INTRODUCTION
Juvenile dermatomyositis (JDM) is an inflammatory, chronic autoimmune multisystem vasculopathy affecting primarily the skin and muscle, causing symmetric proximal weakness and characteristic skin rash (
Chari & Laude, 2000). JDM represents a type of idiopathic myositis, which also comprises of polymyositis, necrotizing autoimmune myopathy and sporadic inclusion body myositis (Malik, Hayat & Kalia, 2016). Onset is usually insidious, with development of progressive muscle weakness and pain; a more acute onset occurs in approximately one third of children (
Cassidy & Lindsley, 2010). The incidence reported in a recent population- based study confirmed an incidence of 9.63 cases per million persons (
Tanaka & Geist, 2012). Our PubMed literature search for search words “juvenile”, “dermatomyositis” and “oral manifestations” revealed scanty literature, out of which only 53 articles discuss the oral manifestations of the disease.
The earliest event in JDM pathogenesis is the immune attack on muscle capillary endothelium, infiltration of plasmacytoid dendritic cells with a resulting type I interferon response, and upregulation of major histocompatibility (MHC) class I expression on the surface of myofibers (
Cassidy & Lindsley, 2010).
The clinical presentation of JDM was first described in 1887. Later, five criteria were given by Bohan and Peter in 1975 aid in its diagnosis. A diagnosis of probable JDM requires the presence of the pathognomonic rash (Gottron papules over the extensor surfaces of the finger joints, elbows, knees or ankles) or the heliotrope rash and two of the other criteria; definite JDM requires the characteristic rash with three other criteria. In general, the first two criteria (i.e., proximal muscle weakness and classic rash) are almost always present; criterion 3 (i.e., elevated serum levels of muscle enzymes), 4 (i.e., electromyographic changes), and 5 (i.e., histopathological changes) provide additional laboratory support for the diagnosis (
Bohan & Peter, 1975).
The onset of JDM is often characterized by fever in the range of 38 °C to 40 °C followed by Malaise, anorexia, and weight loss (
Cassidy & Lindsley, 2010). Cutaneous manifestations include the heliotrope rash (purple pink in color over the eyelids, periorbital oedema, Gottron’s papules (skin thickening over the extensor surfaces of joints) and hypertrichosis (
Chari & Laude, 2000;
Lowry & Pilkington, 2009). Calcinosis, which is the abnormal deposition of insoluble calcium salts within the skin, subcutaneous tissue, myofascia, or muscle occurs in 20–40% of patients (
Lowry & Pilkington, 2009;
Rider &
Jones, 2013). Gower’s sign which is difficulty in climbing stairs and also in raising from floor to standing on the legs to attain a standing position and Trendelenburg sign which is a diagnostic test of function and dysfunction of hip become positive when there is abductor weakness, as in poliomyelitis or muscular dystrophies (Hoeltzel, Oberle, Robinson, Agarwal, Rider, 2014;
Agarwal, 2003).
The oral lesions most commonly manifest as diffuse stomatitis and pharyngitis with halitosis. Telangiectatic lesions on the vermilion border of lips and cheeks may also occur. Capillary abnormalities in the gingiva described in five patients with juvenile dermatomyositis (JDM) were described as analogous to the periungualtelangiectases that are seen in the nail beds of patients with this disease, suggesting that oral symptoms are important diagnostic markers (Vasudevan, Vaidyalingam & Nair, 1997). Pharyngeal, hypopharyngeal, or palatal involvement, causing dysphonia, dysphagia, nasalized speech, and regurgitation of liquids through the nose can be seen in 25% of the patients, due to loss of pharyngo-esophageal muscle tone and abnormal hyolaryngeal excursion (Deepa, Kumar, Chawla, Patil, 2015; Marvi, Chung & Fiorentino, 2012;
Carstens & Schmidt, 2013).
Oral health care providers may play a primary role in the diagnosis of dermatomyositis. The importance of investigations in this area is determined by the fact that, regardless of the restricted number of dermatomyositis patients with oral involvement, the disorder may have acute evolution and may progress with a fatal outcome, or in other cases, with gradual involvement leads to serious invalidity and in the final stage needs special care (Vasudevan, Vaidyalingam, Bhaskaran, 1997).
CASE REPORT
An 8 year old male reported to the Department of Oral Medicine and Radiology, College of Dental Sciences and Research Centre, Bopal, Ahmedabad, with the chief complaint of dental caries. Medical history revealed that the patient complained of mild fever, skin rash and weakness, and was initially diagnosed as a case of virally caused muscle fatigue 2 years before. But since the symptoms worsened and the patient could not write or lift things, had difficulty in holding a comb or a tooth-brush himself, 6 months later they consulted a physiotherapist. A year later, after no resolution in movement limitations and additional complaints of dysphagia, increased intensity of erythematous rash and papules on the elbows and knees, they consulted a general practitioner, who later referred them to a rheumatologist, who diagnosed it as a case of Juvenile Dermatomyositis and initiated treatment for the same. His parents reported that the patient had difficulties in climbing stairs and standing or running for a longer period of time; difficulty in rising from sitting position; problems in swallowing solid food with occasional regurgitation and difficulty in chewing.
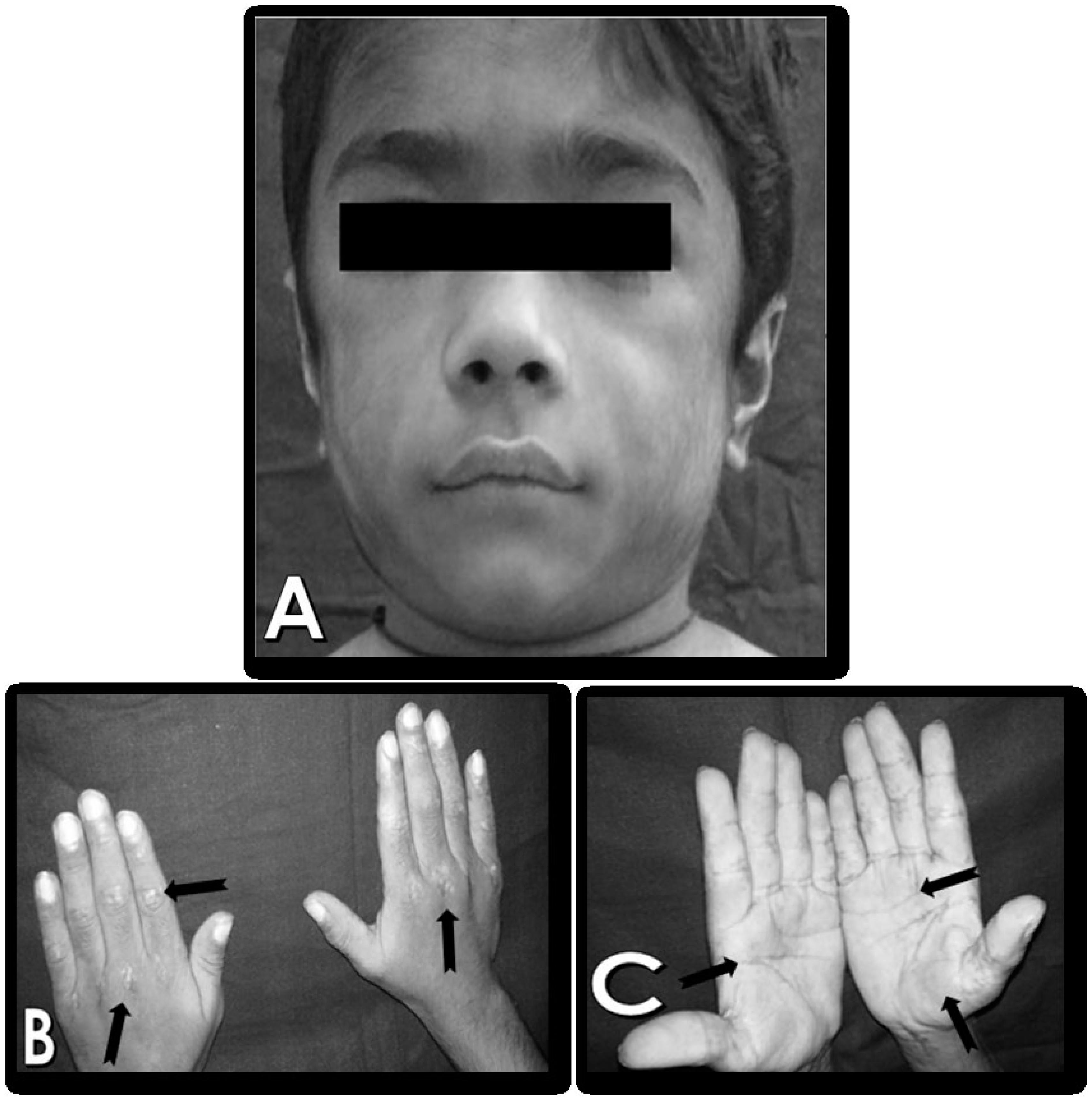
General physical examination revealed Gower’s sign and Trendelenburg sign, Moderate built, low grade fever, the patient had difficulty entering the dental chair due to limited range of motion and hence, had to ask his parents to assist him to get up on the chair by putting their hands under his shoulder blade to lift him up. Extraoral examination revealed, hypertrichosis and characteristic erythematous rash on the palmar surfaces of hands and Gottron’s papules on elbows and knees.
Figure 1 (A - C).
Intraoral examination revealed poor oral hygiene with severe halitosis, retention of food in the vestibule and the teeth due to difficulty in swallowing, leading to drooling and leakage of liquid or food while eating. He also felt fatigued while rinsing his mouth after meals, due to which he was suffering from Early Childhood Caries. The patient attributed this to his inability to brush properly due to proximal muscle weakness of upper limbs. The masseter was tender on palpation, suggestive of myositis, possibly secondary to JDM. Temporomandibular joint function and mandibular movements were limited, with pain during mouth opening, in the pre-auricular region. However, the remainder of the child’s oral examination revealed no other abnormality. The gingival tissue was normal and without inflammation.
Radiographic findings show that 36 and 46 were not erupted suggestive of delayed eruption of permanent teeth. Also, there was no root resorption noted in deciduous central incisors suggestive of delayed root resorption of deciduous teeth. Laboratory investigations showed that the Serum Creatine phosphokinase levels were elevated and there was presence of Jo-1 autoantibodies in the serum. Electromyography was suggestive of motor axonal neuropathy. MRI findings were consistent with inflammatory myopathy. Dental rehabilitation was done after obtaining CBC and platelet counts, without any adverse reactions to local anesthetics. Even though not noted on the radiograph, pulpal calcification was noted in mesiolingual canal of 74, 54 and 64 (FDI tooth numbering system) during pulpectomy. Bite blocks were used to assist in restorative therapy, since the patient could not open the mouth for prolonged duration, owing to masseter involvement. Patient was advised to consume pureed and soft texture foods to manage dysphagia. Oral prophylaxis was done, and patient was instructed to use power brush to maintain a meticulous oral hygiene.
Management under the rheumatologist consisted of high-dose prednisone, i.e., 30mg/day in divided doses of 10mg and 20mg and 0.7mg methotrexate IV per week for steroid-sparing effect. The patient responded to the treatment so the dose of steroids was tapered by 5mg after every 6 weeks and then reduced by 1mg after every 4 weeks. Currently the patient is on maintenance dose and takes prednisolone 5mg/day and 0.4 mg methotrexate IV per week.
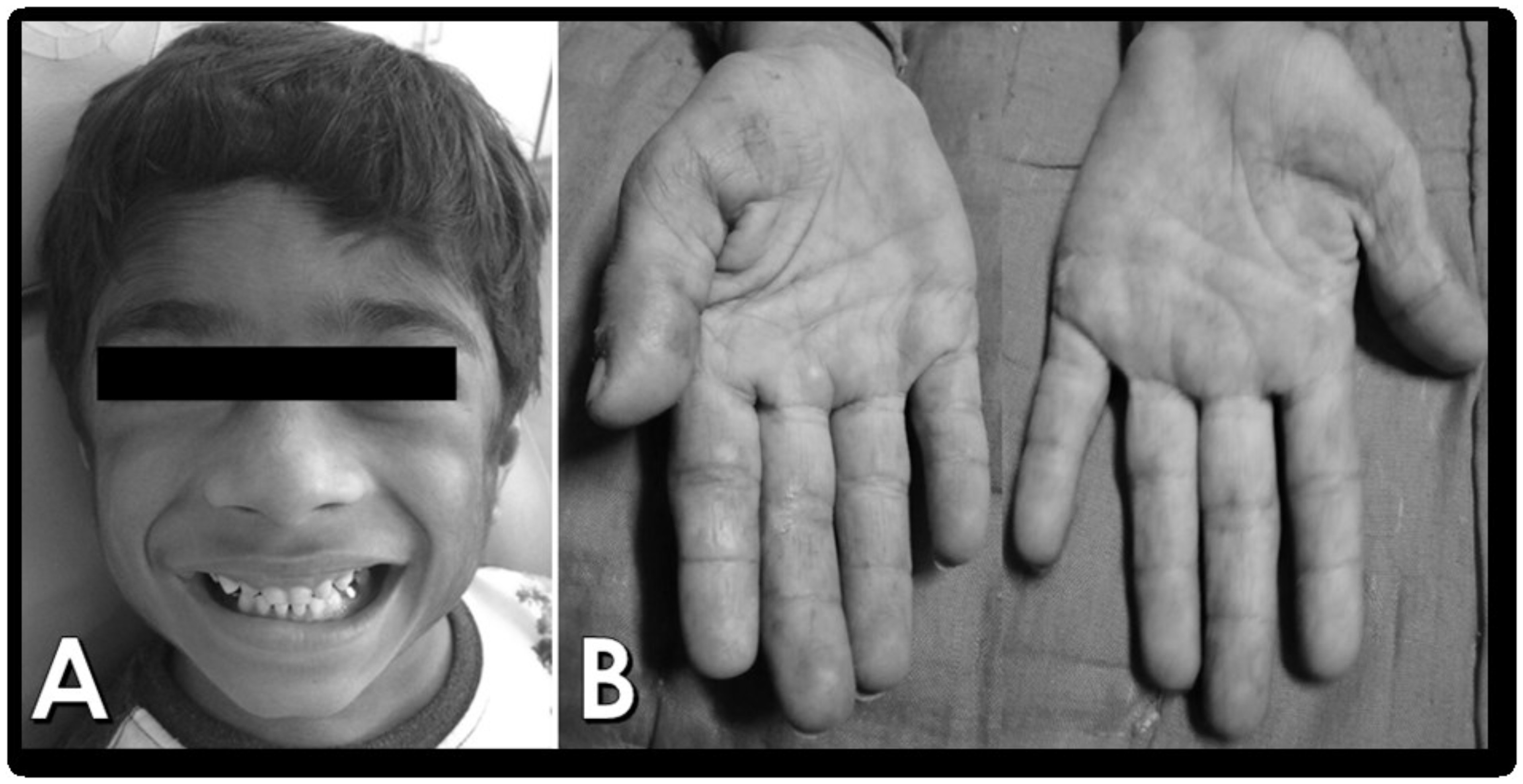
Figure 2 (A & B).
The patient was practicing physiotherapy at home as per the guidance of the pediatrician, and noted significant improvement in the range of motion of the limbs. Our patient showed significant regression of symptoms due to early diagnosis, with reduction in the hypertrichosis and erythematous rashes on the hands, along with improvement in the movements, enabling him to climb the stairs and get up from a sitting position without any difficulty. Patient is kept on follow-up regularly at 3 month intervals.
DISCUSSION
Dermatomyositis is a multi-system disease characterized by vasculopathy of the skin and/or muscles causing subacute progressive, proximal muscle weakness and typical skin rashes, with an incidence of 3.2 per million children per year (
Carstens & Schmidt, 2013); (
Neto & Goldenstein-Schainberg, 2010). JDM is thought to be the result of environmental triggers in genetically susceptible individuals, leading to immune dysfunction and specific tissue responses. Cases of infections with coxsackievirus, influenza, Group A
Streptococcus, Toxoplasmosis, parvovirus, hepatitis B,
Borrelia, and
Leishmania preceding the onset of JDM have been documented (
Cassidy & Lindsley, 2010).
Dermatomyositis has a bimodal age distribution: one peak occurs between 5–14 years of age and a second larger peak occurs between 45–64 years of age (
Carstens & Schmidt, 2013). In our report, the age at the onset of the disease is 6 years which is in congruence with the literature. Age at onset and the sex ratio of Juvenile Polymyositis (PM) are comparable to JDM. But in PM, there are no associated cutaneous abnormalities. Heliotrope rash, periorbital edema and Gottron papules are present only in children with JDM, whereas linear extensor erythema is present in both JDM and SLE. Myositis occurs in systemic scleroderma and juvenile chronic arthritis, but can be differentiated from that of JDM by its severity, greater elevation of serum levels of muscle enzymes and histological examination of muscle (
Cassidy & Lindsley, 2010).
Bohan and Peter’s (
1975) criteria confirms a definite diagnosis of juvenile dermatomyositis for our patient.
The classic heliotrope rash occurs over the upper eyelids as a violaceous, reddish purple suffusion. Pharyngeal, hypopharyngeal, and palatal muscles are frequently affected, leading to difficulty in swallowing i.e., dysphagia. Dysphonia (weakness of the voice or a gurgly voice quality) and nasal speech are also frequent signs. Regurgitation of liquids through the nose increases the threat of aspiration in these children (
Cassidy & Lindsley, 2010). Our patient had characteristic rash on the palmar surfaces of hands and Gottron’s papules along with proximal muscle weakness. In laboratory findings, there is elevation of muscle enzymes (Saghafi, Rezaieyazdi & Hashemzadeh, 2014). CPK was elevated in this case. Dysphagia and esophageal dysmotility are common in children with JDM (
Deepa et al., 2015). Our patient complained of difficulty in swallowing. Similar to the experience reported by Hamlin C and Shelton J, extractions in our patient were accomplished with local anesthetic without any difficulty (Martin, Li & Wedderburn, 2012). Agents used for the management of myositis include glucocorticoids and traditional immunosuppressive or immunomodulatory agents such as methotrexate, azathioprine, mycophenolatemofetil (MMF), cyclosporine, tacrolimus and intravenous immunoglobulin (IVIg) (Robinson, Hoeltzel, Wahezi, et al., 2014); (Hamlin, Jean & Shelton, 1984). Our patient was receiving 30mg/day prednisolone in divided doses of 10mg and 20mg and 0.7mg methotrexate IV per week for steroid-sparing effect. Physical training and rehabilitation should be started early, maintained throughout the treatment (Moghadam-Kia, Aggarwal & Oddis, 2015).
Kim, El-Hallak, Dedeoglu, et al., (2009) suggest that an aggressive treatment to obtain rapid control of muscle weakness and inflammation significantly improves the prognosis and reduces the risk of complications related to the disease. Our findings are compatible with other reports showing JDM.
Table 1.
Oro-facial manifestations of JDM.
Table 1.
Oro-facial manifestations of JDM.
Table 2.
Dental treatment considerations for patients with Juvenile Dermatomyositis.
Table 2.
Dental treatment considerations for patients with Juvenile Dermatomyositis.
CONCLUSION
Juvenile dermatomyositis is a rare and potentially severe disease which requires early diagnosis and management, hence the need to present an already diagnosed case. The aim of presenting this case is to make doctors aware of this condition to aid in early diagnosis of a patient suffering from such myopathy, which can be presented in a dental clinic with the oro-facial manifestations. Juvenile dermatomyositis has the potential to have a significant adverse effect upon the lifestyle of the patient. Oral health professionals are encouraged to keep abreast of new developments in this field and fully participate in the interdisciplinary care of this disease. Early diagnosis and the fast institution of adequate therapy led our patient to achieve good results and quality of life.