Abstract
The purpose of this study was to determine if electropalatography (EPG) would be a useful adjunct and feasible option for those conducting clinical assessments of individuals with suspected nonspeech orofacial myofunctional disorders (NSOMD). Three females (two adults, one child) were referred by their orthodontist for assessment of suspected NSOMD. Three adults and one child without NSOMD were recruited for the purpose of evaluating methodological construct, and to provide comparisons for participants with NSOMD. Using EPG, lingual-palatal timing and contact patterns of 105 saliva swallows (45 with NSOMD, 60 without NSOMD) were analyzed by compartmentalizing the sensor display and tracking the order and duration of activation. Lingual-palatal contact patterns were compared in terms of four stages: prepropulsion, propulsion, postpropulsion, release. Coding the lingual-palatal activation in an operationalized manner was a valuable adjunct for describing lingual-palatal timing and contact patterns. Participants with NSOMD showed unique lingual-palatal contact patterns that differed from the patterns of the participants without NSOMD, and from each other. EPG is a potential adjunct to the non-instrumental assessment of NSOMD. Larger scale investigations using EPG should proceed.
INTRODUCTION
Therapists charged with the responsibility of assessing individuals with suspected nonspeech orofacial myofunctional disorders (NSOMD) face a clinical challenge. Since parting the lips can disrupt the lingual pattern during swallowing (Knosel, Klein, Bleckmann, & Engelke, 2012; Peng, Jost-Brinkmann, Yoshida, Chou, & Lin, 2004), the true movement of the tongue cannot be characterized without instrumentation.
Unfortunately, there are limited options for assessing those with suspected NSOMD in which detailed information regarding the pattern of lingual movement is obtained.
Electropalaography (EPG) is visual feedback device used in clinical and research practices that has shown initial promise in the assessment of individuals with NSOMD. Cayley, Tindall, Sampson, and Butcher (2000) provided a description of “tongue function” of those diagnosed with tongue-thrust using a 62-electrode EPG system. The EPG swallowing data were presented as calculated average lingual-palatal contact durations of electrode rows over time, beginning with the posterior extrusion of the bolus until maximal lingual-palatal contact was achieved. While they noted that their participants with NSOMD swallows had a more posterior pattern of lingual-palatal contact, their system of analysis did not attempt to describe the order of electrode activation, and so a dynamic lingual-palatal timing and contact pattern did not emerge from the study.
Chi-Fishman and Stone (1996), and Chi-Fishman, Stone, and McCall (1998) used EPG for describing dynamic lingual-palatal contact, but only in non-disordered participants. The Kay Elemetrics Palatometer System used in their study had 96 electrodes that were symmetrically embedded in an artificial palate along the medial surface of the teeth and across the plane of the hard palate, extending 5–10 mm beyond the third molars. They characterized the lingual-palatal contact patterns exhibited during the swallow in a quantifiable manner by dividing the visual display of electrode activation into palatal bins. Activation of the bins was then tracked and divided into four stages for analysis. This system provided a detailed description of the swallow pattern in terms of lingual movement and timing of those without a history of swallowing impairment.
Some other modalities used to assess lingual movement related to swallowing include cinefluoroscopy, videofluoroscopy ultrasound, manometric events obtained from tongue-palate pressure appliances, and electromagnetic midsagittal articulography (EMMA). However, a number of these modalities provide limited information during assessment of NSOMD. While fluoroscopy is highly informative for characterizing the position of bony and cartilaginous structures, the resolution for soft tissues such as the tongue is limited (Tasko, Kent, & Westbury, 2002). Positioning participants for optimal imaging of the oral tongue is also a challenge in fluoroscopy because in lateral view the precise location of the anterior border of the tongue is obscured by the teeth, and a well-defined tongue tip is not always visible (Hanson, 1976). Additionally, fluoroscopy poses risks associated with ionizing radiation exposure. Like fluoroscopy, ultrasound can be a challenging method of instrumentation for viewing anterior parts of the tongue during the swallow (Peng, Jost-Brinkmann, Yoshida, Chou, & Lin, 2004). Skeletal abnormalities, the submental contact area, and the quantity of intra-oral saliva can all effect how well the tongue can be visualized when using ultrasound technology (Ardakani, 2006). Manometry obtained during swallowing has been used to provide some information about lingual timing and patterning (Kennedy et al., 2010; Ono, Hori, & Nokubi, 2004; Tamine, Ono, Hori, Kondoh, et al., 2010). However, most manometric assemblies use a limited number of pressure transducers and may not provide the fine-scaled analysis that clinicians desire when assessing clients with NSOMD. EMMA may hold promise for assessing lingual timing and pattern differences in those with NSOMD. EMMA works by recording the movement of electrodes that have been placed directly on the tongue as they move within the electromagnetic field. The technology has been used to study the nature and extent of variability in tongue movement during healthy swallowing (Bennett, Van Lieshout, & Steele, 2007; Steel & Van Lieshout, 2004) but studies outlining the value for populations with NSOMD are still needed.
EPG may be a valuable and practical tool for describing NSOMD lingual-palatal timing and contact patterns and as such, contribute to improved diagnosis and treatment planning. With careful evaluation of lingual movement during the swallow, treatment of those with NSOMD may be tailored to address individual differences. Building upon the work initiated by Chi-Fishman and Stone (1996) who used EPG to quantify dynamic lingual-palatal timing and contact patterns of adults with non-disordered swallows, this feasibility study investigated the use of EPG to quantify the patterns of persons with NSOMD. It expands the work of Cayley et al. (2000) who investigated swallowing patterns of those diagnosed with NSOMD using EPG, but did not provide a quantified, dynamic analysis of the timing and pattern of lingual-palatal contact. The purpose of this study was to determine if it is feasible to use EPG as an adjunct to non-instrumental assessment of NSOMD. If it is found to be feasible, larger scale studies would be recommended.
METHODS
The university institutional research ethics board approved this study.
Research Participants
Three females with suspected NSOMD were referred for the study by their individual dental care providers to determine if their swallow pattern might be contributing to their malocclusions. Participant 1 (P1) was 21 years of age; participant 2 (P2) was 44 years of age; participant (P3) was 8 years of age. A non-instrumental evaluation guided by portions of the Expanded Orofacial Myofunctional Evaluation (OMES-E) (De Felicio, Folha, Ferreira, Medeiros, 2010) was conducted by a certified speech language pathologist. The appearance and posture of the face, cheeks, mandible, lips, and tongue at rest were unremarkable. The palate of P1 and P3 were scored as light dysfunction for width (narrow) and height (deep). The palate of P2 was unremarkable. All three participants had an overjet.
Breathing was nasal for all participants. The strength, range of motion, and coordination of the jaw, lips, and oral tongue were assessed during non-swallowing tasks and were considered unremarkable for all participants. However, all three participants displayed excessive circumoral muscle activity during swallows of thin liquids, honey thickened liquids, and masticated materials. All three participants masticated the solid material (cracker) adequately, but had oral residues post-swallow. P1 and P2 had residues on the lingual surface after the swallow, and P3 had residues in the lateral sulci as well as on the lingual surface. Noise could be heard when P1 swallowed which she indicated was a sucking action that she made with her tongue on the hard palate.
She also indicated that she routinely used a liquid wash to clear these oral residues. No signs or symptoms of pharyngeal or esophageal complications were observed or reported.
Four participants without NSOMD (two adult females aged 17 years and 41 years: one male aged 28 years; one female child aged eight years) were recruited for the study. As our EPG system differed from the technology used by Chi-Fishman and Stone (1996) in the number and layout of electrodes, participants without NSOMD were recruited for the purpose of evaluating methodological construct. The lingual-palatal contact patterns obtained from participants without NSOMD provided information upon which comparisons to the patterns of P1, P2, and P3 could be made.
Electropalatography (EPG) Instrumentation
EPG data were collected using the CompleteSpeech Palatometer V 1.0 system (2012). The CompleteSpeech Palatometer system consisted of an artificial palate (i.e., SmartPalate), DataLink, a USB cable connected between the DataLink and computer, and the associated computer software. The electropalates were ~0.5 mm thick custom formed retainers with thin flexible printed circuits that conformed to the shape of the participants’ palates. The water resistant electropalates contained 126 gold-plated contacts, including 2 lip closure sensors and 2 gum contacts (see Figure 1). For individuals with smaller oral cavities (P3 and the child without NSOMD), the electropalate was modified to 104 gold-plated contacts to accommodate their smaller palates. The removed contacts are displayed in Figure 2.

Figure 1.
Electropalate (Complete Speech, 2012).
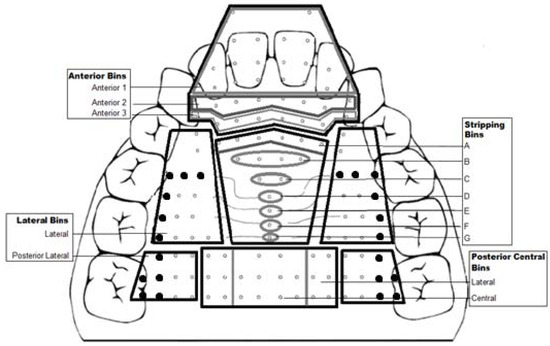
Figure 2.
Compartmentalization of the electropalate into 4 primary palatal bins: anterior, lateral, stripping, and posterior central. The anterior bin is further subdivided into anterior 1, 2 and 3. The lateral bin is further subdivided into lateral and posterior lateral. The stripping bin is further subdivided into A thru G. The posterior central bin is further subdivided into lateral and central. Bolded sensors were not present in the modified palates.
The contact sensors sampled at 100 Hz, a rate that has been suggested as appropriate for assessing lingual-palatal contact for swallowing (Chi-Fishman & Stone, 1996). The CompleteSpeech Palatometer system allows for unlimited length recordings that can be played back in real-time, and slow or stop motion. This information was relayed to a computer, which displayed upon the monitor, a layout closely resembling the actual electrode placement within the oral cavity. Activation of a sensor was accomplished by tongue to artificial palate contact, with a corresponding visual display of the contact location. The information was saved on an external hard drive.
Procedure
Prior to the assessment, the participants wore the pseudopalate for a desensitization period of approximately 20–30 min. This desensitization period has been shown to be suitable in previous studies (Chi-Fishman & Stone, 1996; Searl, Evitts, & Davis, 2006).
Participants performed non-swallow lingual tasks while acclimatizing (ex. tongue clicks, and repeating consonant-vowel sequences). Participants were questioned throughout the study about palatal comfort and swallowing ease. No difficulties were reported by any of the participants.
During the experimental recordings, each participant completed 30 dry swallows divided equally over three separate days (10 swallows/day). Dry swallows were accomplished by swallowing saliva in the mouth. Dry swallows were chosen for this study as Chi-Fishman and Stone (1996) found that their participants’ lingual-palatal contact patterns had no striking differences when swallowing varying amounts of water (5–30 mL) or when performing dry swallows. Therefore, dry swallows were used to eliminate the need to determine optimal bolus sizes for each participant (Peng, Jost-Brinkmann, Yoshida, Chou, & Lin, 2004).
The participants drank small sips of water between the recorded dry swallows to ensure that they maintained a moist oral cavity. A minimum rest period of ten seconds between the water sip and recorded swallow was provided.
Data Analysis
The first 5 swallows that were not interrupted by speech, etc. from each day were selected for analysis. This constituted 60 swallows from participants without NSOMD (4 participants X 5 swallows X 3 days) and 45 with NSOMD (3 participants X 5 swallows X 3 days). For analysis purposes, the sensor display was divided into four palatal bins labeled as follows: anterior, lateral, stripping, and posterior-central. In order to fully represent the pattern of lingual-palatal contact, the bins were further subdivided. The anterior bin was divided into an anterior 1, anterior 2, and anterior 3. The stripping bin was divided in an anterior to posterior manner and labeled A through G. The posterior-central bin was divided into posterior-central-central and posterior-central-lateral. The lateral bin was divided into lateral and posterior lateral. The lateral and posterior lateral bins had fewer sensors in the modified palates (Figure 2).
Bin activation was tracked to describe, in an operationalized manner, the pattern of lingual-palatal contact. The original bins described by Chi-Fishman and Stone (1996) served as a foundation for the bins used in this study.
However, the artificial palate offered by the CompleteSpeech Palatometer system differed in number and layout of electrodes, so modifications were necessary. A priori visual inspection was used to establish bin activation criteria that captured the patterns of those with and without NSOMD. The criteria for activation of the anterior, lateral, and posterior central bins represented the minimum number of activated individual sensors needed to create a lingual-palatal seal. Activation ratios were as follows. The denominator represents the total number of sensors within the bin, and the numerator represents the number of sensors within the bin that had to be activated by lingual-palatal contact; anterior bin: anterior 1 (6/18 sensors), anterior 2 (2/8 sensors), and anterior 3 (2/8 sensors); the lateral bin (12/30 sensors; 10/18 sensors for the modified palates). The value for activation of the stripping bins was a minimum of 50%.
Activation ratios were as follows; stripping bin: A (3/6 sensors), B (2/4 sensors), C (1/2 sensors), D (1/1 sensors), E (1/1 sensors), F (1/1 sensors), G (1/1 sensors); the posterior-lateral bin (6/22 sensors; 6/12 sensors for the modified palates); the posterior central bin: posterior-central-central (4/10 sensors), and posterior-central-lateral (4/12 sensors). Each swallow was analyzed frame by frame, progressing in 1/100 s increments. The order in which the bins activated and/or deactivated was logged. During pilot studies, these values were found to have an inter-rater reliability of 100%.
A modification of Chi-Fishman and Stone’s (1996) four swallowing stages were used to describe the stages of lingual-palatal contact (described below). Durations of these stages were recorded, and then the mean durations and standard deviations were calculated:
- Stage 1. Prepropulsion. Creation of a lingual seal as defined by activation of the lateral, and anterior bins.
- Stage 2. Propulsion. Stripping action as defined by activation of the stripping bin, and posterior central bin until full contact was reached (activation of all bins).
- Stage 3. Postpropulsion. The period between initial full contact and initiation of final release (described below).
- Stage 4. Release. Directional deactivation of all bins.
Reliability
The data were collected, analyzed, and coded together by both researchers. Only two discrepancies in the form of counting errors occurred while the data were coded. These were negotiated until an agreement was reached.
RESULTS
The individual means and standard deviations for all participants with and without NSOMD are presented in Table 1.

Table 1.
Mean Duration of Swallowing Stages for All Participants.
Participants Without NSOMD: Lingual-Palatal Timing and Contact Patterns
Figure 3 represents the lingual-palatal contact pattern of participants without NSOMD, through all four stages. The marginal mean durations for the 3 adults without NSOMD, and the duration for the child without NSOMD are also provided:
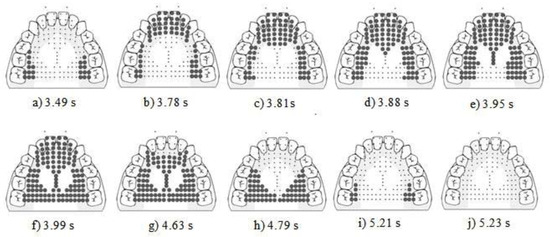
Figure 3.
Display of the raw EPG data for participants without NSOMD. The four stages are prepropulsion (a,b), propulsion (c–f), post propulsion (f), release (g–j). Bolded dots represent activated electrodes.
- Stage 1. Prepropulsion. The marginal mean duration for adults was 0.40 +/− 0.11 s, and the child’s average duration was 0.32 +/− 0.21 s. On 100% (60/60) swallows, the lingual seal was created prior to the initiation of the propulsion stage and the activation was anterior to posterior in direction (see Figure 3a,b).
- Stage 2. Propulsion. The marginal mean duration for adults was 0.31 +/− 0.25 s, and the child’s average duration was 0.36 +/− 0.23 s. On 100% (60/60) swallows the stripping was directional and was initiated in the stripping bin from sub-bins A and moving posteriorly to G, posterior-central-lateral and posterior-central-central (see Figure 3c–f).
- Stage 3. Postpropulsion. The marginal mean duration for adults was 0.72 +/− 0.21 s, and the child’s average duration was 1.43 +/− 0.48 s (see Figure 3f).
- Stage 4. Release. The marginal mean duration for adults was 0.48 +/− 0.04 s, and the child’s average duration was 0.32 +/− 0.18 s. Spontaneous directional deactivation of bins occurred anterior to posterior from the anterior 1 bin to the posterior central bin on 100% (60/60) swallows (see Figure 3g–j).
Participants with NSOMD: Lingual-Palatal Timing and Contact Patterns
Participant 1
- Stage 1. Prepropulsion. The average duration was 0.23 +/− 0.10 s. On 67% (10/15) of the trials, the lingual seal was not completed until after the initiation the stripping action (see Figure 4a–c).
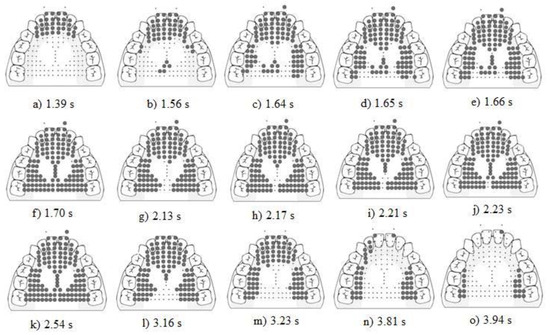 Figure 4. Display of the raw EPG data for a P1. The four stages are prepropulsion (a–c), propulsion (b–f), post propulsion (g–k), release (l–o). Bolded dots represent activated electrodes.
Figure 4. Display of the raw EPG data for a P1. The four stages are prepropulsion (a–c), propulsion (b–f), post propulsion (g–k), release (l–o). Bolded dots represent activated electrodes. - Stage 2. Propulsion. The average duration was 0.24 +/− 0.90 s. The average onset time of propulsion began at 0.19 +/− 0.99 s after the initiation of stage 1 (see Figure 5). The activation of the stripping bin was characterized as follows: 7% of the time (1/15) there was a sequential anterior to posterior strip within the stripping bin. The other 93% (14/15) showed no directional activation (see Figure 4b–f). On 73% (11/15) of occasions the posterior-central-central sub-bin was activated during the stripping action in sub-bins A through G (see Figure 4c–e). On 27% (4/15) of occasions the posterior-central-central sub-bin was activated after the stripping was completed.
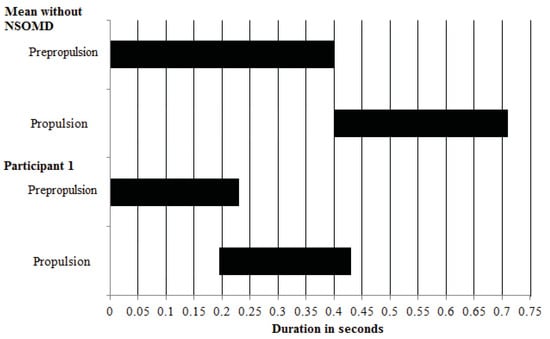 Figure 5. A temporal display in 0.5 s increments comparing the onset of propulsion relative to prepropulsion in P1 versus participants without NSOMD.
Figure 5. A temporal display in 0.5 s increments comparing the onset of propulsion relative to prepropulsion in P1 versus participants without NSOMD. - Stage 3. Postpropulsion. The average duration was 1.99 +/− 0.69 s. A “re-strip” while in postpropulsion stage was noted on 33% (5/15) of occasions (see Figure 4g–k). That is, P1 sequentially activated bins A–G, but unlike 73% of the time in her propulsive stage, she left a small section in the posterior central bin un-activated (see Figure 4h–j).
- Stage 4. Release. The average duration was 0.43 +/− 0.20 s. Spontaneous directional deactivation of bins occurred posterior to anterior on 100% (15/15) of occasions (see Figure 4l–o).
Participant 2
- Stage 1. Prepropulsion. The average duration was 0.32 +/− 0.90 s. The swallow was initiated within the anterior bin on all trials; however, on 53% (8/15) trials, the anterior 2 sub-bin was activated prior to the anterior 1 bin. On 27% (4/15) of occasions, the anterior 3 sub-bin was activated initially, and then activation sequentially moved forward to the anterior 1 (see Figure 6a–d). On 20% (3/15) of occasions, initial contact was made in the anterior 1 sub-bin. After anterior bin contact was made, the lingual seal was accomplished.
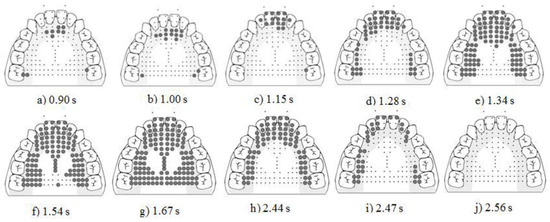 Figure 6. Display of the raw EPG data for P2’s swallow pattern across all four stages. The four stages are prepropulsion (a–d), propulsion (e–g), post propulsion (g), release (h–j). Bolded dots represent activated electrodes.
Figure 6. Display of the raw EPG data for P2’s swallow pattern across all four stages. The four stages are prepropulsion (a–d), propulsion (e–g), post propulsion (g), release (h–j). Bolded dots represent activated electrodes. - Stage 2. Propulsion. The average duration was 0.15 +/− 0.25 s. On all trials, stripping action proceeded in an anterior to posterior motion with sub-bins A through G sequentially activating, followed by the posterior-central-lateral, and then posterior-central-central (see Figure 6e–g).
- Stage 3. Postpropulsion. The average duration was 1.14 +/− 0.23s (see Figure 6g).
- Stage 4. Release. The average duration was 0.49 +/− 0.17 s. Directional deactivation of bins was accomplished with a posterior to anterior deactivation on 20% (3/15) of occasions, and an anterior to posterior deactivation on 80% (12/15) of occasions (see Figure 6h–j).
Participant 3
- Stage 1. Prepropulsion. The average duration was 0.40 +/− 0.24 s. The lingual seal was created prior to the initiation of the propulsion state on all 100% (15/15) swallows (see Figure 7a,b).
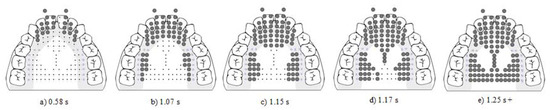 Figure 7. Display of the raw EPG data for P3’s NSOMD swallow pattern across all stages. The stages are prepropulsion (a,b), propulsion (c–e), post propulsion (e). P3 rarely released her swallow spontaneously, and maintained full contact until she began speaking after her swallow. Bolded dots represent activated electrodes.
Figure 7. Display of the raw EPG data for P3’s NSOMD swallow pattern across all stages. The stages are prepropulsion (a,b), propulsion (c–e), post propulsion (e). P3 rarely released her swallow spontaneously, and maintained full contact until she began speaking after her swallow. Bolded dots represent activated electrodes. - Stage 2. Propulsion. The average duration was 0.24 +/− 0.17 s. On all occasions, stripping action proceeded in an anterior to posterior motion with sub-bins A through G of the stripping bin sequentially activating, followed by the posterior-central-lateral, and then posterior-central-central (see Figure 7c–e).
- Stage 3. Postpropulsion. Full contact was made, but it was not spontaneously released; therefore, phase duration could not be established (see Figure 7e).
- Stage 4. Release. Directional deactivation of electrodes was rarely accomplished spontaneously. Full contact was maintained unless verbally prompted by the researchers to release. When prompted, the average duration was 0.33 +/− 0.19 s. Upon release, the pattern was posterior to anterior on 67% (10/15) trials, and anterior to posterior on 7% (1/15) occasions. On 27% (4/15) occasions, full contact was not released during the recording. The examiner confirmed that a full swallow was being completed through laryngeal palpation paired with EPG on 5 additional swallows.
DISCUSSION
The purpose of this study was to determine if EPG would be a useful adjunct and feasible option for those conducting clinical assessments of individuals with suspected NSOMD. This study demonstrates that EPG has potential in adding valuable, quantifiable information to the evaluation. The system of quantifying lingual-palatal contact patterns and measuring the durations within the prepropulsion, propulsion, postpropulsion, and release stages proved efficient for clinical analysis for this small group of participants.
Assessing the lingual-palatal contact and timing patterns from prepropulsion through to release allowed for a systematic method of identifying unique lingual-palatal behaviors in our participants with NSOMD. During prepropulsion, P1 frequently started lingual stripping before the lingual seal was fully created. This may have contributed to the excessive residues in her lateral sulci post swallow that were identified during the clinical evaluation. P2 initiated her swallow with a forward gesture of the tongue between the anterior teeth. She moved her tongue progressively forward from anterior 2 or 3 to anterior 1. This lingual pattern has been characterized as a tongue thrust (Mason & Proffit, 1974). It may contribute to P2’s long history of unsuccessful orthodontic intervention due to the tongue’s impact on dentofacial structures (Jalaly, Ahrari, & Amini, 2009). The prepropulsion stage for P3 mirrored that of her age-matched peer, and the pattern of all adults without NSOMD.
For the propulsion stage, P1 generally lacked a directional stripping action. Rather, P1 made contact with the posterior stripping bin, posterior central, and posterior lateral bins, which interrupted the anterior to posterior propulsion. This may have contributed to her excessive lingual residues and her report of requiring a liquid wash following the swallow of masticated material. Like the participants without NSOMD, P2 and P3 performed sequential anterior to posterior stripping after lingual seal completion.
When the postpropulsion stage was reached P1 was noted to “re-strip”. This may have been necessary because she lacked a sequential strip in the propulsive stage. The period of postpropulsion was maintained longer for all 3 of the participants who had NSOMD. The average postpropulsion time for participants with NSOMD adults were: P1: 1.99 +/− 0.69 s, P2: 1.04 +/− 0.23 s.
While the duration of post propulsion for the child without NSOMD exceeded those of adults without NSOMD, (marginal mean duration for adults was 0.72 +/− 0.21 s compared to 1.43 +/− 0.48 s) a spontaneous release was always achieved. P3 rarely spontaneously released her swallow resulting in excessively long durations in which the tongue was pressing upon the upper incisors. Prolonged durations have been linked to causing dental changes, and may influence the oral occlusion (Jalaly, Ahrari, & Amini, 2009) as was the case for P3. It is generally accepted that forces from unintentional and habitual behaviors constantly acting on the maxillofacial and alveolar regions can cause the bony structures to gradually deform leading to jaw deformity and malocclusion (Yamaguchi & Sueishi, 2003).
All of the participants without NSOMD spontaneously released their swallow and the pattern of release was always anterior to posterior, which mirrored findings of Chi-Fishman and Stone (1996). For our participants with NSOMD, when release occurred the direction was posterior to anterior on at least a proportion of their swallows. P1 always released spontaneously in the direction of posterior to anterior. P2 always spontaneously released but the direction was variable (posterior to anterior on 20% of occasions). P3 failed to spontaneously release. When verbally prompted to release her swallow, her direction of release was posterior to anterior on 67% of occasions. The forces generated by posterior to anterior release may also contribute to malocclusion.
CONCLUSIONS
Clinicians have limited options for assessing lingual-palatal timing and contact patterns of individuals with NSOMD. Determining the physiological abnormalities associated with the NSOMD can lead to greater individualization of the treatment plan that directly targets the needs of the client rather than approaching the disorder in a general manner. The aim of the present investigation was to determine if it is feasible to use EPG as an adjunct to non-instrumental assessment of NSOMD. This was achieved as quantification of the swallow using EPG provided insight into the unique dynamic lingual-palatal timing and contact patterns of individuals with NSOMD. As this was a feasibility study, our finding suggests that it is viable to continue to investigate the clinical utility of EPG for characterization of NSOMD.
Future Directions and Limitations
Larger investigations of EPG as a tool for providing clinically useful information for NSOMD is warranted. In addition to assessment, the visual biofeedback capabilities of EPG may be a useful aid in habilitating or rehabilitating lingual-palatal patterns. While multiple studies have found EPG to be beneficial for the treatment of
speech disorders, currently no investigations have been published on the use of EPG in remediating NSOMD. There is a financial expenditure that may prove cost inhibitive if the palate is used for evaluative purposes only. The financial investment required for the use of EPG may be of increased benefit if future investigations demonstrate usefulness as a remediation tool.
This study focused upon lingual-palatal timing and contact patterns of saliva swallows. For individuals with NSOMD there is interest in understanding the swallow patterns of both non-nutritive and nutritive swallows. However, for other patient populations, the pattern of nutritive swallows may be of primary interest. The feasibility of using EPG with these populations requires investigation.
EPG has been used widely to describe lingual-palatal contact patterns produced during speech with a variety of patient populations, but it is unclear if some might be less able to adapt to the thin oral appliance. While it has been reported that most people can quickly and completely adapt to thin pseudopalates (Fletcher, 1992; Searl et al., 2006) it is possible that individuals with NSOMD rely more heavily on sensory feedback making adaptation a greater challenge.
Finally, it should be noted that EPG displays lingual-palatal contact patterns but does not demonstrate the anatomical portion of the tongue that is creating contact with the palate. This must be deduced from the shape of an individual’s palate, and knowledge of the anatomy and physiology of the tongue (Gibbon & Lee, 2007).
References
- Ardakani, F. E. 2006. Evaluation of swallowing patterns of the tongue using real-time B-mode sonography. Journal of Contemporary Dental Practice 9: 67–74. [Google Scholar] [CrossRef]
- Bennett, J. W., P. Van Lieshout, and C. M. Steel. 2007. Tongue control for speech and swallowing in healthy younger and older subjects. International Journal of Orofacial Myology 33: 5–18. [Google Scholar] [CrossRef]
- Cayley, A. S., A. P. Tindall, W. J. Sampson, and A. R. Butcher. 2000. Electropalatographic and cephalometric assessment of tongue function in open bite and non-open bite subjects. European Journal of Orthodontics 22: 463–474. [Google Scholar] [CrossRef][Green Version]
- Chi-Fishman, G., and M. Stone. 1996. A new application for electropalatography: Swallowing. Dysphagia 11: 239–247. [Google Scholar] [CrossRef]
- Chi-Fishman, G., M. Stone, and G. McCall. 1998. Lingual action in normal sequential swallowing. Journal of Speech and Hearing Research 41: 771–785. [Google Scholar] [CrossRef]
- Complete Speech. 2012. The SmartPalate System. Available online: http://completespeech.com/speech/Product/complete_speech_therapy_system/.
- De Felicio, C. M., G. A. Folha, C. L. P. Ferreira, and A. P. M. Medeiros. 2010. Expanded protocol for oral myofunctional evaluation with scores: Validity and reliability. International Journal of Pediatric Otorhinolarynglogy 74: 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S. 1992. Articulation: A physiological approach. Singular Publishing Group, Inc.: San Diego, CA. [Google Scholar]
- Gibbon, F., and A. Lee. 2007. Electropalatography as a Research and Clinical Tool. Perspectives on Speech Science and Oralfacial Disorders (ASHA Division 5) 17: 7–13. [Google Scholar] [CrossRef]
- Hanson, M. L. 1976. Tongue-thrust: A point of view. Journal of Speech and Hearing Disorders 41: 172–184. [Google Scholar] [CrossRef]
- Jalaly, T., F. Ahrari, and F. Amini. 2009. Effect of tongue-thrust swallowing on position of anterior teeth. Journal of Dental Research, Dental Clinics, Dental Prospects 3: 73–77. [Google Scholar]
- Kennedy, D., J. Kieser, C. Bolter, M. Swain, B. Singh, and J. N. Waddell. 2010. Tongue pressure patterns during water swallowing. Dysphagia 25: 1–19. [Google Scholar] [CrossRef]
- Knosel, M., S. Klein, A. Bleckmann, and W. Engelke. 2012. Coordination of tongue activity during swallowing in mouth-breathing children. Dysphagia 27: 401–407. [Google Scholar] [CrossRef][Green Version]
- Mason, R. M., and W. R. Proffit. 1974. The tongue-thrust controversy: Background and recommendations. Journal of Speech and Hearing Disorders 29: 115–132. [Google Scholar] [CrossRef] [PubMed]
- Ono, T., K. Hori, and T. Nokubi. 2004. Pattern of tongue pressure on the hard palate during swallowing. Dysphagia 19, 259–264. [Google Scholar] [CrossRef]
- Peng, C. L., P. G. Jost-Brinkmann, N. Yoshida, H. H. Chou, and C. T. Lin. 2004. Comparison of tongue functions between mature and tongue-thrust swallowing-an ultrasound investigation. American Journal of Orthodontics and Dentofactial Orthopedics 125: 562–570. [Google Scholar] [CrossRef] [PubMed]
- Searl, J., P. Evitts, and W. J. Davis. 2006. Perceptual and acoustic evidence of speaker adaptation to a think psyedopalate. Logopedics Phoniatrics Vocology 31: 107–116. [Google Scholar] [CrossRef] [PubMed]
- Shawker, T. H., B. Sonies, M. Stone, and B. J. Baum. 1983. Real-time ultrasound visualization of tongue movement during swallowing. Journal of Clinical Ultrasound 11: 485–490. [Google Scholar] [CrossRef]
- Soder, N., and N. Miller. 2002. Using ultrasound to investigate intrapersonal variability in durational aspects of tongue movement during swallowing. Dysphagia 17: 288–297. [Google Scholar] [CrossRef]
- Steele, C. M., and P. H. H. M. Van Lieshout. 2004. The use of electromagnetic midsagittal articulography in the study of swallowing. Journal of Speech, Language, and Hearing Research 47: 342–352. [Google Scholar] [CrossRef][Green Version]
- Tamine, K., T. Ono, K. Hori, J. Kondoh, S. Hamanaka, and Y. Maeda. 2010. Age-related changes in tongue pressure during swallowing. Journal of Dental Research 89: 1097–1101. [Google Scholar] [CrossRef]
- Tasko, S. M., R. D. Kent, and J. Westbury. 2002. Variability in tongue movement kinematics during normal liquid swallowing. Dysphagia 17: 126–1138. [Google Scholar] [CrossRef]
- Wilson, E. M., and J. R. Green. 2006. Coordinative organization of lingual propulsion during the normal adult swallow. Dysphagia 21: 226–236. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H., and K. Sueishi. 2003. Malocclusion associated with abnormal posture. Bulletin of Tokyo Dental College 44: 43–54. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the author. 2013 Alana Mantie-Kozlowski, Kevin Pitt