Unveiling the Molecular Mechanism of Azospirillum in Plant Growth Promotion
Abstract
1. Introduction
2. Comparative Genomics and Proteomics Study Among Different Azospirillum Strains
3. Plant Growth Promoting Hormones and Regulators Produced by Azospirillum Species
3.1. Phytohormone Production
| Phytohormones | Function | Molecules Present in Phytohormones | Reference |
|---|---|---|---|
| Auxin | Growth and development of different plant tissues, Cell Division | IAA, PAA, IBA | [29,36] |
| Gibberellic Acid | Cell division, breaking dormancy | GA3, GA1 | [37,38] |
| Cytokines | Leaf growth, chloroplast maturation, and shoot and root morphogenesis | iP, iPr, Z, t-Zr | [39,40] |
| Abscisic acid | Phytohormone production in response to environmental stress | ABA | [41,42] |
| Polyamines | Root growth, control stomata | Cad, Spm, Spd | [29,41] |
| Ethylene | Breaking of seed dormancy, growth modulation, stress responses | Et | [43,44] |
3.2. Siderophore Production
3.3. Other Plant Regulators for Plant Growth
3.3.1. Nitric Oxide
3.3.2. Polyamines
3.3.3. Phosphate Solubilization
4. Genetics of Nitrogen Fixation by Azospirillum

| Gene | Function | References |
|---|---|---|
| nifH | Dinitrogenase reductase. | [21] |
| nifD | Subunit of dinitrogenase, FeMo-co biosynthesis, | [96] |
| nifK | Subunit of dinitrogenase | [96] |
| nifA | Nitrogen fixation (nif) genes transcriptional activator (Regulatory element) | [21] |
| nifN | Synthesis of FeMo-co | [96] |
| nifX | Involved in FeMo cosynthesis | [96] |
| nifB | Required for Fe-Mo cosynthesis | [96] |
| nifU | Participates in the mobilization of iron for the production and repair of Fe-S clusters | [96] |
| nifS | Participates in the mobilization of S for the production and repair of Fe-S clusters | [96] |
| glnA | Glutamine synthetase structural gene | [21] |
| amtB | Transporter of the structural gene ammonium | [21] |
| glnZ | PII homologue | [21] |
| glnD | The enzyme uridylyl-removing/uridylyl-transferase (UTase/UR) | [21] |
| ntrB | The two-component regulatory system sensor protein involved in overall nitrogen regulation | [21] |
| ntrC | The two-component regulatory system regulator protein involved in overall nitrogen regulation | [21] |
| ntrA, rpoN | A different sigma factor | [21] |
| draT | Reduced dinitrogenase ADP ribosyl-transferase | [21] |
| draG | Glycohydrolase activation by dinitrogenase reductase | [21] |
5. Application of Azospirillum spp. In Stress Management, Phytoremediation, Biofortification, and Biocontrol in Modern Agriculture
6. Molecular Tools Techniques to Study Azospirillum Species
7. Genetic Engineering in Azospirillum and Its Benefits
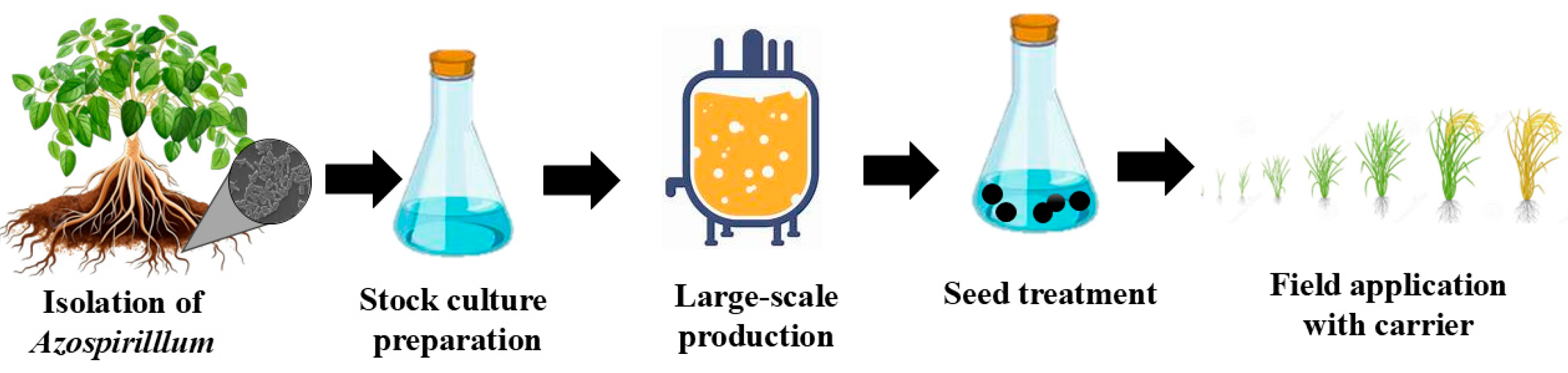
8. Formulation of Inocula and Industrial Production of Azospirillum Species
9. Field Applications, Commercial Products, and Barriers to Adoption
10. Novel Nitrogen Fixing Azospirillum Species and Their Efficiency
11. Quorum Sensing (AHLs Pathway) in Azospirillum
12. Effects of Azospirillum-Based Nano-Biofertilizers on Crop Growth
13. Ecological Effects of the Application of Azospirillum spp.
14. Future Prospects
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugavel, D.; Rusyn, I.; Solorza-Feria, O.; Kamaraj, S.K. Sustainable SMART fertilizers in agriculture systems: A review on fundamentals to in-field applications. Sci. Total Environ. 2023, 904, 166729. [Google Scholar] [CrossRef]
- Pramanik, B.; Sar, P.; Bharti, R.; Gupta, R.K.; Purkayastha, S.; Sinha, S.; Chattaraj, S.; Mitra, D. Multifactorial role of nanoparticles in alleviating environmental stresses for sustainable crop production and protection. Plant Physiol. Biochem. 2023, 201, 107831. [Google Scholar] [CrossRef]
- Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Mitra, D.; Pellegrini, M.; Olatunbosun, A.N.; Mondal, R.; Del Gallo, M.; Chattaraj, S.; Chakroborty, D.; Priyadarshini, A.; Khoshru, B.; Sierra, B.G.; et al. Seed priming with microbial inoculants for enhanced crop yield. In Microbial Inoculants; Academic Press: Cambridge, MA, USA, 2023; pp. 99–123. [Google Scholar]
- Kumar, G.; Baweja, P. Biofertilizer: A tool for sustainable agriculture in changing environment. Bioeng. Res. 2024, 11, 1. [Google Scholar]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Nad, S.; Konar, U.; Chattaraj, S.; Ganguly, A. Valorization of feather waste by microbial enzymatic activity: Bioconversion, production and application. In Agro-Waste to Microbe Assisted Value Added Product: Challenges and Future Prospects: Recent Developments in Agro-Waste Valorization Research; Springer Nature: Cham, Switzerland, 2024; pp. 337–363. [Google Scholar]
- Suhameena, B.; Devi, S.; Gowri, R.; Kumar, S.D. Utilization of Azospirillum as a Biofertilizer–An overview. Int. J. Pharm. Sci. Rev. Res. 2020, 62, 141–145. [Google Scholar]
- Jehani, M.D.; Singh, S.; Kumar, D.; Kumar, G. Azospirillum—A free-living nitrogen-fixing bacterium. In Rhizobiome; Academic Press: Cambridge, MA, USA, 2023; pp. 285–308. [Google Scholar]
- Nievas, S.; Coniglio, A.; Takahashi, W.Y.; López, G.A.; Larama, G.; Torres, D.; Rosas, S.; Etto, R.M.; Galvão, C.W.; Mora, V.; et al. Unraveling Azospirillum’s colonization ability through microbiological and molecular evidence. J. Appl. Microbiol. 2023, 134, lxad071. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, X.J.; Liu, H.C.; Zhou, Y.G.; Wu, X.L.; Nie, Y.; Kang, Y.Q.; Cai, M. Azospirillum oleiclasticum sp. nov, a nitrogen-fixing and heavy oil degrading bacterium isolated from an oil production mixture of Yumen Oilfield. Syst. Appl. Microbiol. 2021, 44, 126171. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef]
- Martin-Didonet, C.C.; Chubatsu, L.S.; Souza, E.M.; Kleina, M.; Rego, F.G.; Rigo, L.U.; Yates, M.G.; Pedrosa, F.O. Genome structure of the genus Azospirillum. J. Bacteriol. 2000, 182, 4113–4116. [Google Scholar] [CrossRef]
- Caballero-Mellado, J.; López-Reyes, L.; Bustillos-Cristales, R. Presence of 16S rRNA genes in multiple replicons in Azospirillum brasilense. FEMS Microbiol. Lett. 1999, 178, 283–288. [Google Scholar] [CrossRef]
- Wisniewski-Dyé, F.; Borziak, K.; Khalsa-Moyers, G.; Alexandre, G.; Sukharnikov, L.O.; Wuichet, K.; Hurst, G.B.; McDonald, W.H.; Robertson, J.S.; Barbe, V.; et al. Azospirillum genomes reveal transition of bacteria from aquatic to terrestrial environments. PLOS Genet. 2011, 7, e1002430. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.W.; Lower, R.P.; Kim, N.K.; Young, J.P.W. Introducing the bacterial ‘chromid’: Not a chromosome, not a plasmid. Trends Microbiol. 2010, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, A.; Mora, V.; Puente, M.; Cassán, F. Azospirillum as biofertilizer for sustainable agriculture: Azospirillum brasilense AZ39 as a model of PGPR and field traceability. In Microbial Probiotics for Agricultural Systems: Advances in Agronomic Use; Springer: Cham, Switzerland, 2019; pp. 45–70. [Google Scholar]
- Wisniewski-Dyé, F.; Lozano, L.; Acosta-Cruz, E.; Borland, S.; Drogue, B.; Prigent-Combaret, C.; Rouy, Z.; Barbe, V.; Herrera, A.M.; González, V.; et al. Genome sequence of Azospirillum brasilense CBG497 and comparative analyses of Azospirillum core and accessory genomes provide insight into niche adaptation. Genes 2012, 3, 576–602. [Google Scholar] [CrossRef] [PubMed]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Cesari, A.B.; Paulucci, N.S.; Yslas, E.I.; Dardanelli, M.S. Immobilization of Bradyrhizobium and Azospirillum in alginate matrix for long time of storage maintains cell viability and interaction with peanut. Appl. Microbiol. Biotechnol. 2020, 104, 10145–10164. [Google Scholar] [CrossRef]
- Damam, M.; Kaloori, K.; Gaddam, B.; Kausar, R. Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. Int. J. Pharm. Sci. Rev. Res. 2016, 37, 130–136. [Google Scholar]
- Nath, D.; Maurya, B.R.; Meena, V.S. Documentation of five potassium-and phosphorus-solubilizing bacteria for their K and P-solubilization ability from various minerals. Biocatal. Agric. Biotechnol. 2017, 10, 174–181. [Google Scholar] [CrossRef]
- Molina, R.; Rivera, D.; Mora, V.; López, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassán, F. Regulation of IAA biosynthesis in Azospirillum brasilense under environmental stress conditions. Curr. Microbiol. 2018, 75, 1408–1418. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harbor Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Zhang, P.; Jin, T.; Kumar Sahu, S.; Xu, J.; Shi, Q.; Liu, H.; Wang, Y. The distribution of tryptophan-dependent indole-3-acetic acid synthesis pathways in bacteria unraveled by large-scale genomic analysis. Molecules 2019, 24, 1411. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of A rabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium A zospirillumbrasilense. New Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; De-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Singh, M.; Klingmüller, W. Isolation and characterization of Azospirillum mutants excreting high amounts of indoleacetic acid. Can. J. Microbiol. 1983, 29, 916–923. [Google Scholar] [CrossRef]
- Creus, C.M.; Graziano, M.; Casanovas, E.M.; Pereyra, M.A.; Simontacchi, M.; Puntarulo, S.; Barassi, C.A.; Lamattina, L. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 2005, 221, 297–303. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Lanteri, M.L.; Lamattina, L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003, 132, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, E.; Costacurta, A.; Michiels, K.; Vanderleyden, J.; Van Onckelen, H. Azospirillum brasilense indole-3-acetic acid biosynthesis: Evidence for a non-tryptophan dependent pathway. Mol. Plant Microbe Interact. 1993, 6, 609. [Google Scholar] [CrossRef]
- Bacilio, M.; Vazquez, P.; Bashan, Y. Alleviation of noxious effects of cattle ranch composts on wheat seed germination by inoculation with Azospirillum spp. Biol. Fertil. Soils 2003, 38, 261–266. [Google Scholar] [CrossRef]
- Mehnaz, S. Azospirillum: A biofertilizer for every crop. In Plant Microbes Symbiosis: Applied Facets; Springer India: New Delhi, India, 2014; pp. 297–314. [Google Scholar]
- Horemans, S.; De koninck, K.A.T.L.E.E.N.; Neuray, J.; Hermans, R.; Vlassak, K. Production of Plant Growth Substances by Azospirillum sp., and Other Rhizosphere Bacteria. Symbiosis 1986, 2, 341–346. [Google Scholar]
- Spaepen, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting actions of rhizobacteria. Adv. Bot. Res. 2009, 51, 283–320. [Google Scholar]
- Kolb, W.; Martin, P. Response of plant roots to inoculation with Azospirillum brasilense and to application of indole acetic acid. In Azospirillum III: Genetics·Physiology Ecology Proceedings of the Third Bayreuth Azospirillum Workshop; Springer: Berlin/Heidelberg, Germany, 1985; pp. 215–221. [Google Scholar]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Strzelczyk, E.; Kampert, M.; Pachlewski, R. The influence of pH and temperature on ethylene production by mycorrhizal fungi of pine. Mycorrhiza 1994, 4, 193–196. [Google Scholar] [CrossRef]
- Glick, B.R.; Patten, C.L.; Holguin, G.; Penrose, D.M. Biochemical and Genetic Mechanisms Used by Plant Growth Promoting Bacteria; World Scientific: London, UK, 1999. [Google Scholar]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Ghosh, S. Isolation and characterization of the outer membrane proteins of Azospirillum brasilense. Microbiology 1987, 133, 1751–1758. [Google Scholar] [CrossRef][Green Version]
- Bachhawat, A.K.; Ghosh, S. Iron transport in Azospirillum brasilense: Role of the siderophore spirilobactin. Microbiology 1987, 133, 1759–1765. [Google Scholar] [CrossRef]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Arch. Microbiol. 2011, 193, 275–286. [Google Scholar] [CrossRef]
- Saxena, B.; Modi, M.; Modi, V.V. Isolation and characterization of siderophores from Azospirillum lipoferum D-2. Microbiology 1986, 132, 2219–2224. [Google Scholar] [CrossRef]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassán, F.D.; Luna, M.V. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.; Soares, E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Delaporte-Quintana, P.; Lovaisa, N.C.; Rapisarda, V.A.; Pedraza, R.O. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Regul. 2020, 91, 185–199. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric oxide: The versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Lamattina, L.; Polacco, J.C. (Eds.) Nitric Oxide in Plant Growth, Development and Stress Physiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 6. [Google Scholar]
- Correa-Aragunde, N.; Graziano, M.; Chevalier, C.; Lamattina, L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 2006, 57, 581–588. [Google Scholar] [CrossRef]
- Dunn, M.F.; Becerra-Rivera, V.A. The biosynthesis and functions of polyamines in the interaction of plant growth-promoting rhizobacteria with plants. Plants 2023, 12, 2671. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bera, D.; Roy, L.; Ghosh, C.K. Biomimetic and bioinspired nanostructures: Recent developments and applications. In Bioinspired and Green Synthesis of Nanostructures: A Sustainable Approach; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 353–404. [Google Scholar]
- Khan, N.; Siddiqui, M.H.; Ahmad, S.; Ahmad, M.M.; Siddiqui, S. New insights in enhancing the phosphorus use efficiency using phosphate-solubilizing microorganisms and their role in cropping system. Geomicrobiol. J. 2024, 41, 485–495. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, K.; Chandra, R. Plant growth–promoting rhizobacteria (PGPR) and bioremediation of industrial waste. In Microbes for Sustainable Development and Bioremediation; CRC Press: Boca Raton, FL, USA, 2019; pp. 207–241. [Google Scholar]
- Ponmurugan, P.; Gopi, C. Distribution pattern and screening of phosphate solubilizing bacteria isolated from different food and forage crops. J. Agron. 2006, 5, 600–604. [Google Scholar] [CrossRef]
- Iftikhar, A.; Aijaz, N.; Farooq, R.; Aslam, S.; Zeeshan, A.; Munir, M.; Irfan, M.; Mehmood, T.; Atif, M.; Ali, M.; et al. Beneficial role of phosphate solubilizing bacteria (PSB) in enhancing soil fertility through a variety of actions on plants growth and ecological perspective: An updated review. J. Xi’an Shiyou Univ. Nat. Sci. Ed. 2023, 19, 520–547. [Google Scholar]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total. Environ. 2023, 901, 166468. [Google Scholar] [CrossRef]
- Etesami, H. Enhanced phosphorus fertilizer use efficiency with microorganisms. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 215–245. [Google Scholar]
- Shahid, R.; Zia, H.U.U.R.; Bilal, H. Insect Pest Complex of Wheat Crop. In Current Trends in Wheat Research; IntechOpen: London, UK, 2022; p. 47. [Google Scholar]
- Kim, Y.N.; Yoon, J.H.; Kim, K.H.J. Microplastic contamination in soil environment–a review. Soil Sci. Annu. 2021, 71, 300–308. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef]
- Chakraborty, T.; Akhtar, N. Biofertilizers: Characteristic features and applications. In Biofertilizers: Study and Impact; Scrivener Publishing LLC: Austin, TX, US, 2021; pp. 429–489. [Google Scholar]
- Khoshru, B.; Nosratabad, A.F.; Mitra, D.; Chaithra, M.; Danesh, Y.R.; Boyno, G.; Chattaraj, S.; Priyadarshini, A.; Anđelković, S.; Pellegrini, M.; et al. Rock phosphate solubilizing potential of soil microorganisms: Advances in sustainable crop production. Bacteria 2023, 2, 98–115. [Google Scholar] [CrossRef]
- Boujenna, A.; del Moral, L.F.G. Biotechnological approaches to develop nitrogen-fixing cereals: A review. Span. J. Agric. Res. 2021, 19, e08R01. [Google Scholar] [CrossRef]
- Zehr, J.P.; Capone, D.G. Marine Nitrogen Fixation; Springer: New York, NY, USA, 2021; p. 184. [Google Scholar]
- Quiviger, B.; Franche, C.; Lutfalla, G.; Rice, D.; Haselkorn, R.; Elmerich, C. Cloning of a nitrogen fixation (nif) gene cluster of Azospirillum brasilense. Biochimie 1982, 64, 495–502. [Google Scholar] [CrossRef]
- Fahsold, R.; Singh, M.; Klingmüller, W. Cosmid cloning of nitrogenase structural genes of Azospirillum lipoferum. In Azospirillum III: Genetics Physiology·Ecology Proceedings of the Third Bayreuth Azospirillum Workshop; Springer: Berlin/Heidelberg, Germany, 1985; pp. 30–40. [Google Scholar]
- Schrank, I.S.; Zaha, A.; De Araujo, E.F.; Santos, D.S. Construction of a gene library from Azospirillum brasilense and characterization of a recombinant containing the nif structural genes. Brazilian J. Med. Biol. Res. Rev. Pesqui. Biol. 1987, 20, 321–330. [Google Scholar]
- Nichio, B.T.D.L.; Chaves, R.B.R.; Pedrosa, F.D.O.; Raittz, R.T. Exploring diazotrophic diversity: Unveiling Nif core distribution and evolutionary patterns in nitrogen-fixing organisms. BMC Genom. 2025, 26, 81. [Google Scholar] [CrossRef]
- Passaglia, L.M.; Nunes, C.P.; Zaha, A.; Schrank, I.S. The nifHDK operon in the free-living nitrogen-fixing bacteria Azospirillum brasilense sequentially comprises genes H, D, K, an 353 bp orf and gene Y. Brazilian J. Med. Biol. Res. Rev. Pesqui. Biol. 1991, 24, 649–675. [Google Scholar]
- Milcamps, A.; Keyers, V.; Vanderleyden, J. Identification of a nifW-like gene in Azospirillum brasilense. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1993, 1173, 237–238. [Google Scholar] [CrossRef]
- Broek, A.V.; Vanderleyden, J. The role of bacterial motility, chemotaxis, and attachment in bacteria-plant interactions. Mol. Plant-Microbe Interact. 1995, 8, 800–810. [Google Scholar] [CrossRef]
- de Zamaroczy, M.; Delorme, F.; Elmerich, C. Regulation of transcription and promoter mapping of the structural genes for nitrogenase (nifHDK) of Azospirillum brasilense Sp7. Mol. Gen. Genet. MGG 1989, 220, 33–42. [Google Scholar] [CrossRef]
- Fani, R.; Allotta, G.; Bazzicalupo, M.; Ricci, F.; Schipani, C.; Polsinelli, M. Nucleotide sequence of the gene encoding the nitrogenase iron protein (nifH) of Azospirillum brasilense and identification of a region controlling nifHtranscription. Mol. Genet. Genom. 1989, 220, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; de Zamaroczy, M.; Arséne, F.; Paquelin, A.; Elmerich, C. Regulation of nitrogen fixation in Azospirillum brasilense Sp7: Involvement of nifA, glnA and glnB gene products. FEMS Microbiol. Lett. 1992, 100, 113–119. [Google Scholar] [CrossRef]
- Milcamps, A.; Banderleyden, J. In vitro construction of LacZ gene fusions with the nif HDK-operon of Azospirillum brasilense. FEMS Microbiol. Lett. 1991, 77, 79–84. [Google Scholar] [CrossRef]
- Broek, A.V.; Michiels, J.; De Faria, S.M.; Milcamps, A.; Vanderleyden, J. Transcription of the Azospirillum brasilense nifH gene is positively regulated by NifA and NtrA and is negatively controlled by the cellular nitrogen status. Mol. Genet. Genom. 1992, 232, 279–283. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Kaminski, P.A.; Elmerich, C. Identification of a nifA-like regulatory gene of Azospirillum brasilense Sp7 expressed under conditions of nitrogen fixation and in the presence of air and ammonia. Mol. Microbiol. 1991, 5, 2735–2744. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Kreutzer, R.; Klingmüller, W. Identification of a promoter dependent on NifA and σ 54 upstream of nifH in Azospirillum lipoferum. Mol. Gen. Genet. MGG 1991, 227, 86–90. [Google Scholar] [CrossRef]
- Vande Broek, A.; Vanderleyden, J. Genetics of the Azospirillum-plant root association. Crit. Rev. Plant Sci. 1995, 14, 445–466. [Google Scholar] [CrossRef]
- de Zamaroczy, M.; Delorme, F.; Elmerich, C. Characterization of three different nitrogen-regulated promoter regions for the expression of glnB and glnA in Azospirillum brasilense. Mol. Genet. Genom. 1990, 224, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Elmerich, C.; de Zamaroczy, M.; Vieille, C.; Delorme, F.; Onyeocha, I.; Liang, Y.Y.; Zimmer, W. Nif and nod genes in Azospirillum. In Nitrogen Fixation: Proceedings of the Fifth International Symposium on Nitrogen Fixation with Non-Legumes, Florence, Italy, 10–14 September 1990; Springer: Dordrecht, The Netherlands, 1991; pp. 79–87. [Google Scholar]
- Fischer, H.M.; Hennecke, H. Direct response of Bradyrhizobium japonicum nifA-mediated nif gene regulation to cellular oxygen status. Mol. Genet. Genom. 1987, 209, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; D’hooghe, I.; Verreth, C.; Pelemans, H.; Vanderleyden, J. Characterization of the Rhizobium leguminosarum biovar phaseoli nifA gene, a positive regulator of nif gene expression. Arch. Microbiol. 1994, 161, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Gussin, G.N.; Ronson, C.W.; Ausubel, F.M. Regulation of nitrogen fixation genes. Annu. Rev. Genet. 1986, 20, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Arsène, F.; Elmerich, C. Characterization of the ntrBC genes of Azospirillambrasilense Sp7: Their involvement in the regulation of nitrogenase synthesis and activity. Mol. Genet. Genom. 1993, 240, 188–196. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Huang, K.; Wang, F.; Mei, Z. Molecular mechanism and agricultural application of the NifA–NifL system for nitrogen fixation. Int. J. Mol. Sci. 2023, 24, 907. [Google Scholar] [CrossRef]
- Kneip, C.; Lockhart, P.; Voß, C.; Maier, U.G. Nitrogen fixation in eukaryotes–new models for symbiosis. BMC Evol. Biol. 2007, 7, 55. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Dangar, T.K.; Tuteja, N.A.R.E.N.D.R.A. Formulation of associative nitrogen fixing Azotobacter and Azospirillum biofertilizers for improvement of rice production—A review. In Advances in Biotechnology; Studium Press LLC: Houston, TX, USA, 2013. [Google Scholar]
- Rueda, D.; Valencia, G.; Soria, N.; Rueda, B.B.; Manjunatha, B.; Kundapur, R.R.; Selvanayagam, M. Effect of Azospirillum spp.; Azotobacter spp. on the growth and yield of strawberry (Fragaria vesca) in hydroponic system under different nitrogen levels. J. Appl. Pharm. Sci. 2016, 6, 048–054. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.A.; Mendoza-Herrera, A.; Bocanegra-García, V.; Rivera, G. Azospirillum spp. from plant growth-promoting bacteria to their use in bioremediation. Microorganisms 2022, 10, 1057. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation–A comprehensive evaluation. Appl. Soil Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
- Lourenzi, C.R.; Loss, A.; Souza, M.; Comin, J.J.; Lovato, P.E.; Soares, C.R.F.S. The role of PGPR secondary metabolites in alleviating Allelopathic effects (biotic stress) and induced tolerance in plants. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Springer International Publishing: Cham, Switzerland, 2022; pp. 133–152. [Google Scholar]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.; Muratova, A.; Turkovskaya, O. Degradation of polycyclic aromatic hydrocarbons by co-culture of Pleurotus ostreatus Florida and Azospirillum brasilense. Appl. Microbiol. 2022, 2, 735–748. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Hernandez, J.P.; Nelson, K.N.; Bashan, Y.; Maier, R.M. Growth of quailbush in acidic, metalliferous desert mine tailings: Effect of Azospirillum brasilense Sp6 on biomass production and rhizosphere community structure. Microb. Ecol. 2010, 60, 915–927. [Google Scholar] [CrossRef]
- Muratova, A.Y.; Turkovskaya, O.V.; Antonyuk, L.P.; Makarov, O.E.; Pozdnyakova, L.I.; Ignatov, V.V. Oil-oxidizing potential of associative rhizobacteria of the genus Azospirillum. Microbiology 2005, 74, 210–215. [Google Scholar] [CrossRef]
- Eckford, R.; Cook, F.D.; Saul, D.; Aislabie, J.; Foght, J. Free-living heterotrophic nitrogen-fixing bacteria isolated from fuel-contaminated Antarctic soils. Appl. Environ. Microbiol. 2002, 68, 5181–5185. [Google Scholar] [CrossRef]
- Al-Mailem, D.M.; Kansour, M.K.; Radwan, S.S. Cross-bioaugmentation among four remote soil samples contaminated with oil exerted just inconsistent effects on oil-bioremediation. Front. Microbiol. 2019, 10, 2827. [Google Scholar] [CrossRef]
- Saeed, M.; Ilyas, N.; Arshad, M.; Sheeraz, M.; Ahmed, I.; Bhattacharya, A. Development of a plant microbiome bioremediation system for crude oil contamination. J. Environ. Chem. Eng. 2021, 9, 105401. [Google Scholar] [CrossRef]
- Young, C.C.; Hupfer, H.; Siering, C.; Ho, M.J.; Arun, A.B.; Lai, W.A.; Rekha, P.D.; Shen, F.T.; Hung, M.H.; Chen, W.M.; et al. Azospirillumrugosum sp. nov.; isolated from oil-contaminated soil. Int. J. Syst. Evol. Microbiol. 2008, 58, 959–963. [Google Scholar] [CrossRef][Green Version]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Dhawi, F.; Datta, R.; Ramakrishna, W. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of sorghum grown in marginal soil. Chemosphere 2016, 157, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.S.; Sankaranarayanan, C.; Jothi, G. Management of Pratylenchuszeae on maize by biofertilizers and VAM. Indian J. Nematol. 1998, 28, 77–80. [Google Scholar]
- Bouillant, M.L.; Miché, L.; Ouedraogo, O.; Alexandre, G.; Jacoud, C.; Sallé, G.; Bally, R. Inhibition of Striga seed germination associated with sorghum growth promotion by soil bacteria. Comptes Rendus L’académie Sci.-Ser. III-Sci. Vie 1997, 320, 159–162. [Google Scholar] [CrossRef]
- Glick, B.R.; Karaturovíc, D.M.; Newell, P.C. A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can. J. Microbiol. 1995, 41, 533–536. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Ross, A.F. Systemic acquired resistance induced by localized virus infections in plants. Virology 1961, 14, 340–358. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. In New Perspectives and Approaches in Plant Growth-promoting Rhizobacteria Research; Springer: Dordrecht, The Netherlands, 2007; pp. 243–254. [Google Scholar]
- Van Loon, L.C.; Bakker, P.A.H.M. Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2006; pp. 39–66. [Google Scholar]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Rodrigues, E.P.; Rodrigues, L.S.; De Oliveira, A.L.M.; Baldani, V.L.D.; Teixeira, K.R.D.S.; Urquiaga, S.; Reis, V.M. Azospirillum amazonense inoculation: Effects on growth, yield and N2 fixation of rice (Oryza sativa L.). Plant Soil 2008, 302, 249–261. [Google Scholar] [CrossRef]
- Gureeva, M.V.; Kirillova, M.S.; Trandina, V.A.; Kryukova, V.A.; Eremina, A.A.; Alimova, A.A.; Grabovich, M.Y.; Gureev, A.P. Effect of Bacteria from the Genus Azospirillum on Oxidative Stress Levels in Wheat Triticum aestivum L. in the Presence of Copper, Nickel, and Lead. Microorganisms 2025, 13, 334. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Wang, N. A Study of the Different Strains of the Genus Azospirillum spp. on Increasing Productivity and Stress Resilience in Plants. Plants 2025, 14, 267. [Google Scholar] [CrossRef]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- da Cunha, E.T.; Pedrolo, A.M.; Arisi, A.C.M. Thermal and salt stress effects on the survival of plant growth-promoting bacteria Azospirillum brasilense in inoculants for maize cultivation. J. Sci. Food Agric. 2024, 104, 5360–5367. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, S.; Mitra, D.; Chattaraj, M.; Ganguly, A.; Thatoi, H.; Mohapatra, P.K.D. Brewers’ spent grain as fish feed ingredient: Evaluation of bio-safety and analysis of its impact on gut bacteria of Cirrhinus reba by 16S Metagenomic sequencing. Curr. Res. Microb. Sci. 2024, 7, 100286. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, S.; Chattaraj, M.; Mitra, D.; Ganguly, A.; Thatoi, H.; Das Mohapatra, P.K. 16S amplicon sequencing of the gastrointestinal microbiota of Cirrhinus reba and isolation of an autochthonous probiotic using culture based approaches. Syst. Microbiol. Biomanuf. 2025, 5, 156–170. [Google Scholar] [CrossRef]
- Ferrarezi, J.A.; Defant, H.; De Souza, L.F.; Azevedo, J.L.; Hungria, M.; Quecine, M.C. Meta-omics integration approach reveals the effect of soil native microbiome diversity in the performance of inoculant Azospirillum brasilense. Front. Plant Sci. 2023, 14, 1172839. [Google Scholar] [CrossRef]
- Coniglio, A.; Larama, G.; Molina, R.; Mora, V.; Torres, D.; Marin, A.; Avila, A.I.; Lede NoirCarlan, C.; Erijman, L.; Figuerola, E.L.; et al. Modulation of maize rhizosphere microbiota composition by inoculation with Azospirillum argentinense Az39 (formerly A. brasilense Az39). Soil Sci. Plant Nutr. 2022, 22, 3553–3567. [Google Scholar] [CrossRef]
- Bigatton, E.D.; Verdenelli, R.A.; Haro, R.J.; Ayoub, I.; Barbero, F.M.; Martín, M.P.; Dubini, L.E.; Jorriín Novo, J.V.; Lucini, E.I.; Castillejo, M.Á. Metagenomic Analysis to Assess the Impact of Plant Growth-Promoting Rhizobacteria on Peanut (Arachis hypogaea L.) Crop Production and Soil Enzymes and Microbial Diversity. J. Agric. Food Chem. 2024, 72, 22385–22397. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Igiehon, O.N.; Babalola, O.O. Microbial genes of agricultural importance in maize rhizosphere unveiled through shotgun metagenomics. Span. J. Soil Sci. 2022, 12, 10427. [Google Scholar] [CrossRef]
- Elmerich, C.; Franche, C. Azospirillum genetics: Plasmids, bacteriophages and chromosome mobilization. Experientia 1982, 9–17. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19831975781 (accessed on 4 June 2025).
- Elmerich, G. Azospirillum. In Nitrogen Fixation; Broughton, W.J., Pühler, S., Eds.; Clarendon Press: Oxford, UK, 1986; Volume 4 Molecular Biology; pp. 106–126. [Google Scholar]
- Croes, C.; Van Bastelaere, E.; DeClercq, E.; Eyers, M.; Vanderleyden, J.; Michiels, K. Identification and mapping of loci involved in motility, adsorption to wheat roots, colony morphology, and growth in minimal medium on the Azospirillum brasilense Sp7 90-MDa plasmid. Plasmid 1991, 26, 83–93. [Google Scholar] [CrossRef]
- Revers, L.F.; Passaglia, L.M.P.; Marchal, K.; Frazzon, J.; Blaha, C.G.; Vanderleyden, J.; Schrank, I.S. Characterization of an Azospirillum brasilense Tn5 mutant with enhanced N2 fixation: The effect of ORF280 on nifHexpression. FEMS Microbiol. Lett. 2000, 183, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.U.P.A.P.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Martinez, E.; Romero, D.; Palacios, R. The rhizobium genome. Crit. Rev. Plant Sci. 1990, 9, 59–93. [Google Scholar] [CrossRef]
- Jang, J.; Sakai, Y.; Senoo, K.; Ishii, S. Potentially mobile denitrification genes identified in Azospirillum sp. strain TSH58. Appl. Environ. Microbiol. 2019, 85, e02474-18. [Google Scholar] [CrossRef]
- Koul, V.; Srivastava, D.; Singh, P.P.; Kochar, M. Genome-wide identification of Azospirillum brasilense Sp245 small RNAs responsive to nitrogen starvation and likely involvement in plant-microbe interactions. BMC Genom. 2020, 21, 821. [Google Scholar] [CrossRef]
- Gullett, J.; O’Neal, L.; Mukherjee, T.; Alexandre, G. Azospirillum brasilense: Laboratory Maintenance and Genetic Manipulation. Curr. Protoc. Microbiol. 2017, 47, 3E.2.1–3E.2.17. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Katsy, E.I.; Petrova, L.P. Genome rearrangements in Azospirillum brasilense Sp7 with the involvement of the plasmid pRhico and the prophage ΦAb-Cd. Russ. J. Genet. 2015, 51, 1165–1171. [Google Scholar] [CrossRef]
- Faure, D.; Bouillant, M.L.; Bally, R. Isolation of Azospirillum lipoferum 4T Tn5 mutants affected in melanization and laccase activity. Appl. Environ. Microbiol. 1994, 60, 3413–3415. [Google Scholar] [CrossRef] [PubMed]
- Katsy, E.I.; Prilipov, A.G. Mobile elements of an Azospirillum brasilense Sp245 85-MDa plasmid involved in replicon fusions. Plasmid 2009, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Katzy, E.I.; Iosipenko, A.D.; Egorenkov, D.A.; Zhuravleva, E.A.; Panasenko, V.I.; Ignatov, V.V. Involvement of Azopirillumbrasilense plasmid DNA in the productio of indole acetic acid. FEMS Microbiol. Lett. 1990, 72, 1–4. [Google Scholar] [CrossRef]
- Schnabel, T.; Sattely, E. Improved stability of engineered ammonia production in the plant-symbiont Azospirillum brasilense. ACS Synth. Biol. 2021, 10, 2982–2996. [Google Scholar] [CrossRef]
- Barbieri, P.; Zanelli, T.; Galli, E.; Zanetti, G. Wheat inoculation with Azospirillum brasilense Sp6 and some mutants altered in nitrogen fixation and indole-3-acetic acid production. FEMS Microbiol. Lett. 1986, 36, 87–90. [Google Scholar] [CrossRef]
- Spaepen, S.; Dobbelaere, S.; Croonenborghs, A.; Vanderleyden, J. Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 2008, 312, 15–23. [Google Scholar] [CrossRef]
- Yahalom, E.; Dovrat, A.; Okon, Y.; Czosnek, H. Effect of inoculation with Azospirillum brasilense strain Cd and Rhizobium on the root morphology of burr medic (Medicago polymorpha L.). Israel J. Bot. 1991, 40, 155–164. [Google Scholar]
- Bar, T.; Okon, Y. Tryptophan conversion to indole-3-acetic acid via indole-3-acetamide in Azospirillum brasilense Sp7. Can. J. Microbiol. 1993, 39, 81–86. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mukherjee, A.; Ghosh, S. Molecular cloning and sequencing of an operon, carRS of Azospirillum brasilense, that codes for a novel two-component regulatory system: Demonstration of a positive regulatory role of carR for global control of carbohydrate catabolism. J. Bacteriol. 1994, 176, 7484–7490. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.; Chaudhary, N.; Shams, R.; Dash, K.K. Genetically modified crops and sustainable development: Navigating challenges and opportunities. Food Sci. Biotechnol. 2025, 34, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, G.G.; Braccini, A.L.; Dan, L.G.; Scapim, C.A.; Ricci, T.T.; and Bazo, G.L. Efficiency of seed inoculation with Azospirillum brasilense on agronomic characteristics and yield of wheat. Ind. Crop. Prod. 2013, 43, 393–397. [Google Scholar] [CrossRef]
- da Silva, M.F.; Antônio, C.d.S.; de Oliveira, P.J.; Xavier, G.R.; Rumjanek, N.G.; Soares, L.H.d.B.; Reis, V.M. Survival of endophytic bacteria in polymerbased inoculants and efficiency of their application to sugarcane. Plant Soil 2012, 356, 231–243. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial bioformulation: A microbial assisted biostimulating fertilization technique for sustainable agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef]
- Samantaray, A.; Chattaraj, S.; Mitra, D.; Ganguly, A.; Kumar, R.; Gaur, A.; Mohapatra, P.K.D.; de Los Santos-Villalobos, S.; Rani, A.; Thatoi, H. Advances in microbial based bio-inoculum for amelioration of soil health and sustainable crop production. Curr. Res. Microb. Sci. 2024, 7, 100251. [Google Scholar] [CrossRef]
- Bashan, Y. Interactions of Azospirillum spp. in soils: A review. Biol. Fertil. Soils 1999, 29, 246–256. [Google Scholar] [CrossRef]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- Binodh, A.K.; Thankappan, S.; Ravichandran, A.; Mitra, D.; Alagarsamy, S.; Panneerselvam, P.; Senapati, A.; Sami, R.; Al-Mushhin, A.A.; Aljahani, A.H.; et al. Synergistic modulation of seed metabolites and enzymatic antioxidants tweaks moisture stress tolerance in non-cultivated traditional rice genotypes during germination. Plants 2022, 11, 775. [Google Scholar] [CrossRef]
- Bashan, Y.J.S.B. Significance of timing and level of inoculation with rhizosphere bacteria on wheat plants. Soil Biol. Biochem. 1986, 18, 297–301. [Google Scholar] [CrossRef]
- Yang, L.Y.; Lin, C.S.; Huang, X.R.; Neilson, R.; Yang, X.R. Effects of biofertilizer on soil microbial diversity and antibiotic resistance genes. Sci. Total. Environ. 2022, 820, 153170. [Google Scholar] [CrossRef]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.A. Recent advances in seed coating technologies: Transitioning toward sustainable agriculture. Green Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Kupryashina, M.A.; Ponomareva, E.G.; Pylaev, T.E. Immobilization of Azospirillum Bacteria on Various Carriers. Microbiology 2024, 93, 104–111. [Google Scholar] [CrossRef]
- Freeman, A.; Lilly, M.D. Effect of processing parameters on the feasibility and operational stability of immobilized viable microbial cells. Enzym. Microb. Technol. 1998, 23, 335–345. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of plant growth promoting Rhizobacteria—Prospects and potential in agricultural sector: A review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- El-Katatny, M.H. Enzyme production and nitrogen fixation by free, immobilized and coimmobilized inoculants of Trichoderma harzianum and Azospirillum brasilense and their possible role in growth promotion of tomato. Food Technol. Biotechnol. 2010, 48, 161–174. [Google Scholar]
- GII Global Information (2024). Azospirillum Bacteria Fertilizers Market—2023–2030. Available online: https://www.giiresearch.com/report/dmin1447986-azospirillum-bacteria-fertilizers-market (accessed on 4 June 2025).
- Chattaraj, S.; Samantaray, A.; Ganguly, A.; Thatoi, H. Employing plant growth-promoting rhizobacteria for abiotic stress mitigation in plants: With a focus on drought stress. Discov. Appl. Sci. 2025, 7, 68. [Google Scholar] [CrossRef]
- Chattaraj, S.; Mitra, D.; Ganguly, A.; Mohapatra, P.K.D.; Thatoi, H. Harnessing microbe-based soil inoculums, strigolactones, and nanotechnology for sustainable agriculture: Mechanisms, innovations, and challenges. Pedosphere 2025. [Google Scholar] [CrossRef]
- Lei, Y.; Kuai, Y.; Guo, M.; Zhang, H.; Yuan, Y.; Hong, H. Phosphate-solubilizing microorganisms for soil health and ecosystem sustainability: A forty-year scientometric analysis (1984–2024). Front. Microbiol. 2025, 16, 1546852. [Google Scholar] [CrossRef]
- Databridgemarketresearch. Global Azospirillum Bacteria Fertilizers Market—Industry Trends and Forecast to 2029. 2022. Available online: https://www.databridgemarketresearch.com/reports/global-azospirillum-bacteria-fertilizers-market (accessed on 4 June 2025).
- Ferreira, N.D.S. Taxonomy and reclassification of strains of the genus Azospirillum and Nitrospirillum belonging to the Johanna Döbereiner Center for Biological Resources. Ph.D. Thesis, Universidade Federal Rural do Rio de Janeiro, Seropédica, Brazil, 2022. Available online: https://rima.ufrrj.br/jspui/handle/20.500.14407/20209 (accessed on 4 June 2025).
- Kim, H.; Park, Y.H.; Yang, J.E.; Kim, H.S.; Kim, S.C.; Oh, E.J.; Moon, J.; Cho, W.; Shin, W.; Yu, C. Analysis of major bacteria and diversity of surface soil to discover biomarkers related to soil health. Toxics 2022, 10, 117. [Google Scholar] [CrossRef]
- Baldani, J.I.; dos Santos Ferreira, N.; Shwab, S.; Reis, V.M.; de Barros Soares, L.H.; Simões-Araujo, J.L.; dos Santos Dourado, F.; Bach, E.; Camacho, N.N.; de Oliveira, A.M.; et al. Nitrospirillumviridazoti sp. nov.; an Efficient Nitrogen-Fixing Species Isolated from Grasses. Curr. Microbiol. 2024, 81, 144. [Google Scholar] [CrossRef]
- dos Santos Ferreira, N.; Hayashi Sant’Anna, F.; Massena Reis, V.; Ambrosini, A.; GazollaVolpiano, C.; Rothballer, M.; Schwab, S.; Baura, V.A.; Balsanelli, E.; Pedrosa, F.D.O.; et al. Genome-based reclassification of Azospirillum brasilense Sp245 as the type strain of Azospirillum baldaniorum sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 6203–6212. [Google Scholar] [CrossRef]
- Tikhonova, E.N.; Grouzdev, D.S.; Kravchenko, I.K. Azospirillum palustre sp. nov.; a methylotrophic nitrogen-fixing species isolated from raised bog. Int. J. Syst. Evol. Microbiol. 2019, 69, 2787–2793. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hameed, A.; Liu, Y.C.; Hsu, Y.H.; Lai, W.A.; Shen, F.T.; Young, C.C. Azospirillum soli sp. nov.; a nitrogen-fixing species isolated from agricultural soil. Int. J. Syst. Evol. Microbiol. 2015, 65 Pt 12, 4601–4607. [Google Scholar] [CrossRef] [PubMed]
- Bano, Q.U.D.S.I.A.; Ilyas, N.; Bano, A.; Zafar, N.A.D.I.A.; Akram, A.B.I.D.A.; Hassan, F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013, 45 Suppl. S1, 13–20. [Google Scholar]
- Pan, W.; Lu, Q.; Xu, Q.R.; Zhang, R.R.; Li, H.Y.; Yang, Y.H.; Liu, H.J.; Du, S.T. Abscisic acid-generating bacteria can reduce Cd concentration in pakchoi grown in Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 177, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dietz, K.J. Effect of associative bacteria on element composition of barley seedlings grown in solution culture at toxic cadmium concentrations. Microbiol. Res. 2000, 155, 113–121. [Google Scholar] [CrossRef] [PubMed]
- AlzateZuluaga, M.Y.; Miras-Moreno, B.; Monterisi, S.; Rouphael, Y.; Colla, G.; Lucini, L.; Cesco, S.; Pii, Y. Integrated metabolomics and morpho-biochemical analyses reveal a better performance of Azospirillum brasilense over plant-derived biostimulants in counteracting salt stress in tomato. Int. J. Mol. Sci. 2022, 23, 14216. [Google Scholar] [CrossRef]
- Marks, B.B.; Nogueira, M.A.; Hungria, M. Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges. Agronomy 2025, 15, 1350. [Google Scholar] [CrossRef]
- Djedidi, S.; Terasaki, A.; Aung, H.P.; Kojima, K.; Yamaya, H.; Ohkama-Ohtsu, N.; Bellingrath-Kimura, S.D.; Meunchang, P.; Yokoyama, T. Evaluation of the possibility to use the plant–microbe interaction to stimulate radioactive 137 Cs accumulation by plants in a contaminated farm field in Fukushima, Japan. J. Plant Res. 2015, 128, 147–159. [Google Scholar] [CrossRef]
- Marastoni, L.; Pii, Y.; Maver, M.; Valentinuzzi, F.; Cesco, S.; Mimmo, T. Role of Azospirillum brasilense in triggering different Fe chelate reductase enzymes in cucumber plants subjected to both nutrient deficiency and toxicity. Plant Physiol. Biochem. 2019, 136, 118–126. [Google Scholar] [CrossRef]
- Romero, A.M.; Correa, O.S.; Moccia, S.; Rivas, J.G. Effect of Azospirillum-mediated plant growth promotion on the development of bacterial diseases on fresh-market and cherry tomato. J. Appl. Microbiol. 2003, 95, 832–838. [Google Scholar] [CrossRef]
- Khan, M.R.; Kounsar, K.; Hamid, A. Effect of certain rhizobacteria and antagonistic fungi on root-modulation and root-knot nematode disease of green gram. Nematologia Mediterranea. 2002, 30, 85–89. [Google Scholar]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski-Dyé, F.; Vial, L.; Burdman, S.; Okon, Y.; Hartmann, A. Phenotypic variation in Azospirillum spp. other root-associated bacteria. In Biological Nitrogen Fixation; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1047–1054. [Google Scholar]
- Hartmann, A.; Klink, S.; Rothballer, M. Importance of N-acyl-homoserine lactone-based quorum sensing and quorum quenching in pathogen control and plant growth promotion. Pathogens 2021, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, N.; Aswathy, S.; Malaikozhundan, B.; Boopathi, T. Nano-zinc oxide synthesized using diazotrophic Azospirillum improves the growth of mung bean, Vigna radiata. Int. Nano Lett. 2021, 11, 405–415. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Manoharadas, S.; Hameed, A.; Riaz, H.; Manzoor, M.A.; Rehman, S.; Riaz, M.W.; Sabir, S.; Munir, A.; et al. Exploring the impact of titanium dioxide nanoparticles (nTiO2) at varied concentrations in combination with Azospirillum brasilense on wheat growth and physiology. J. King Saud Univ.-Sci. 2024, 36, 103189. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Waraky, E.A.; Almutairi, H.H.; Al-Daej, M.I.; El-Nady, M.F.; Shehata, W.F.; Belal, E.B.; El-Mogy, M.M.; El-Mehasseb, I.; Metwaly, M.M.S. Morphophysiological and biochemical responses of cotton (Gossypium barbadense L.) to nano zinc (ZnO-NPs) and Azospirillum sp. under water deficit stress conditions. J. Plant Nutr. 2025, 48, 326–344. [Google Scholar] [CrossRef]
- Aghaei, F.; Seyed Sharifi, R.; Farzaneh, S. The effect of some nanoparticles and biofertilizers on chlorophyll fluorescence components and some physiological traits of Triticale (Triticosecale Wittmack) at different irrigation levels. J. Plant Biol. Sci. 2022, 14, 13–40. [Google Scholar]
- Seyed Sharifi, R.; Khalilzadeh, R.; Pirzad, A.; Anwar, S. Effects of biofertilizers and nano zinc-iron oxide on yield and physicochemical properties of wheat under water deficit conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 2511–2524. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Jia, Z.; Tang, Y.; Lin, J.; Liu, X.; Zhang, J.; Müller, C. Microbial Inoculants Modify the Functions of Resident Soil Microbes to Expedite the Field Restoration of the Abandoned Mine. Land Degrad. Dev. 2025, 36, 307–317. [Google Scholar] [CrossRef]
- Ali, R.; Zulaykha, K.D.; Sajjad, N. Genetically modified microbes as biofertilizers. In Bioremediation and Biotechnology, Vol 4: Techniques for Noxious Substances Remediation; Springer: Cham, Switzerland, 2020; pp. 275–293. [Google Scholar]
- Zaied, K.A.; Abd El-Hady, A.H.; Sharief, A.E.; Ashour, E.H.; Nassef, M.A. Effect of horizontal DNA transfer in Azospirillum and Azotobacter strains on biological and biochemical traits of non-legume plants. J. Appl. Sci. Res. 2007, 3, 73–86. [Google Scholar]
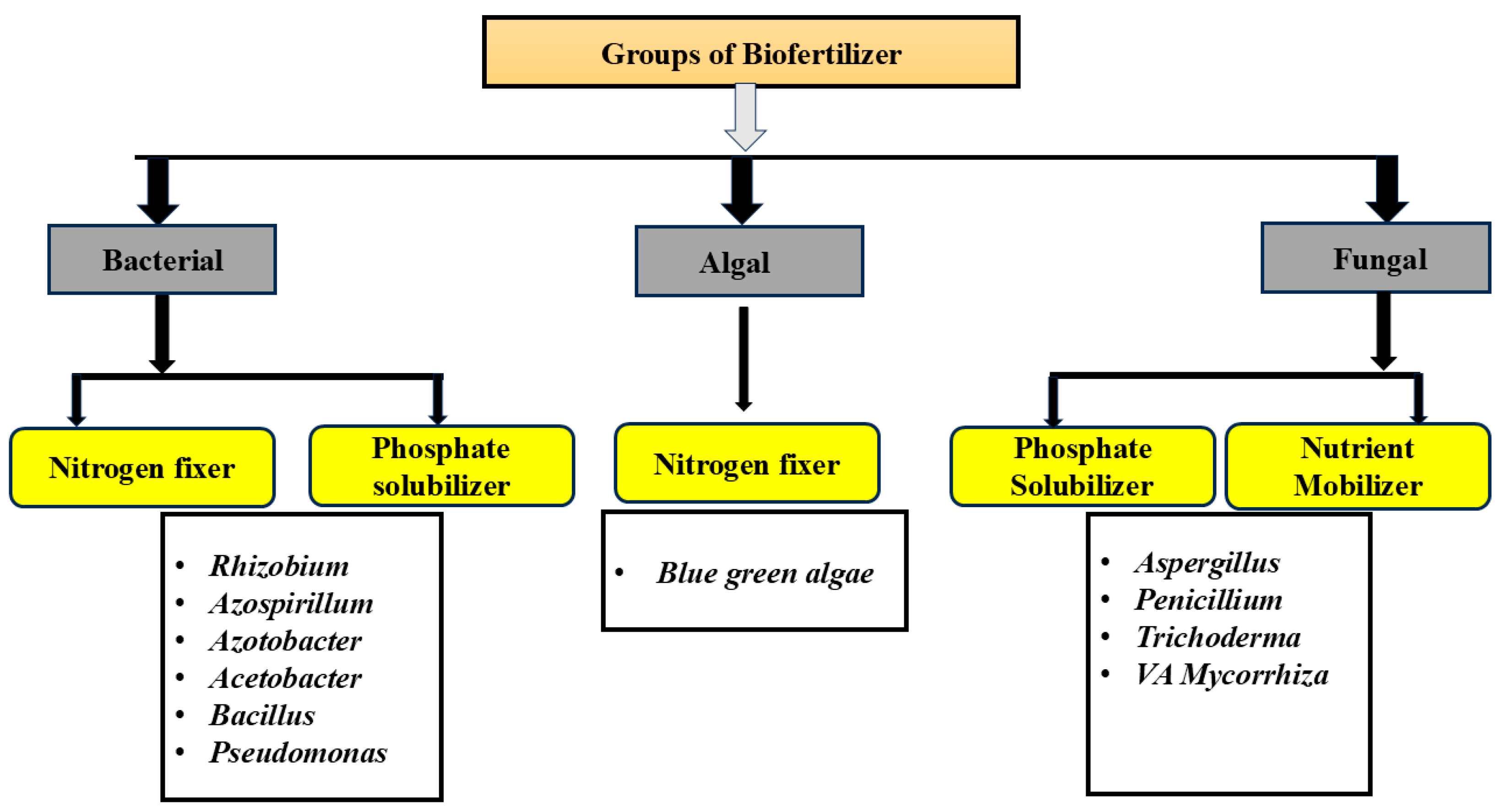
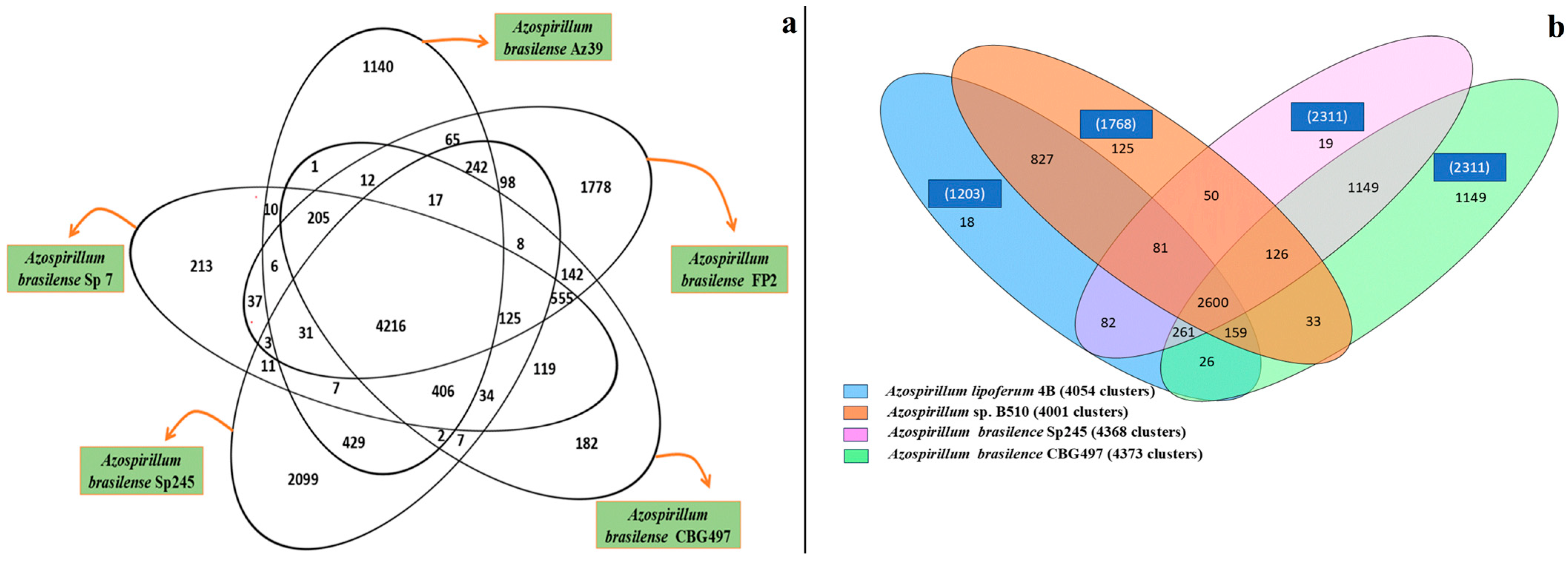
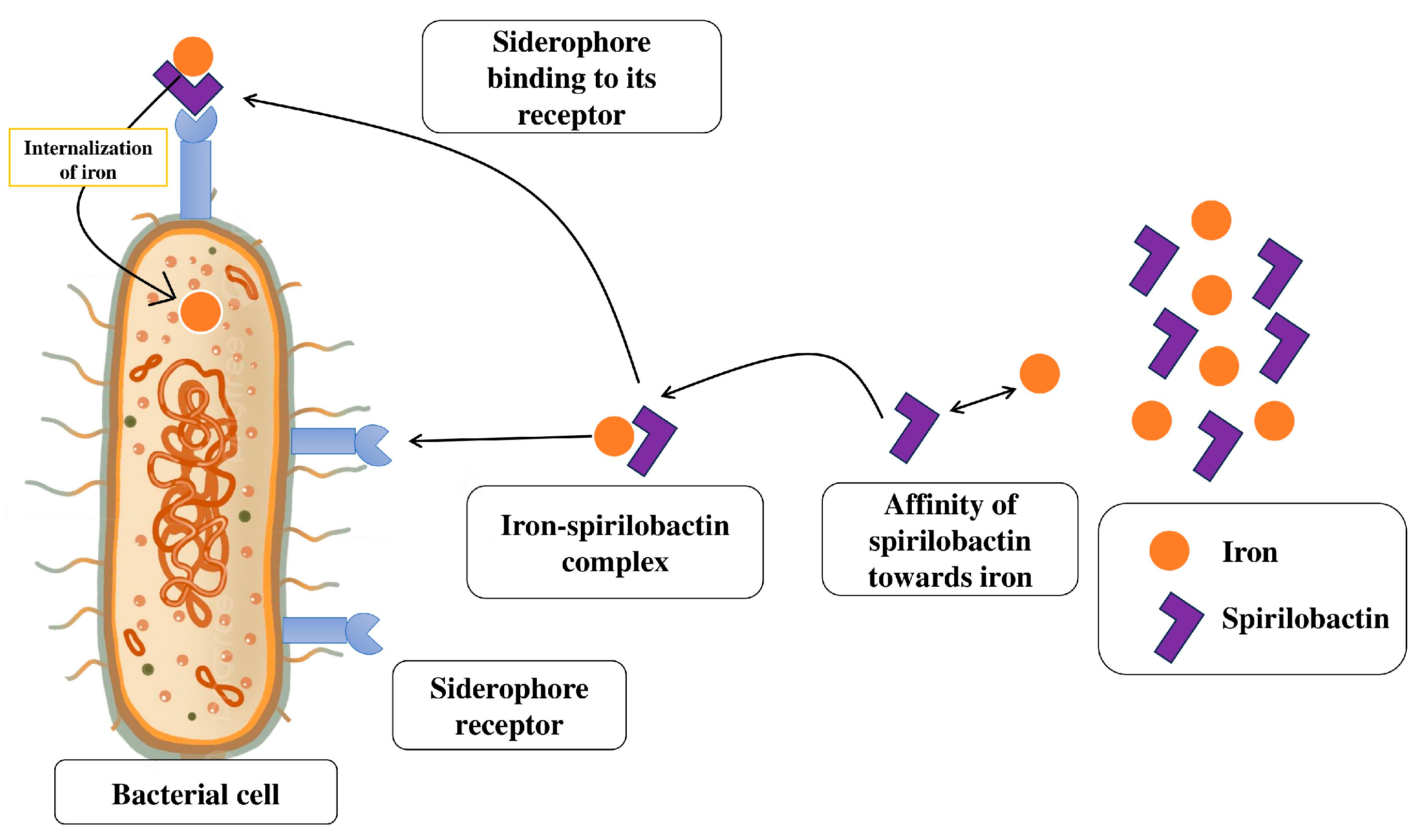
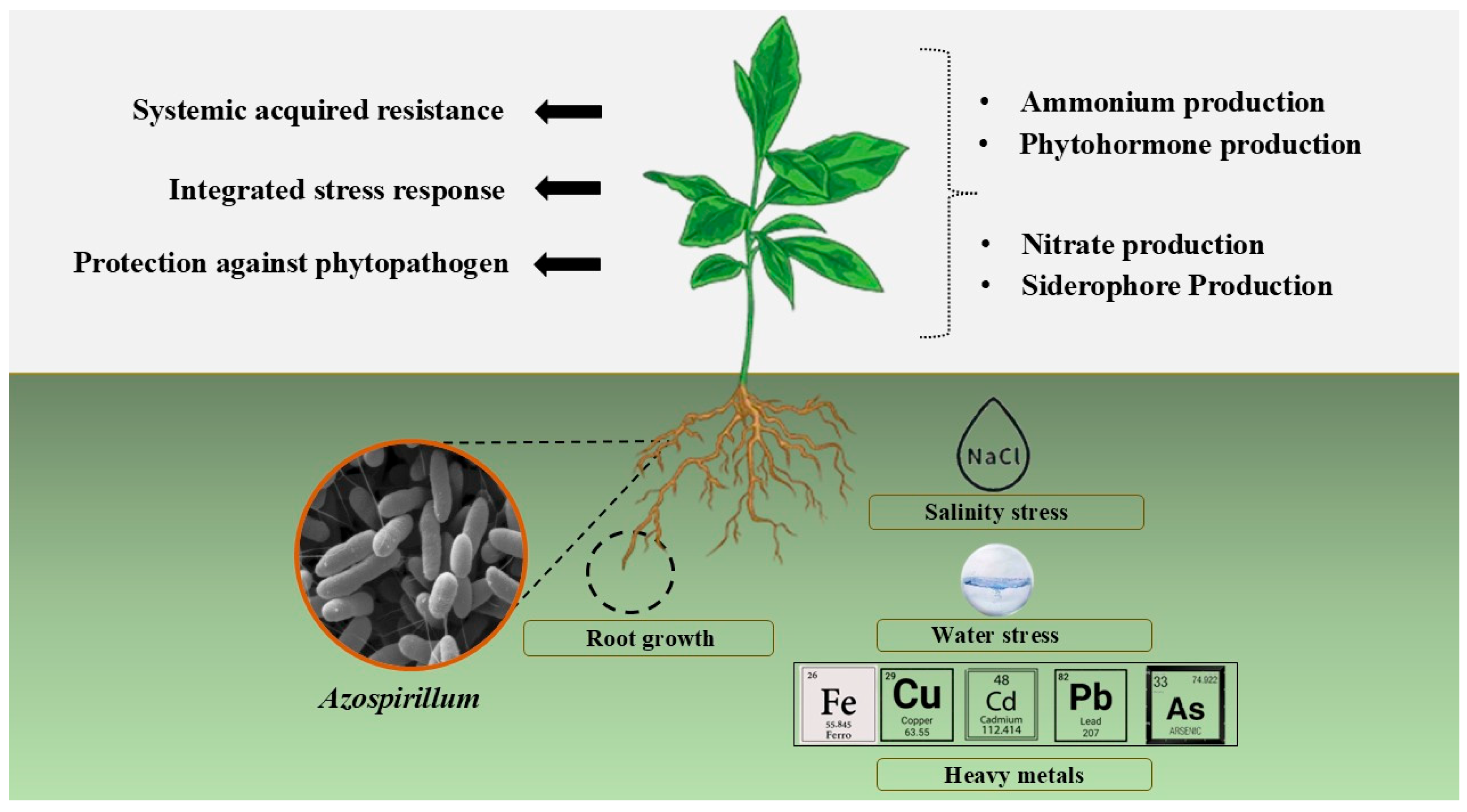
| Species | Applied Crops | Improved Growth/Yield | Reference |
|---|---|---|---|
| A. lipoferum | Maize | Height 35.33–43.89% | [180] |
| A. brasilense | Pak choi | Biomass 26–255% | [181] |
| A. lipoferum | Barley | Root elongation 12.5%, Root biomass 22.22% | [182] |
| A. brasilense | Tomato | Root biomass 118% | [183] |
| A. lipoferum | Wheat | Wheat yields up to 109% | [184] |
| Azospirillum sp. TS13 | Komatsuna | Dry weight 40–51% | [185] |
| A. brasilense | Cucumber | Root length 73.65% Root weight 55.32%, root tips 35.85% | [186] |
| A. brasilense and Azospirillum sp. BNM-65 | Cherry tomato | Dry weight 81–107%, leaves 32–43%, Shoot root dry weight 37–80%, Height 12–143% | [187] |
| A. lipoferum | Green gram | 10.26% of shoot length, 18.28% of fresh weight, 18.45% of dry weight | [188] |
| Crop | Nanoparticles Used | Biofertilizer Used | Irrigation Level | Growth Parameters Improved | Effects | Notable Outcomes | References |
|---|---|---|---|---|---|---|---|
| Not specified | Azospirillum-capped ZnO NPs | Azospirillum strains | Not specified | Seed germination (95%), LAI (45.6%), root/shoot length, biomass | Eco-friendly, increased chlorophyll and carotenoids | Potential for sustainable agriculture | [192] |
| Wheat | Titanium dioxide (nTiO2) | Azospirillum brasilense | Field trial (normal irrigation) | LAI, photosynthesis, nutrient uptake, and antioxidant enzymes | nTiO2 > 40 mg/L harmful; mitigated by A. brasilense | Best combo: 30 mg/L nTiO2 + A. brasilense | [193] |
| Cotton (Giza 96) | Zinc oxide (ZnO-NPs) | Azospirillum sp. | 15, 30, 45-day intervals | Plant height, dry weight, leaf area, chlorophyll, seed yield | CAT, POD, PPO, proline increased under drought | Combined treatment, most effective in both seasons | [194] |
| Triticale | Nano Fe-Si oxide | A. lipoferum, P. putida | Full, moderate, severe (booting/head) | Chlorophyll index (50.23%), RWC (43.97%), stomatal conductance | Reduced F0, electrolyte leakage; improved FV, yield | Improved physiology and yield under drought | [195] |
| Wheat | Nano Zn, Fe, Zn-Fe oxide | Azotobacter, Azospirillum, Pseudomonas | Normal, moderate, severe | Yield (88%), photosynthetic pigments, PSII efficiency, RWC | Proline, sugars, CAT, POD, and PPO increased under drought | Zn-Fe oxide + Azotobacter best under stress | [196] |
| Molecular Engineering | Despite the nitrogen-fixing capabilities of Azospirillum, the efficiency of this process can vary under different environmental conditions. Engineering strains with enhanced nitrogenase activity or greater tolerance to environmental stressors (e.g., heat, oxygen, nutrient limitations) could significantly improve their performance in agricultural settings. |
| Co-expression of Nitrogen Fixation Pathways | A lot of Azospirillum strains depend on the nif gene cluster to fix nitrogen. However, more studies could be conducted on co-expressing other nitrogenase systems, like the vnf or anf clusters, to make them work better in places with few nutrients. |
| Oxygen Sensitivity and Adaptation | Azospirillum’s nitrogenase is highly sensitive to oxygen, making its activity difficult to maintain under oxygen-rich conditions. Innovative strategies to improve the bacterium’s tolerance to oxidative stress, such as the development of oxygen-scavenging systems or genetic modifications to enhance its ability to withstand aerobic conditions, could lead to more effective applications in diverse environments. |
| Abiotic Stress Resistance | Improving the tolerance of Azospirillum to abiotic stresses (such as salinity, drought, or extreme temperatures) would make the bacteria more versatile and beneficial for plants growing in challenging environments. |
| Symbiotic Relationships with Plants | Research into the specific signaling mechanisms between Azospirillum and host plants can help optimize their symbiotic interactions. Developing customised inoculants that are more efficient for crops may result from a thorough understanding of the molecular interactions of Azospirillum with various plant species. |
| Plant Growth-Promotion Mechanisms | Azospirillum is known for fixing nitrogen, but it also produces siderophores, plant hormones, and other secondary metabolites that aid in plant growth. Researchers may be able to increase the positive effects of these extra processes on plant development and stress resistance by recognising and comprehending them. |
| Nitrogenase Pathways | Alternative nitrogenases (V- and Fe-dependent) could be more efficient than Mo-dependent pathways. |
| Metagenomics of Soil and Rhizosphere Communities | Studying the complex microbiomes in the rhizosphere and soil can provide insights into how Azospirillum interacts with other microbes. By analyzing metagenomic data from natural environments, researchers can uncover new, more effective strains or microbial consortia that enhance nitrogen fixation or have synergistic effects on plant growth. |
| Crop Specific Nitrogen Fixation | There is a limitation in transferring the nif genes into cereals like rice and wheat. Need crop-specific engineering to optimize nitrogenase activity under different conditions. |
| Gene Editing | Optimised Azospirillum strains may be produced by selectively altering genes related to nitrogen fixation, stress tolerance, or plant signalling using CRISPR/Cas9 or other genome-editing methods. This would make it possible to adjust gene expression and produce strains that are suited to particular crops or environmental circumstances. |
| Cross-species Genetic Exchange | Research into the possibility of transferring beneficial genes from other nitrogen-fixing organisms (like Rhizobium or other Diazotrophs) into Azospirillum could create super-efficient strains capable of better adaptation to different environmental conditions. |
| Efficient Mass Production | To make Azospirillum inoculants commercially viable, efficient large-scale production methods are to be developed. Optimising culture media, fermentation procedures, and downstream processing are all part of this strategy to increase the production and activity of Azospirillum strains. |
| Formulation of Bioinoculants | It is important to make stable Azospirillum mixtures that are easy to use on plants or soil while still keeping the bacteria’s effectiveness and viability. |
| Long-term Effectiveness | Further research is necessary to determine the long-term efficacy of Azospirillum in various soil types and habitats. For sustainable agricultural methods, it is crucial to pursue further research on the persistence of these bacteria in the soil and their potential for either long-term advantages or detrimental effects on soil health. |
| Biosafety Considerations | The possible environmental impact of releasing transgenic Azospirillum strains must be carefully evaluated. Further research is needed to assess the biosafety of these strains, including the potential for horizontal gene transfer to nontarget organisms. |
| Carbon–Nitrogen Interaction | Understanding the reduced impacts of rising CO2 levels and improving nitrogen fixation by Azospirillum in the development of plants is an interesting topic of study now. This in-depth understanding could help to trap carbon in soil and lessen the impact of chemical fertilisers on the environment. |
| Adapting to Changing Environments | The development of Azospirillum strains capable of efficiently fixing nitrogen in higher temperatures, altering precipitation patterns, and elevating salinity will be essential in sustaining agricultural productivity under altered climate conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, B.R.; Chattaraj, S.; Rath, S.; Pattnaik, M.M.; Mitra, D.; Thatoi, H. Unveiling the Molecular Mechanism of Azospirillum in Plant Growth Promotion. Bacteria 2025, 4, 36. https://doi.org/10.3390/bacteria4030036
Giri BR, Chattaraj S, Rath S, Pattnaik MM, Mitra D, Thatoi H. Unveiling the Molecular Mechanism of Azospirillum in Plant Growth Promotion. Bacteria. 2025; 4(3):36. https://doi.org/10.3390/bacteria4030036
Chicago/Turabian StyleGiri, Bikash Ranjan, Sourav Chattaraj, Subhashree Rath, Mousumi Madhusmita Pattnaik, Debasis Mitra, and Hrudayanath Thatoi. 2025. "Unveiling the Molecular Mechanism of Azospirillum in Plant Growth Promotion" Bacteria 4, no. 3: 36. https://doi.org/10.3390/bacteria4030036
APA StyleGiri, B. R., Chattaraj, S., Rath, S., Pattnaik, M. M., Mitra, D., & Thatoi, H. (2025). Unveiling the Molecular Mechanism of Azospirillum in Plant Growth Promotion. Bacteria, 4(3), 36. https://doi.org/10.3390/bacteria4030036







