Comparative Antimicrobial Effects of Dimethylsulfoxide and Dimethylsulfone on the Planktonic Growth and Viability of Porphyromonas gingivalis and Their Cytotoxic Effects on Human Oral Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Supplies
2.2. Preparation of Stock DMSO-2
2.3. Planktonic Growth of P. gingivalis
2.4. Viability of P. gingivalis
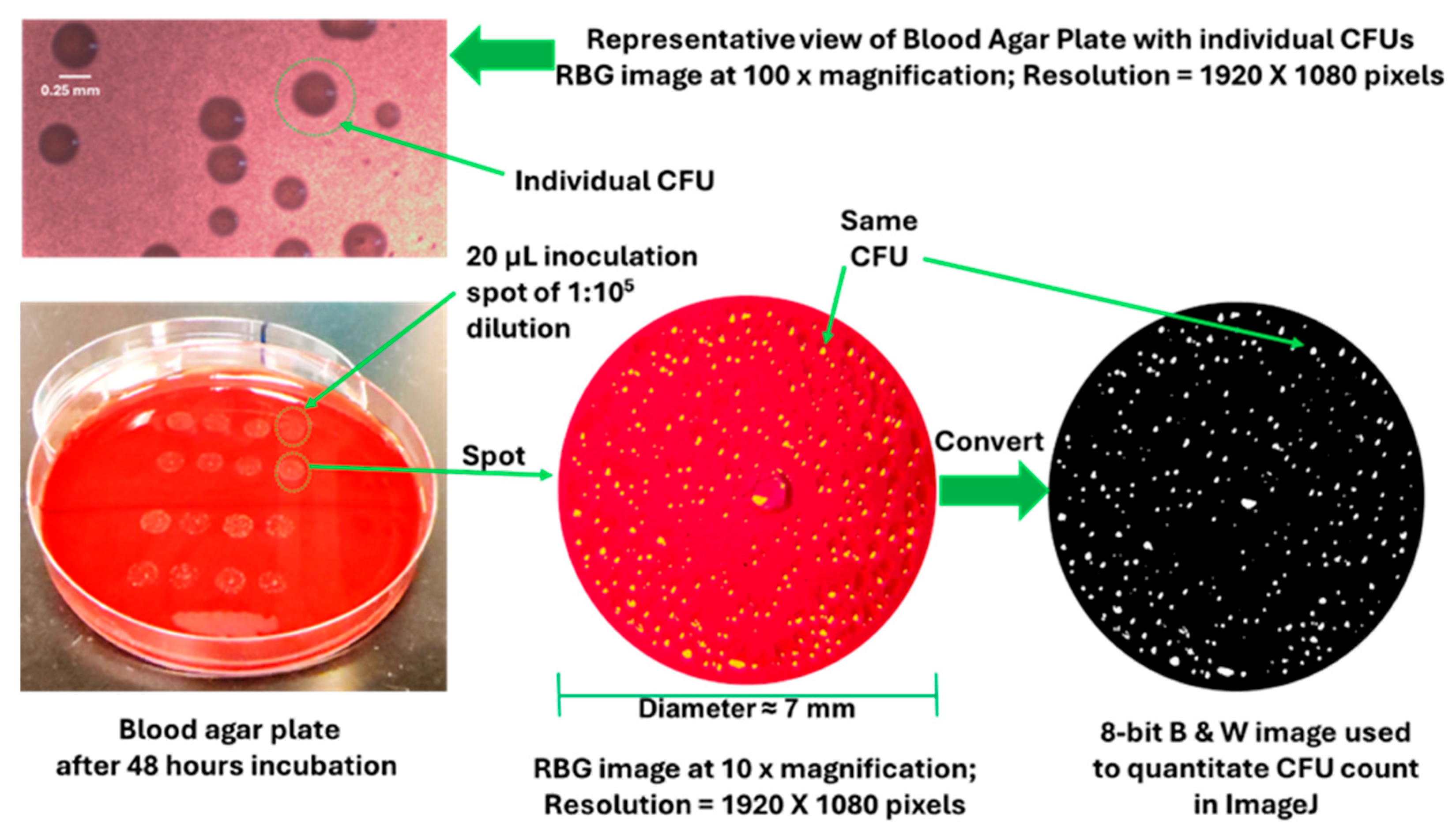
2.5. CFU Analysis of P. gingivalis
2.6. Morphology and Viability of OKF6/TERT-2 Cells
2.7. Statistical Analysis
3. Results
3.1. DMSO and DMSO-2 Inhibit Planktonic Growth of P. gingivalis
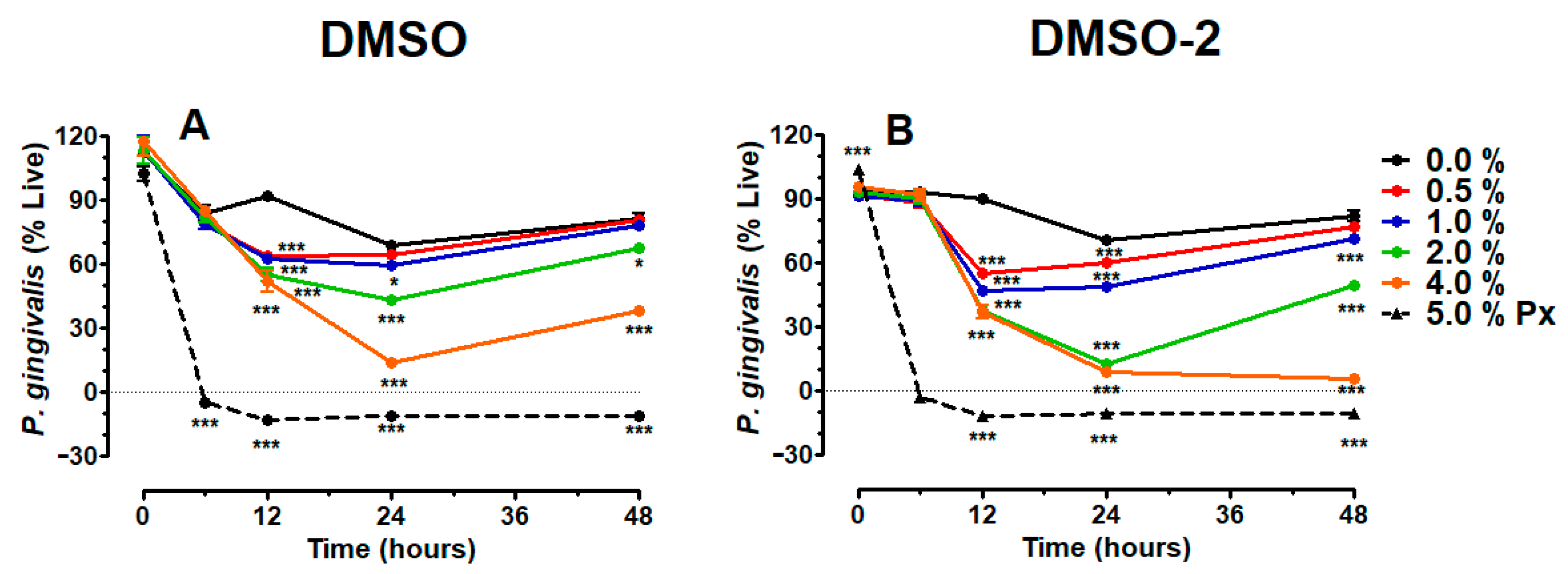
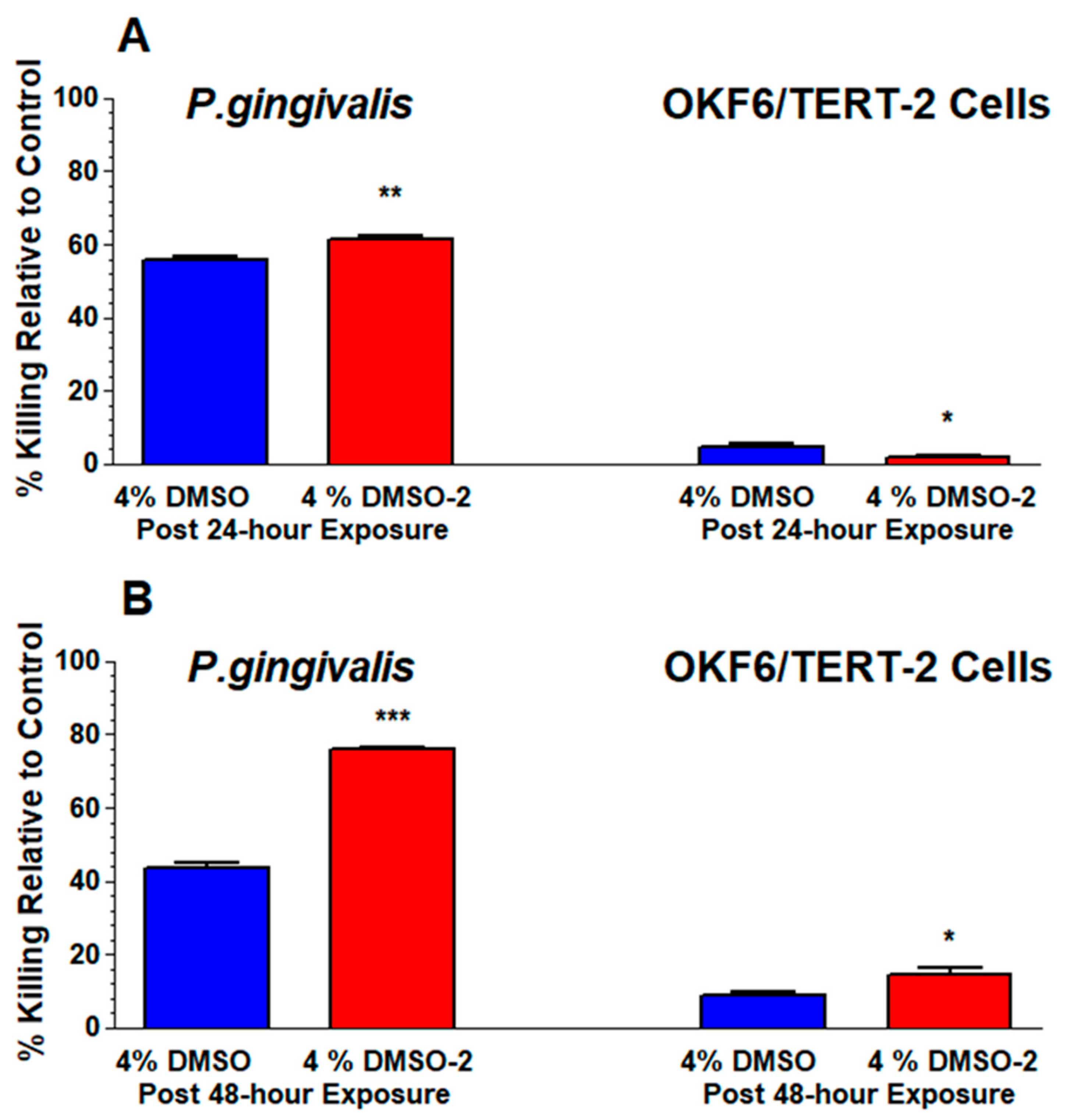
3.2. DMSO and DMSO-2 Suppress Viability of P. gingivalis
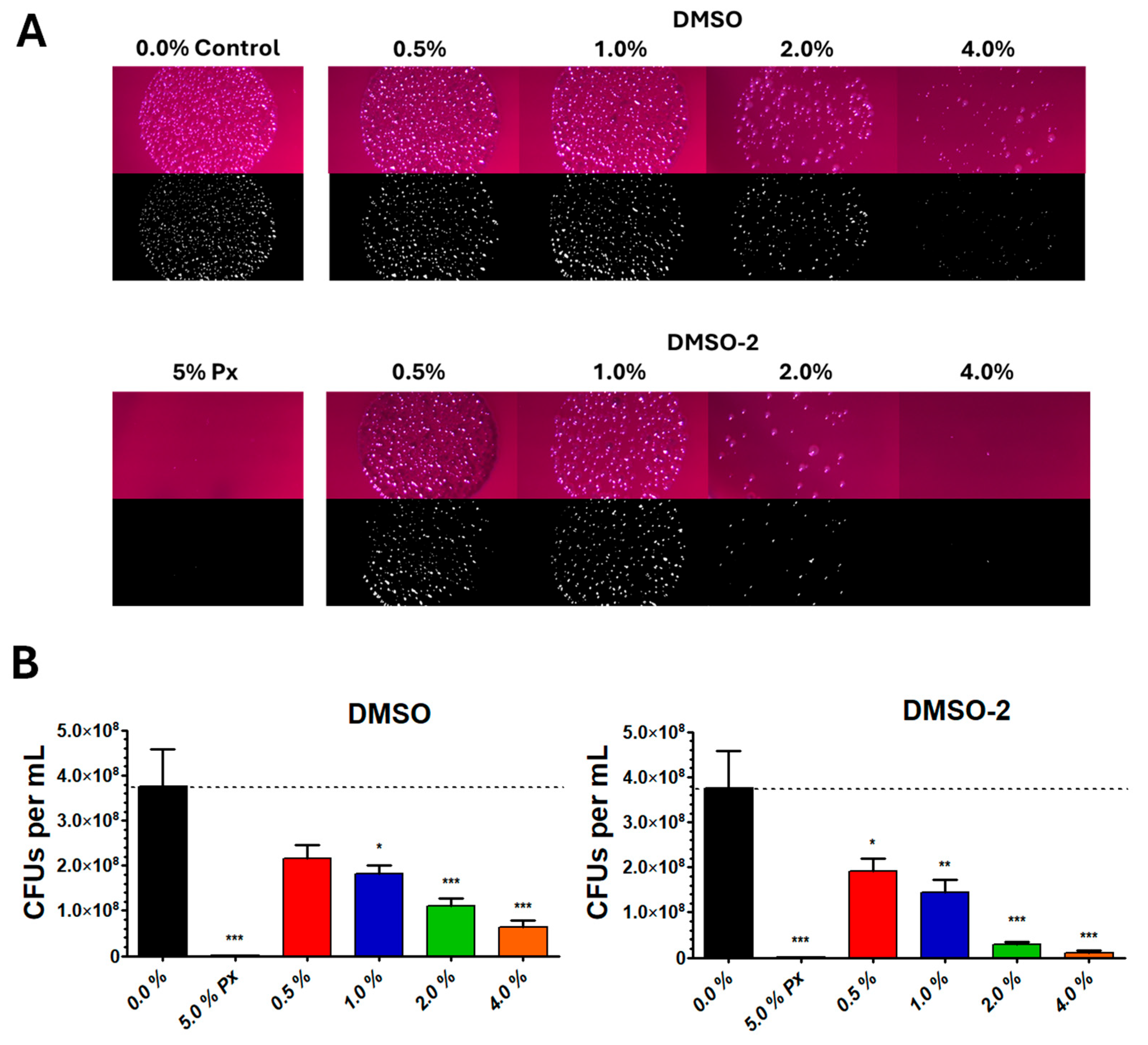
3.3. DMSO and DMSO-2 Reduce CFU Count of P. gingivalis
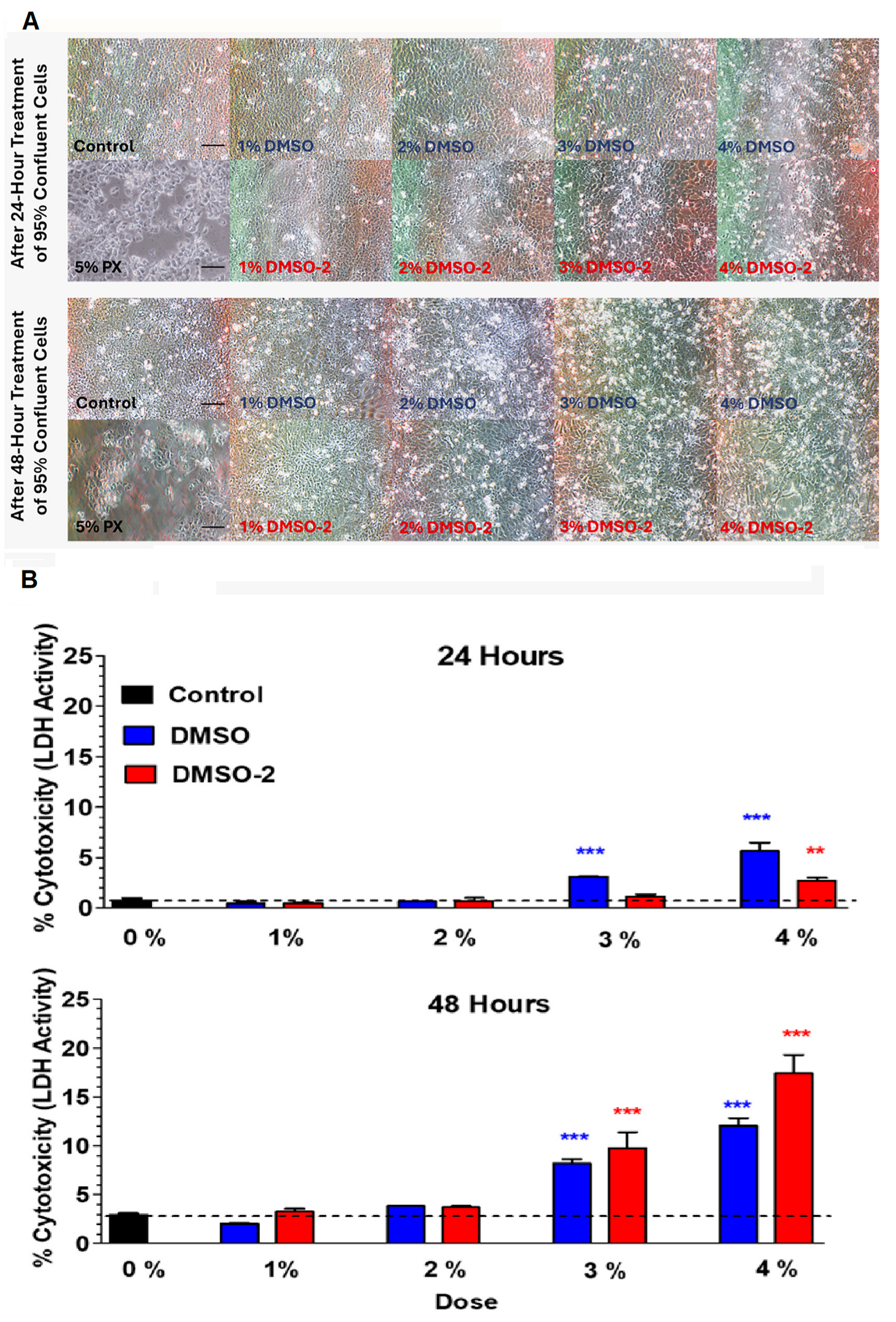
3.4. Morphology and Viability of OKF6/TERT-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clifford, K.; Dy-Boarman, E.; Haase, K.; Maxvill, K.; Pass, S.; Alvarez, C. Challenges with Diagnosing and Managing Sepsis in Older Adults. Expert Rev. Anti-Infect. Ther. 2016, 14, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Church, D.L. Selected Topics in Anaerobic Bacteriology. Microbiol. Spectr. 2016, 4, 493–535. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Treatment of Anaerobic Infection. Expert Rev. Anti-Infect. Ther. 2007, 5, 991–1006. [Google Scholar] [CrossRef]
- Noor, A.; Khetarpal, S. Anaerobic Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The Keystone-Pathogen Hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Cuadra, G.; Hanel, A.; Christian, N.; Pecorelli, S.; Ha, V.; Tomov, S.; Palazzolo, D. Perspective Chapter: The Impact of Electronic Cigarettes on the Oral Microenvironment. In Periodontal Frontiers [Working Title]; IntechOpen: London, UK, 2025. [Google Scholar]
- Madruga, G.M.; Crivellenti, L.Z.; Borin-Crivellenti, S.; Cintra, C.A.; Gomes, L.G.; Spiller, P.R. Comparative Use of Dimethyl Sulphoxide (DMSO) in Different Animal Species. Vet. Med. 2017, 62, 179–185. [Google Scholar] [CrossRef]
- Jacob, S.W.; Torre, J.C. Dimethyl Sulfoxide (DMSO) in Trauma and Disease; CRC press: Boca Raton, FL, USA, 2015; ISBN 978-1-4987-1467-9. [Google Scholar]
- Carrol, D.H.; Chassagne, F.; Dettweiler, M.; Quave, C.L. Antibacterial Activity of Plant Species Used for Oral Health against Porphyromonas Gingivalis. PLoS ONE 2020, 15, e0239316. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Hollanders, M.; Benkendorff, K. Out of Control: The Need for Standardised Solvent Approaches and Data Reporting in Antibiofilm Assays Incorporating Dimethyl-Sulfoxide (DMSO). Biofilm 2022, 4, 100081. [Google Scholar] [CrossRef]
- Wadhwani, T.; Desai, K.; Patel, D.; Lawani, D.; Bahaley, P.; Joshi, P.; Kothari, V. Effect of Various Solvents on Bacterial Growth in Context of Determining MIC of Various Antimicrobials. Int. J. Microbiol. 2008, 7, 1–6. [Google Scholar]
- Feldman, W.E.; Punch, J.D.; Holden, P.C. In Vivo and In Vitro Effects of Dimethyl Sulfoxide on Streptomycin-Sensitive and -Resistant Escherichia coli. Ann. N. Y. Acad. Sci. 1975, 243, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ansel, H.C.; Norred, W.P.; Roth, I.L. Antimicrobial Activity of Dimethyl Sulfoxide Against Escherichia Coli, Pseudomonas Aeruginosa, and Bacillus Megaterium. J. Pharm. Sci. 1969, 58, 836–839. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected Low-dose Toxicity of the Universal Solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO Induces Drastic Changes in Human Cellular Processes and Epigenetic Landscape in Vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed]
- Kollerup Madsen, B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse Reactions of Dimethyl Sulfoxide in Humans: A Systematic Review. F1000Research 2019, 7, 1746. [Google Scholar] [CrossRef]
- Vaidya, S.; Tozer, K.; Chen, J. An Overview of Embolic Agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Butawan, M.; Benjamin, R.; Bloomer, R. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef]
- Williams, K.I.H.; Burstein, S.H.; Layne, D.S. Metabolism of Dimethyl Sulfide, Dimethyl Sulfoxide, and Dimethyl Sulfone in the Rabbit. Arch. Biochem. Biophys. 1966, 117, 84–87. [Google Scholar] [CrossRef]
- He, X.; Slupsky, C.M. Metabolic Fingerprint of Dimethyl Sulfone (DMSO2) in Microbial–Mammalian Co-Metabolism. J. Proteome Res. 2014, 13, 5281–5292. [Google Scholar] [CrossRef]
- HOMD: Human Oral Microbiome Database. Available online: https://www.homd.org/# (accessed on 15 August 2025).
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral Microbiomes: More and More Importance in Oral Cavity and Whole Body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Zamanian Azodi, M. Study of Porphyromonas Gingivalis in Periodontal Diseases: A Systematic Review and Meta-Analysis. Med. J. Islam. Repub. Iran. 2017, 31, 62. Available online: https://pubmed.ncbi.nlm.nih.gov/29445691/ (accessed on 25 October 2025). [CrossRef] [PubMed]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human Keratinocytes That Express hTERT and Also Bypass a P16INK4a -Enforced Mechanism That Limits Life Span Become Immortal yet Retain Normal Growth and Differentiation Characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. [Google Scholar] [CrossRef]
- Werheim, E.R.; Senior, K.G.; Shaffer, C.A.; Cuadra, G.A. Oral Pathogen Porphyromonas Gingivalis Can Escape Phagocytosis of Mammalian Macrophages. Microorganisms 2020, 8, 1432. [Google Scholar] [CrossRef]
- Singer Instruments. How to Count Colonies with ImageJ (and Fiji); Singer Instruments: Roadwater, UK; Available online: https://www.singerinstruments.com/resource/counting-colonies-with-imagej/ (accessed on 25 October 2025).
- Cuadra, G.A.; Shamim, A.; Shah, R.; Morgan, J.; Palazzolo, D.L. Comparison of Culture Media for In Vitro Expansion of Oral Epithelial Keratinocytes. Appl. Biosci. 2023, 2, 308–327. [Google Scholar] [CrossRef]
- Shamim, A.; Herzog, H.; Shah, R.; Pecorelli, S.; Nisbet, V.; George, A.; Cuadra, G.A.; Palazzolo, D.L. Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids. Dent. J. 2025, 13, 60. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021, 30, 096368972199961. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; McKenzie, K.; Ma, X. Effect of Dimethyl Sulfoxide on in Vitro Proliferation of Skin Fibroblast Cells. J. Biotech. Res. 2017, 8, 78–82. [Google Scholar]
- Gallardo-Villagrán, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. [Google Scholar] [CrossRef]
- Nguyen, T.; Parat, M.-O.; Hodson, M.; Pan, J.; Shaw, P.; Hewavitharana, A. Chemical Characterization and in Vitro Cytotoxicity on Squamous Cell Carcinoma Cells of Carica Papaya Leaf Extracts. Toxins 2015, 8, 7. [Google Scholar] [CrossRef]
- Poole, T.L.; Benjamin, R.; Genovese, K.J.; Nisbet, D.J. Methylsulfonylmethane Exhibits Bacteriostatic Inhibition of Escherichia Coli, and Salmonella Enterica Kinshasa, in Vitro. J. Appl. Microbiol. 2019, 127, 1677–1685. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, H.; Ren, T.; Kim, I.H. Protective Effects of Methylsulfonylmethane (MSM) on Barrier Function Injury of Porcine Intestinal Epithelial Cells (IPEC-J2) Induced by Lipopolysaccharide (LPS). Can. J. Anim. Sci. 2023, 103, 262–272. [Google Scholar] [CrossRef]
- Jacob, S.W.; Bischel, M.; Herschler, R.J. Dimethyl Sulfoxide (DMSO): A New Concept in Pharmacotherapy. Curr. Ther. Res. Clin. Exp. 1964, 6, 134–135. [Google Scholar] [PubMed]
- Jacob, S.W.; Herschler, R. Pharmacology of DMSO. Cryobiology 1986, 23, 14–27. [Google Scholar] [CrossRef]
- Ghajar, B.M.; Harmon, S.A. The Effect of Dimethyl Sulfoxide (DMSO) on Permeability of Staphylococcusaureus. Biochem. Biophys. Res. Commun. 1968, 32, 940–944. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Anwar, J. Ion Transport through Chemically Induced Pores in Protein-Free Phospholipid Membranes. J. Phys. Chem. B 2007, 111, 13379–13382. [Google Scholar] [CrossRef] [PubMed]
- Tunçer, S.; Gurbanov, R. Non-Growth Inhibitory Doses of Dimethyl Sulfoxide Alter Gene Expression and Epigenetic Pattern of Bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 299–312. [Google Scholar] [CrossRef]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Antibiofilm Activity and Mode of Action of DMSO Alone and Its Combination with Afatinib against Gram-Negative Pathogens. Folia Microbiol. 2018, 63, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Li, Y.-S.; Song, M.-F.; Kasai, H. DNA Methylation by Dimethyl Sulfoxide and Methionine Sulfoxide Triggered by Hydroxyl Radical and Implications for Epigenetic Modifications. Bioorganic Med. Chem. Lett. 2010, 20, 260–265. [Google Scholar] [CrossRef]
- Cloutier, J.F.; Castonguay, A.; O’Connor, T.R.; Drouin, R. Alkylating Agent and Chromatin Structure Determine Sequence Context-Dependent Formation of Alkylpurines. J. Mol. Biol. 2001, 306, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, J.J.; Harkaway, S.; Snyder, R. Biological Effects of the Metabolites of Dimethyl Sulfoxide. Ann. N. Y. Acad. Sci. 1975, 243, 104–109. [Google Scholar] [CrossRef]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary Utilization of Dimethyl Sulfoxide: Pharmacological, Cellular, and Molecular Aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Muir, M. DMSO: Many Uses, Much Controversy. Altern. Complement. Ther. 1996, 2, 230–235. [Google Scholar] [CrossRef]
- Davis, P.W. An Incipient “Wonder Drug” Movement: DMSO and the Food and Drug Administrat. Soc. Probl. 1984, 32, 197–212. [Google Scholar] [CrossRef]
- Swanson, B.N. Medical Use of Dimethyl Sulfoxide (DMSO). Rev. Clin. Basic. Pharm. 1985, 5, 1–33. [Google Scholar]
- Jacob, S.W.; Appleton, J. MSM The Definitive Guide (A Comprehensive Review of a Science and Therapeutics of Methylsulfonylmethane), 1st ed.; Freedom Press: London, UK, 2003; ISBN 1-893910-21-0. [Google Scholar]
- Still, A.B.; Sellers, J.T.; Chrenek, M.; Nickerson, J.M.; Boatright, J.H. Systemic Treatment with Methylsulfonylmethane Protects against Light-Induced Retinal Degeneration. In Proceedings of the ARVO Annual Meeting, Denver, CO, USA, 1–4 May 2022. [Google Scholar]
- Borzelleca, J.; Spies, I.; Wallace, K. Dossier in Support of the Generally Recognized as Safe (Gras) Status of Optimsm (Methylsulfonylmethane; Msm) as a Food Ingredient; Food and Drug Administration: Vero Beach, FL, USA, 2007. [Google Scholar]
- Kim, L.S.; Axelrod, L.J.; Howard, P.; Buratovich, N.; Waters, R.F. Efficacy of Methylsulfonylmethane (MSM) in Osteoarthritis Pain of the Knee: A Pilot Clinical Trial. Osteoarthr. Cartil. 2006, 14, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Debbi, E.M.; Agar, G.; Fichman, G.; Ziv, Y.B.; Kardosh, R.; Halperin, N.; Elbaz, A.; Beer, Y.; Debi, R. Efficacy of Methylsulfonylmethane Supplementation on Osteoarthritis of the Knee: A Randomized Controlled Study. BMC Complement. Altern. Med. 2011, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- MSM JointGel. Available online: https://www.amazon.com/JointGel-Formula-Flavored-Combination-Bioactive/dp/B075FFBXD3/ref=sr_1_1_sspa?crid=1YGG5ONOPJSJR&dib=eyJ2IjoiMSJ9.zCJGi2t1lgay6SrpOWw641tPeE0vgaIHOMRpH4r6U_o7AKvm87XedNYNBVADhblbmfkhDVuAu2Za_v_1VPD53EOGK21xFW9AjCMopSsWUOUvh7-xj1cPxWMp49yrpQjOG__PGLvhVCgtnZDvSaQIL6SkbLLqCc5dvXIJqTLc8cZJYCLS96VeVn0xIDR8-1q9ZxEMt8qyT-stvGuHV73Yinz_qCdN-RaGVPx5m37_gFI-nnZwp3YowpYML4TvuXeyKTCInxoPHqYVGp9P9rmNnzVM0r1qs9amh66VxH7FZZ8U.mBaC6tv8wWlPayNmsf3soGnLMEuXAhzAou1zGCwlG7A&dib_tag=se&keywords=msm+products&qid=1711552097&s=beauty&sprefix=msm+products%2Cbeauty%2C95&sr=1-1-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 (accessed on 25 October 2025).
- MSM Skin Enhance Cream. Available online: https://www.amazon.com/At-Last-Naturals-Enhance-Cream/dp/B00854H6GY/ref=sr_1_5?crid=1YGG5ONOP-JSJR&dib=eyJ2IjoiMSJ9.zCJGi2t1lgay6SrpOWw641tPeE0vgaIHOMRpH4r6U_o7AKvm87XedNYNBVADhblbmfkhDVuAu2Za_v_1VPD53KJH_x-a3Anu1R4nvhT3_uWU7s8mmpJb9A7tdc1kYjmUR1PMKvuQZLLGDx5rbS-pnaSkbLLqCc5dvXIJqTLc8cZJYCLS96VeVn0xIDR8-1q9ZxEMt8qyT-stvGuHV73YimIfDDaOwv8NyGPzXUxCYOKMnxWDEv-zUDJFW4BoiFVv-7pE2gDZEwzWY3vcou44QxlM0r1qs9amh66VxH7FZZ8U.qAisAHG8lNA5NHutHJ2Rzs6HAj0n6rsWmkOYMdW4QnE&dib_tag=se&keywords=msm+products&qid=1711552755&s=beauty&sprefix=msm+products%2Cbeauty%2C95&sr=1-5 (accessed on 25 October 2025).
- MSM Shampoo Bar. Available online: https://www.amazon.com/Buffalo-Gal-Grassfed-Beauty-Unscented/dp/B0BSTG6L6S/ref=sr_1_1_sspa?crid=1GFCKFTWDW5RU&dib=eyJ2IjoiMSJ9.6e0FqrxJUEIPa4qS97jewK_TYotOhD-fUQMuU9iK0JoDbeIngJMv55oDY-NAD6CPzq4zDTzNz8OvacBhQiDde_GPuiO9O3kqNVHOKXeQIDrbvcEx8aRg8HTQlbXqTSCcl8FHavilcyEFjRTffG4K91pvXea7YmYEZO4d9rFM8_1O7AnZO9nHptvF--yKK9JSLMDo9nqSCUujDG1OjvUGgeLoicS7p4DtD4IxGKPpi7iwnly_JJ6lt5o93BRjRtOw8S2_xLkXPcWLdcxWJs6S5dHi2zCIlaW7j5FozW1skrR0.lbFIwaV7xsbe1d0F-o0hKcFhDpUW-gaU8leVDFgSI22s&dib_tag=se&keywords=msm+shampoo&qid=1711552981&s=beauty&sprefix=msm+shampoo%2Cbeauty%2C82&sr=1-1-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 (accessed on 25 October 2025).
- MSM Nasal Spray. Available online: https://www.socalhealthyhideaway.net/product/msm-nasal-spray/34 (accessed on 29 October 2025).
- MSM Toothpaste. Available online: https://www.amazon.com/Trimedica-MSM-Toothpaste-3-oz/dp/B0013QRZRQ/ref=cm_cr_arp_d_product_top?ie=UTF8 (accessed on 25 October 2025).
- MSM Mouthwash. Available online: https://store.turbify.net/drkowalski/coolmout16oz.html (accessed on 25 October 2025).
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J. Communication among Oral Bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Walker, J.T.; Paolini, D.L.; Segretario, J. Quantitation of Residual Dimethylsulfoxide in a Drug Substance (Bisnaf Ide) by Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. Sci. 1996, 34, 513–516. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yamamoto, S.; Narai, R.; Nishida, M.; Yashiki, M.; Sakui, N.; Namera, A. Determination of Dimethyl Sulfoxide and Dimethyl Sulfone in Urine by Gas Chromatography–Mass Spectrometry after Preparation Using 2,2-dimethoxypropane. Biomed. Chromatogr. 2010, 24, 465–471. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzolo, D.L.; Jorratt, A.; Patel, D.; Hoover, M.; Mondal, D.; Tabakha, M.; Tran, C.; Amram, J.R.; Cuadra, G.A. Comparative Antimicrobial Effects of Dimethylsulfoxide and Dimethylsulfone on the Planktonic Growth and Viability of Porphyromonas gingivalis and Their Cytotoxic Effects on Human Oral Epithelial Cells. Bacteria 2025, 4, 57. https://doi.org/10.3390/bacteria4040057
Palazzolo DL, Jorratt A, Patel D, Hoover M, Mondal D, Tabakha M, Tran C, Amram JR, Cuadra GA. Comparative Antimicrobial Effects of Dimethylsulfoxide and Dimethylsulfone on the Planktonic Growth and Viability of Porphyromonas gingivalis and Their Cytotoxic Effects on Human Oral Epithelial Cells. Bacteria. 2025; 4(4):57. https://doi.org/10.3390/bacteria4040057
Chicago/Turabian StylePalazzolo, Dominic L., Andrea Jorratt, Deneil Patel, Makenna Hoover, Debasis Mondal, Maya Tabakha, Cathy Tran, Juliette R. Amram, and Giancarlo A. Cuadra. 2025. "Comparative Antimicrobial Effects of Dimethylsulfoxide and Dimethylsulfone on the Planktonic Growth and Viability of Porphyromonas gingivalis and Their Cytotoxic Effects on Human Oral Epithelial Cells" Bacteria 4, no. 4: 57. https://doi.org/10.3390/bacteria4040057
APA StylePalazzolo, D. L., Jorratt, A., Patel, D., Hoover, M., Mondal, D., Tabakha, M., Tran, C., Amram, J. R., & Cuadra, G. A. (2025). Comparative Antimicrobial Effects of Dimethylsulfoxide and Dimethylsulfone on the Planktonic Growth and Viability of Porphyromonas gingivalis and Their Cytotoxic Effects on Human Oral Epithelial Cells. Bacteria, 4(4), 57. https://doi.org/10.3390/bacteria4040057







