Molecular Detection of Colistin-Resistant E. coli in Village Chickens from Kelantan, Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Phenotypic Identification of E. coli
2.2. Antimicrobial Susceptibility Test
2.3. Determination of Multiple Antibiotic Resistance (MAR) Index
2.4. Minimum Inhibitory Concentration (MIC) Test of Colistin in MCR-EC
2.5. Genetic Characteristics of E. coli
2.5.1. Genomic DNA Extraction
2.5.2. Molecular Confirmation of E. coli Using Specie-Specific Gene
2.5.3. Detection of Colistin Resistance Gene (mcr) in E. coli Isolates
2.5.4. Phylogenetic Grouping of E. coli
2.5.5. Multi-Locus Sequence Typing (MLST)
3. Results
3.1. Molecular Occurrence of E. coli and Colistin Resistant mcr-E. coli (MCREC)
3.2. Phylogenetic Grouping of E. coli and MCREC
3.3. Antibiotic Susceptibility Testing
3.4. Multiple Antimicrobial Resistance Index
3.5. Minimum Inhibitory Concentration (MIC)
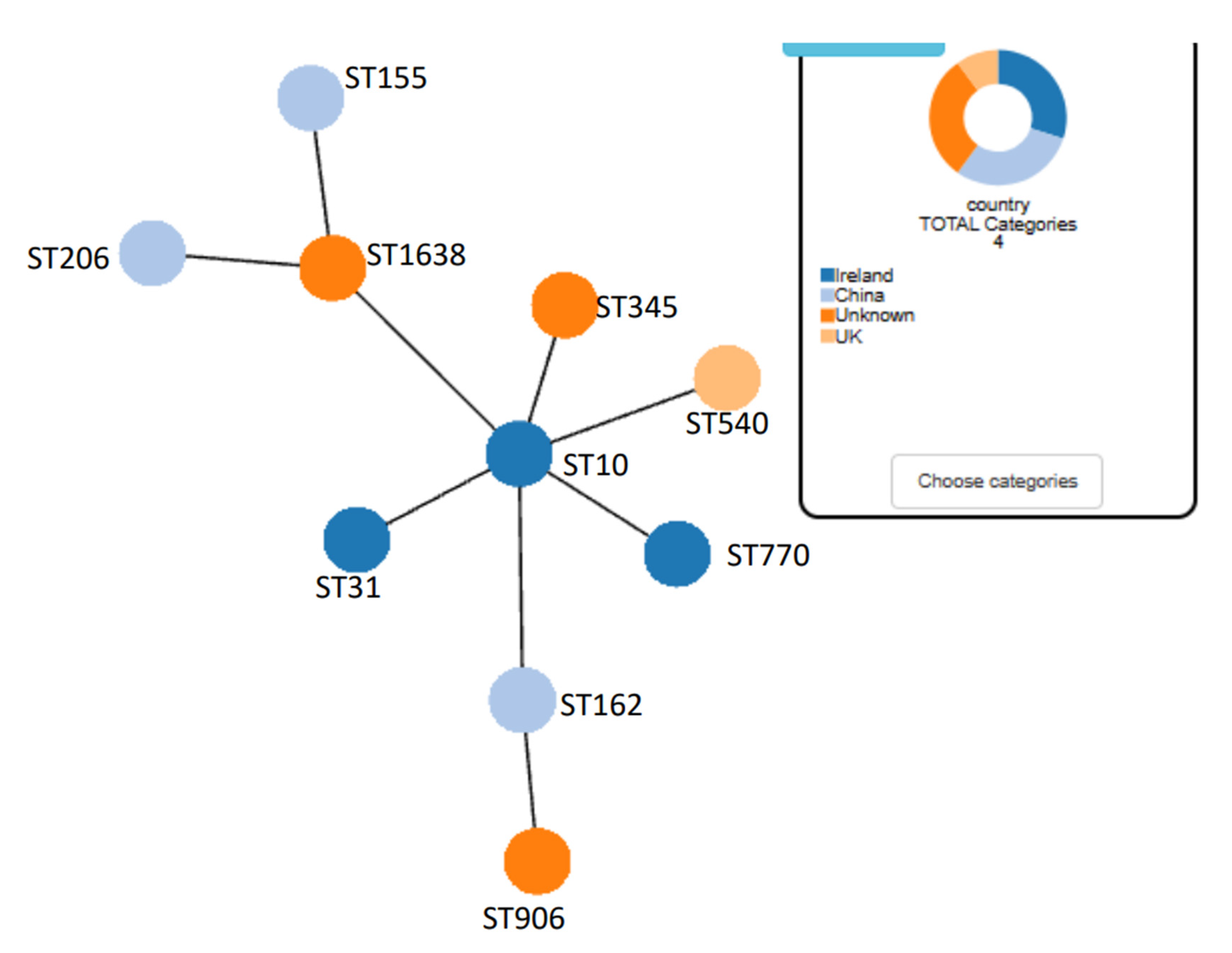
3.6. Identification of STs for the Colistin Resistant E. coli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valiakos, G.; Kapna, I. Colistin resistant mcr genes prevalence in livestock animals (swine, bovine, poultry) from a multinational perspective. A systematic review. Vet. Sci. 2021, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Doi, Y.; Zeng, L.; Lv, L.; Liu, J.H. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect. Dis. 2016, 16, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Elabbasy, M.T.; Hussein, M.A.; Algahtani, F.D.; Abd El-Rahman, G.I.; Morshdy, A.E.; Elkafrawy, I.A.; Adeboye, A.A. MALDI-TOF MS based typing for rapid screening of multiple antibiotic resistance E. coli and virulent non-O157 shiga toxin-producing E. coli isolated from the slaughterhouse settings and beef carcasses. Foods 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Kempf, F.; Slugocki, C.; Blum, S.E.; Leitner, G.; Germon, P. Genomic comparative study of bovine mastitis Escherichia coli. PLoS ONE 2016, 11, e0147954. [Google Scholar] [CrossRef]
- Ahmed, S.; Das, T.; Islam, Z.; Herrero-Fresno, A.; Biswas, P.K.; Olsen, J.E. High prevalence of mcr-1-encoded colistin resistance in commensal Escherichia coli from broiler chicken in Bangladesh. Sci. Rep. 2020, 10, 18637. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Shivashekaregowda, N.K.H.; Cock, I.E. Bacterial Foodborne Illness in Malaysia: Terminalia spp. as a Potential Resource for Treating Infections and Countering Antibiotic Resistance. Malays. J. Med. Sci. 2023, 30, 42–54. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Saleha, A.A.; Jalila, A.; Zunita, Z. Risk factors and spatial distribution of extended spectrum β-lactamase-producing- Escherichia coli at retail poultry meat markets in Malaysia: A cross-sectional study. BMC Public Health 2016, 16, 699. [Google Scholar] [CrossRef]
- Elmi, S.A.; Simons, D.; Elton, L.; Haider, N.; Hamid, M.M.A.; Shuaib, Y.A.; Khan, M.A.; Othman, I.; Kock, R.; Osman, A.Y. Identification of risk factors associated with resistant Escherichia coli isolates from poultry farms in the east coast of peninsular malaysia: A cross sectional study. Antibiotics 2021, 10, 117. [Google Scholar] [CrossRef]
- Rahman, M.; Husna, A.; Elshabrawy, H.A.; Alam, J.; Runa, N.Y.; Badruzzaman, A.T.M.; Banu, N.A.; Al Mamun, M.; Paul, B.; Das, S.; et al. Isolation and molecular characterization of multidrug-resistant Escherichia coli from chicken meat. Sci. Rep. 2020, 10, 21999. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Inf. Suppl. 2021, 31, 100–121. [Google Scholar]
- Piryaei, M.R.; Peighambari, S.M.; Razmyar, J. Drug resistance and genotyping studies of Salmonella enteritidis isolated from broiler chickens in Iran. Front. Veter. Sci. 2025, 12, 1542313. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mediavilla, J.R.; Oliveira, D.C.; Willey, B.M.; de Lencastre, H.; Kreiswirth, B.N. Multiplex real-time PCR for rapid Staphylococcal cassette chromosome mec typing. J. Clin. Microbiol. 2009, 47, 3692–3706. [Google Scholar] [CrossRef] [PubMed]
- Aklilu, E.; Harun, A.; Singh, K.K.B. Molecular characterization of bla NDM, bla OXA-48, mcr-1 and bla TEM-52 positive and concurrently carbapenem and colistin resistant and extended spectrum beta-lactamase producing Escherichia coli in chicken in Malaysia. BMC Vet. Res. 2022, 18, 190. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microb. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Liu, X.; Thungrat, K.; Boothe, D.M. Multilocus sequence typing and virulence profiles in uropathogenic Escherichia coli isolated from cats in the United States. PLoS ONE 2015, 10, e0143335. [Google Scholar] [CrossRef]
- Lincopan, N.; Fuentes-Castillo, D.; Espinoza-Muñoz, M.; Gonzales-Zubiate, F.; Gonzales-Escalante, E.; Maturrano, L.; Vignoli, R.; Di Conza, J.; Gutkind, G. WHO Critical Priority Escherichia coli in Latin America: A One Health Challenge for a Post-Pandemic World. In Trending Topics in Escherichia coli Research: The Latin American Perspective; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–32. [Google Scholar]
- Sobur, M.A.; Ievy, S.; Haque, Z.F.; Nahar, A.; Zaman, S.B.; Rahman, M.T. Emergence of colistin-resistant Escherichia coli in poultry, house flies, and pond water in Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 50. [Google Scholar]
- Wang, D.; Berglund, B.; Li, Q.; Shangguan, X.; Li, J.; Liu, F.; Yao, F.; Li, X. Transmission of clones of carbapenem-resistant Escherichia coli between a hospital and an urban wastewater treatment plant. Environ. Pollut. 2023, 336, 122455. [Google Scholar] [CrossRef]
- Karim, R.; Zakaria, Z.; Hassan, L.; Faiz, N.M.; Ahmad, N.I. Antimicrobial resistance profiles and co-existence of multiple antimicrobial resistance genes in mcr-Harbouring Colistin-resistant Enterobacteriaceae isolates recovered from poultry and poultry meats in Malaysia. Antibiotics 2023, 12, 1060. [Google Scholar] [CrossRef]
- Devan, S.S.; Aklilu, E.; Zakaria, Z.; Hamdan, R.H.; Lemlem, M.; Kamaruzzaman, N.F.; Kamaruzzaman, I.N.A.; Reduan, M.F.H. Emergence of mcr-1,-3,-6,-8 and-9) in Escherichia coli isolated from live chickens, raw chicken meat and vegetables from Kelantan, Malaysia. J. Microb. Biotech. Food Sci. 2023, 12, 9829. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Emergence of colistin-resistant bacteria in humans without colistin usage: A new worry and cause for vigilance. Int. J. Antimicrob. Agents 2016, 47, 1–3. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Asante, J. Emerging mechanisms of antimicrobial resistance in bacteria and fungi: Advances in the era of genomics. Futur. Microbiol. 2018, 13, 241–262. [Google Scholar] [CrossRef]
- Amin, M.B.; Sraboni, A.S.; Hossain, M.I.; Roy, S.; Mozmader, T.A.U.; Unicomb, L.; Rousham, E.K.; Islam, M.A. Occurrence and genetic characteristics of mcr-1-positive colistin-resistant E. coli from poultry environments in Bangladesh. J. Glob. Antimicrob. Resist. 2020, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Aklilu, E.; Raman, K. MCR-1 gene encoded colistin-resistant Escherichia coli in raw chicken meat and bean sprouts in Malaysia. Int. J. Microbiol. 2020, 2020, 8853582. [Google Scholar] [CrossRef]
- Monte, D.F.; Fernandes, M.R.; Cerdeira, L.; de Souza, T.A.; Mem, A.; Franco, B.D.G.M.; Landgraf, M.; Lincopan, N. Draft genome sequences of colistin-resistant MCR-1-producing Escherichia coli ST1850 and ST74 strains isolated from commercial chicken meat. Genome Announc. 2017, 5, e00329-17. [Google Scholar] [CrossRef]
- Odoi, J.O.; Takayanagi, S.; Sugiyama, M.; Usui, M.; Tamura, Y.; Asai, T. Prevalence of colistin-resistant bacteria among retail meats in Japan. Food Saf. 2021, 9, 48–56. [Google Scholar] [CrossRef]
- Schrauwen, E.J.A.; Huizinga, P.; van Spreuwel, N.; Verhulst, C.; Bergh, M.F.Q.K.-V.D.; Kluytmans, J.A.J.W. High prevalence of the mcr-1 gene in retail chicken meat in the Netherlands in 2015. Antimicrob. Resist. Infect. Ctrl. 2017, 6, 83. [Google Scholar] [CrossRef]
- Joshi, P.R.; Thummeepak, R.; Leungtongkam, U.; Pooarlai, R.; Paudel, S.; Acharya, M.; Dhital, S.; Sitthisak, S. The emergence of colistin-resistant Escherichia coli in chicken meats in Nepal. FEMS Microbiol. Lett. 2019, 366, 237. [Google Scholar] [CrossRef]
- Nakayama, T.; Le Thi, H.; Thanh, P.N.; Minh, D.T.N.; Hoang, O.N.; Hoai, P.H.; Yamaguchi, T.; Jinnai, M.; Do, P.N.; Van, C.D.; et al. Abundance of colistin-resistant Escherichia coli harbouring mcr-1 and extended-spectrum β-lactamase-producing E. coli co-harbouring bla CTX-M-55 or-65 with bla TEM isolates from chicken meat in Vietnam. Archv. Microb. 2022, 204, 137. [Google Scholar]
- Dominguez, J.E.; Redondo, L.M.; Espinosa, R.A.F.; Cejas, D.; Gutkind, G.O.; Chacana, P.A.; Di Conza, J.A.; Miyakawa, M.E.F. Simultaneous carriage of mcr-1 and other antimicrobial resistance determinants in Escherichia coli from poultry. Front. Microbiol. 2018, 9, 1679. [Google Scholar] [CrossRef]
- Ejaz, H.; Younas, S.; Qamar, M.U.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Alameen, A.A.M.; Elamir, M.Y.M.; Ahmad, N.; Hamam, S.S.M.; et al. Molecular epidemiology of extensively drug-resistant mcr encoded colistin-resistant bacterial strains co-expressing multifarious β-lactamases. Antibiotics 2021, 10, 467. [Google Scholar] [CrossRef]
- Hussain, A.; Shaik, S.; Ranjan, A.; Nandanwar, N.; Tiwari, S.K.; Majid, M.; Baddam, R.; Qureshi, I.A.; Semmler, T.; Wieler, L.H.; et al. Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free-range retail chicken in India. Front. Microbiol. 2017, 8, 2120. [Google Scholar] [CrossRef]
- Murase, T.; Ozaki, H. Relationship between phylogenetic groups of Escherichia coli and Pathogenicity among Isolates from chickens with Colibacillosis and healthy chickens. Poult. Sci. 2022, 101, 102007. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Goswami, S.; Sirajee, A.S.; Ahsan, S. Phylotyping, Pathotyping and Phenotypic Characteristics of Escherichia coli Isolated from Various Street Foods in Bangladesh. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e4619. [Google Scholar] [CrossRef]
- Grakh, K.; Mittal, D.; Prakash, A.; Jindal, N. Characterization and antimicrobial susceptibility of biofilm-producing Avian Pathogenic Escherichia coli from broiler chickens and their environment in India. Veter. Res. Commun. 2022, 46, 537–548. [Google Scholar] [CrossRef]
- Raimondi, S.; Righini, L.; Candeliere, F.; Musmeci, E.; Bonvicini, F.; Gentilomi, G.; Erjavec, M.S.; Amaretti, A.; Rossi, M. Antibiotic resistance, virulence factors, phenotyping, and genotyping of E. coli isolated from the feces of healthy subjects. Microorganisms 2019, 7, 251. [Google Scholar] [CrossRef]
- Sary, K.; Fairbrother, J.M.; Arsenault, J.; de Lagarde, M.; Boulianne, M. Antimicrobial resistance and virulence gene profiles among Escherichia coli isolates from retail chicken carcasses in Vietnam. Foodborne Pathog. Dis. 2019, 16, 298–306. [Google Scholar] [CrossRef]

| Isolate Identification | Resistance Phenotype | MIC Colistin | Resistance Genes Detected | Phylogenetic Group | MLST Sequence Type | MLST Clonal Complex |
|---|---|---|---|---|---|---|
| C5 | Ery-tet-Ofl-Amp-Kan-Amk-Chl-Ctz-Amc-AmS-Cfx | ≥4 | mcr1, sul1, tetA, genta (aac3), chloram (catA1) | A, C, E, B2, B1 | 345 | - |
| C6 | Ery-tet-Ofl-Amp-Kan-Chl-Ctz-Amc-AmS-Cfx | ≥4 | mcr1, sul1, tetA, genta (aac3), | A, C, E, B2 | 770 | - |
| C7 | Ery-tet-Ofl-Amp-Kan-Amk-Chl-AmS-Cfx | ≥4 | mcr4, sul1, tetA, genta (aac3), | A, C, E, B1 | 10 | - |
| C9 | Ery-tet-Ofl-Amp-Kan-Amk-Chl-Amc-AmS-Cfx | ≥4 | mcr1, sul1, tetA, genta (aac3), chloram (catA1) | A, C, E, B2, B1 | 540 | - |
| C10 | Ery-tet-Ofl-Amp-Kan-Amk-Chl-Ctz-Amc-AmS-Cfx | ≥4 | mcr4, sul1, tetA, genta (aac3), chloram (catA1) | A, C, E, B1 | 155 | ST155cplx |
| C11 | Ery-tet-Ofl-Amp-Kan-Chl-AmS-Cfx | ≥4 | mcr9, sul1, tetA, genta (aac3), | A, C, E, B2 | 31 | - |
| C12 | Ery-tet-Ofl-Amp-Kan-Chl-Ctz-Amc-AmS-Cfx | ≤4 | mcr1, sul1, tetA, genta (aac3), | A, C, E, B1 | 1638 | ST10cplx |
| C15 | Ery-tet-Ofl-Amp-Kan-Amk-Chl-Amc-AmS | ≥4 | mcr4, sul1, tetA, genta (aac3), | A, C, E, B1 | 906 | ST11cplx |
| C18 | Ery-Tet-Ofl-Amp-Kan-Chl-Ctz-Amc-Ams | ≥4 | mcr9, sul1, tetA, genta (aac3), chloram (catA1) | A, C, E, B1 | 206 | - |
| C19 | Tet-Cft-Amp-Kan-Chl-Amc-Ams | ≥4 | mcr1, sul1, tetA, genta (aac3), chloram (catA1) | A, C, E, B1 | 162 | - |
| Class of Antibiotics | Mechanism of Action | Antibiotics (Dosage) | Resistance Rate (%) |
|---|---|---|---|
| Inhibition of Cell Wall Synthesis | |||
| Penicillin | Amoxicillin (10 µg) | 0% | |
| Carbapenems | Imipenem (10 µg) | 18% | |
| Cephems (Parental) | Cefotaxime (30 µg) | 63.15% | |
| Cephalosporins III | Ceftazadime (30 µg) | 5.26% | |
| Ceftriazone (30 µg) | 63.15% | ||
| Ceftiofur (30 µg) | 0% | ||
| β–lactams combination agents | Amoxicillin/Clavulanic acid (30 µg) | 63.15% | |
| Ampicillin/Sulbactam (20 µg) | 100% | ||
| Disruption of DNA synthesis and DNA replication | |||
| Fluoroquinolones | Ofloxacin (5 µg) | 63.15% | |
| Inhibition of protein synthesis | |||
| Tetracyclines | Tetracycline (30 µg) | 89.4% | |
| Phenicols | Chloramphenicol (30 µg) | 100% | |
| Macrolides | Erythromycin (30 µg) | 89.4% | |
| Aminoglycoside | Kanamycin (30 µg) | 100% | |
| Aminoglycoside | Gentamicin (10 µg) | 100% | |
| Aminoglycoside | Amikacin (10 µg) | 26.31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawal, H.; Saeed, S.I.; Kamaruzzaman, N.F.; Suhaili, Z.; Sani, G.M.; Lemlem, M.; Yang, Q.; Aklilu, E. Molecular Detection of Colistin-Resistant E. coli in Village Chickens from Kelantan, Malaysia. Bacteria 2025, 4, 19. https://doi.org/10.3390/bacteria4020019
Lawal H, Saeed SI, Kamaruzzaman NF, Suhaili Z, Sani GM, Lemlem M, Yang Q, Aklilu E. Molecular Detection of Colistin-Resistant E. coli in Village Chickens from Kelantan, Malaysia. Bacteria. 2025; 4(2):19. https://doi.org/10.3390/bacteria4020019
Chicago/Turabian StyleLawal, Habiba, Shamsaldeen Ibrahim Saeed, Nor Fadhilah Kamaruzzaman, Zarizal Suhaili, Gaddafi Mohammed Sani, Mulu Lemlem, Qiya Yang, and Erkihun Aklilu. 2025. "Molecular Detection of Colistin-Resistant E. coli in Village Chickens from Kelantan, Malaysia" Bacteria 4, no. 2: 19. https://doi.org/10.3390/bacteria4020019
APA StyleLawal, H., Saeed, S. I., Kamaruzzaman, N. F., Suhaili, Z., Sani, G. M., Lemlem, M., Yang, Q., & Aklilu, E. (2025). Molecular Detection of Colistin-Resistant E. coli in Village Chickens from Kelantan, Malaysia. Bacteria, 4(2), 19. https://doi.org/10.3390/bacteria4020019







