealth | Mobile Health |
| RCT | Randomized controlled trial |
| Ahus | Akershus University Hospital |
| FPH | Fertilitas Private Hospital |
| BMI | Body mass index |
| MIO | Maximal interincisal opening |
| MOTTIP | Maxillary incisive papillae at the roof of the mouth |
| PSG | Polysomnography |
References
- Hrubos-Strom, H.; Randby, A.; Namtvedt, S.K.; Kristiansen, H.A.; Einvik, G.; Benth, J.; Somers, V.K.; Nordhus, I.H.; Russell, M.B.; Dammen, T.; et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP). J. Sleep. Res. 2011, 20 Pt 2, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Kendzerska, T.; Mollayeva, T.; Gershon, A.S.; Leung, R.S.; Hawker, G.; Tomlinson, G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: A systematic review. Sleep Med. Rev. 2013, 18, 49–59. [Google Scholar] [CrossRef]
- Giles, T.L.; Lasserson, T.J.; Smith, B.H.; White, J.; Wright, J.; Cates, C.J. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst. Rev. 2006, 25, CD001106. [Google Scholar]
- Kribbs, N.B.; Pack, A.I.; Kline, L.R.; Smith, P.L.; Schwartz, A.R.; Schubert, N.M.; Redline, S.; Henry, J.N.; Getsy, J.E.; Dinges, D.F. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 1993, 147, 887–895. [Google Scholar] [CrossRef]
- Thomasouli, M.A.; Brady, E.M.; Davies, M.J.; Hall, A.P.; Khunti, K.; Morris, D.H.; Gray, L.J. The impact of diet and lifestyle management strategies for obstructive sleep apnoea in adults: A systematic review and meta-analysis of randomised controlled trials. Sleep Breath. Schlaf Atm. 2013, 17, 925–935. [Google Scholar] [CrossRef]
- Ravesloot, M.J.; van Maanen, J.P.; Dun, L.; de Vries, N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea—A review of the literature. Sleep Breath. Schlaf Atm. 2013, 17, 39–49. [Google Scholar]
- Lim, J.; Lasserson, T.J.; Fleetham, J.; Wright, J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2004, 2006, CD004435. [Google Scholar] [CrossRef]
- Sundaram, S.; Bridgman, S.A.; Lim, J.; Lasserson, T.J. Surgery for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2005, 19, CD001004. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.G.; de la Torre Díez, I.; Zapiraín, B.G. Predictive, Personalized, Preventive and Participatory (4P) Medicine Applied to Telemedicine and eHealth in the Literature. J. Med. Syst. 2019, 43, 140. [Google Scholar] [PubMed]
- Wannheden, C.; Åberg-Wennerholm, M.; Dahlberg, M.; Revenäs, Å.; Tolf, S.; Eftimovska, E.; Brommels, M. Digital Health Technologies Enabling Partnerships in Chronic Care Management: Scoping Review. J. Med. Internet Res. 2022, 24, e38980. [Google Scholar] [CrossRef]
- Mediano, O.; Romero-Peralta, S.; Resano, P.; Cano-Pumarega, I.; Sanchez-de-la-Torre, M.; Castillo-Garcia, M.; Martinez-Sanchez, A.B.; Ortigado, A.; Garcia-Rio, F. Obstructive Sleep Apnea: Emerging Treatments Targeting the Genioglossus Muscle. J. Clin. Med. 2019, 8, 1754. [Google Scholar] [CrossRef]
- Rueda, J.R.; Mugueta-Aguinaga, I.; Vilaró, J.; Rueda-Etxebarria, M. Myofunctional therapy (oropharyngeal exercises) for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2020, 11, CD013449. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. Lancet 2025, 405, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, K.C.; Drager, L.F.; Genta, P.R.; Marcondes, B.F.; Lorenzi-Filho, G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2009, 179, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M.J.S. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.L.; Sacco, W.P. Measuring Sleep Efficiency: What Should the Denominator Be? J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 12, 263–266. [Google Scholar] [CrossRef]
- Schmitz, L.; Sveinbjarnarson, B.F.; Gunnarsson, G.N.; Davíðsson, Ó.A.; Davíðsson, Þ.B.; Arnardottir, E.S.; Óskarsdóttir, M. Towards a Digital Sleep Diary Standard. In Proceedings of the Americas Conference on Information Systems (AMCIS), Minneapolis, MN, USA, 10–14 August 2022. [Google Scholar]
- Yoon, A.; Zaghi, S.; Weitzman, R.; Ha, S.; Law, C.S.; Guilleminault, C.; Liu, S.Y.C. Toward a functional definition of ankyloglossia: Validating current grading scales for lingual frenulum length and tongue mobility in 1052 subjects. Sleep Breath. Schlaf Atm. 2017, 21, 767–775. [Google Scholar]
- Albrecht, B.M.; Stalling, I.; Bammann, K. Sex- and age-specific normative values for handgrip strength and components of the Senior Fitness Test in community-dwelling older adults aged 65–75 years in Germany: Results from the outdoor active study. BMC Geriatr. 2021, 21, 273. [Google Scholar] [CrossRef]
- Felício, C.M.; Ferreira, C.L. Protocol of orofacial myofunctional evaluation with scores. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 367–375. [Google Scholar] [CrossRef]
- van Erp, L.W.; van Gerven, J.; Bloem, S.; Groenen, M.J.M.; Wahab, P.J. Acceptance and Perceived Control are Independently Associated with Quality of Life in Inflammatory Bowel Disease: Introduction of a New Segmentation Model. J. Crohns Colitis 2021, 15, 1837–1845. [Google Scholar] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar]
- Denollet, J. DS14: Standard Assessment of Negative Affectivity, Social Inhibition, and Type D Personality. Psychosom. Med. 2005, 67, 89–97. [Google Scholar] [CrossRef]
- Arnardottir, E.S.; Islind, A.S.; Óskarsdóttir, M. The Future of Sleep Measurements: A Review and Perspective. Sleep Med. Clin. 2021, 16, 447–464. [Google Scholar] [CrossRef]
- Berry, R.; Quan, S.; Abreu, A. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications, version 2.6. Health 2020, 12. [Google Scholar]
- Kristbergsdottir, H.; Schmitz, L.; Arnardottir, E.S.; Islind, A.S. Evaluating User Compliance in Mobile Health Apps: Insights from a 90-Day Study Using a Digital Sleep Diary. Diagnostics 2023, 13, 2883. [Google Scholar] [CrossRef]
- Arnardottir, E.S.; Islind, A.S.; Oskarsdottir, M.; Olafsdottir, K.A.; August, E.; Jonasdottir, L.; Hrubos-Strom, H.; Saavedra, J.M.; Grote, L.; Hedner, J.; et al. The Sleep Revolution project: The concept and objectives. J. Sleep. Res. 2022, 31, e13630. [Google Scholar]
- Fridgeirsdottir, K.Y.; Murphy, C.J.; Islind, A.S.; Árnadóttir, B.S.; Hrubos-Strøm, H.; Arnardottir, E.S.; Saavedra, J.M. Effects of Exercise and a Lifestyle App on Sleep-Disordered Breathing, Physical Health, and quality of Life. ERJ Open Res. 2024, 11, 01134–02024. [Google Scholar]
- Dietsch, A.M.; Solomon, N.P.; Sharkey, L.A.; Duffy, J.R.; Strand, E.A.; Clark, H.M. Perceptual and instrumental assessments of orofacial muscle tone in dysarthric and normal speakers. J. Rehabil. Res. Dev. 2014, 51, 1127–1142. (In English) [Google Scholar] [CrossRef]
- de Felicio, C.M.; da Silva Dias, F.V.; Trawitzki, L.V.V. Obstructive sleep apnea: Focus on myofunctional therapy. Nat. Sci. Sleep 2018, 10, 271–286. [Google Scholar] [CrossRef]
- O’Connor-Reina, C.; Ignacio Garcia, J.M.; Rodriguez Ruiz, E.; Morillo Dominguez, M.D.C.; Ignacio Barrios, V.; Baptista Jardin, P.; Casado Morente, J.C.; Garcia Iriarte, M.T.; Plaza, G. Myofunctional Therapy App for Severe Apnea-Hypopnea Sleep Obstructive Syndrome: Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e23123. [Google Scholar] [CrossRef]
- Kim, H.; Cho, N.B.; Kim, J.; Kim, K.M.; Kang, M.; Choi, Y.; Kim, M.; You, H.; Nam, S.I.; Shin, S. Implementation of a Home-Based mHealth App Intervention Program with Human Mediation for Swallowing Tongue Pressure Strengthening Exercises in Older Adults: Longitudinal Observational Study. JMIR Mhealth Uhealth 2020, 8, e22080. (In English) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Association of Orofacial Myology (IAOM). Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrubos-Strøm, H.; Hansen, D.D.; Feng, X.; Mäkinen, H.; Tinbod, U.; Köster, A.; Vaher, H.; Klungsøyr, O.; Saavedra, J.M.; Skirbekk, H.; et al. Effect of Orofacial Myofunctional Therapy with Auto-Monitoring on the Apnea–Hypopnea Index and Secondary Outcomes in Treatment-Naïve Patients with Mild to Moderate Obstructive Sleep Apnea (OMTaOSA): A Multicenter Randomized Controlled Trial Protocol. Int. J. Orofac. Myol. Myofunct. Ther. 2025, 51, 8. https://doi.org/10.3390/ijom51020008
Hrubos-Strøm H, Hansen DD, Feng X, Mäkinen H, Tinbod U, Köster A, Vaher H, Klungsøyr O, Saavedra JM, Skirbekk H, et al. Effect of Orofacial Myofunctional Therapy with Auto-Monitoring on the Apnea–Hypopnea Index and Secondary Outcomes in Treatment-Naïve Patients with Mild to Moderate Obstructive Sleep Apnea (OMTaOSA): A Multicenter Randomized Controlled Trial Protocol. International Journal of Orofacial Myology and Myofunctional Therapy. 2025; 51(2):8. https://doi.org/10.3390/ijom51020008
Chicago/Turabian StyleHrubos-Strøm, Harald, Diana Dobran Hansen, Xin Feng, Hanna Mäkinen, Unn Tinbod, Andres Köster, Heisl Vaher, Ole Klungsøyr, Jose M. Saavedra, Helge Skirbekk, and et al. 2025. "Effect of Orofacial Myofunctional Therapy with Auto-Monitoring on the Apnea–Hypopnea Index and Secondary Outcomes in Treatment-Naïve Patients with Mild to Moderate Obstructive Sleep Apnea (OMTaOSA): A Multicenter Randomized Controlled Trial Protocol" International Journal of Orofacial Myology and Myofunctional Therapy 51, no. 2: 8. https://doi.org/10.3390/ijom51020008
APA StyleHrubos-Strøm, H., Hansen, D. D., Feng, X., Mäkinen, H., Tinbod, U., Köster, A., Vaher, H., Klungsøyr, O., Saavedra, J. M., Skirbekk, H., Dammen, T., & Jagomägi, T. (2025). Effect of Orofacial Myofunctional Therapy with Auto-Monitoring on the Apnea–Hypopnea Index and Secondary Outcomes in Treatment-Naïve Patients with Mild to Moderate Obstructive Sleep Apnea (OMTaOSA): A Multicenter Randomized Controlled Trial Protocol. International Journal of Orofacial Myology and Myofunctional Therapy, 51(2), 8. https://doi.org/10.3390/ijom51020008





