Molecular Characterization of Phosphate Solubilizing Bacteria Klebsiella variicola PSEG-1 Associated with Aporrectodea rosea Gastrointestinal Tract
Abstract
:1. Introduction
2. Materials and Methods
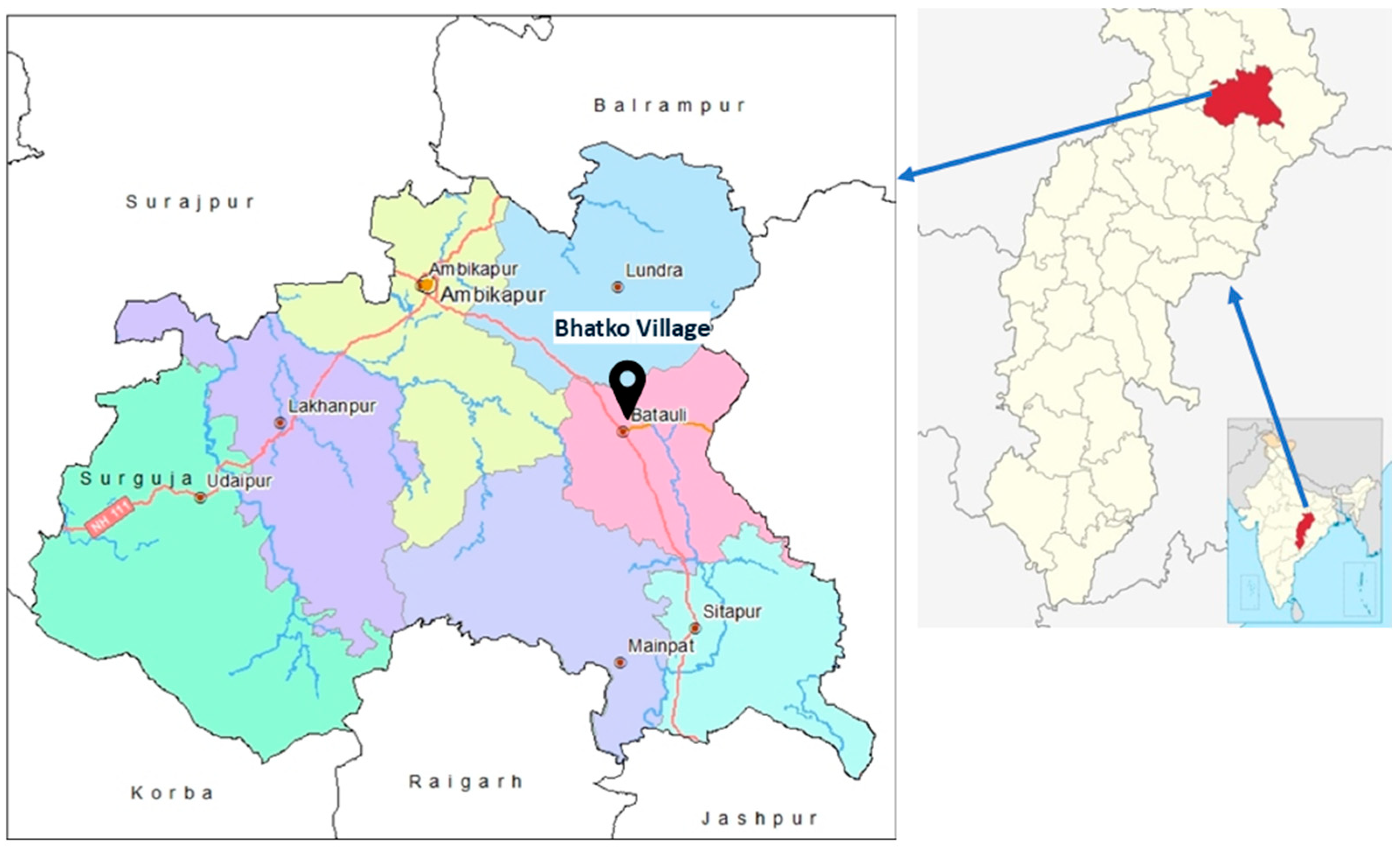
2.1. Sample Collection and Characterization of Earthworms
2.2. Identification of Earthworms
2.3. Isolation of Phosphate Solubilizing Bacteria from Earthworm Gut
2.4. Detection of Organic Acid Production
2.5. Qualitative Assessment of Phosphate Solubilization
2.6. Determination of Optimum pH for PSB Strain
2.7. Quantitative Estimation of Phosphate and Ammonium Ion
2.8. Morphological and Biochemical Characterization of Potent Isolate
2.9. Molecular Characterization of Potent Isolate
3. Results
3.1. Morphological Characteristics of the Earthworm
3.2. Isolation of Phosphate Solubilizing Bacteria
3.3. Effect of pH on Isolated PSB Strain
3.4. Quantitative Estimation of Phosphate Solubilization
3.5. Quantitative Estimation of Ammonium Ion
3.6. Morphological, Biochemical, and Molecular Characterization of the Bacterial Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology; Springer: Cham, Switzerland, 2014; pp. 31–62. [Google Scholar]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.M. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán, P.; Peirce, C.A.E.; McBeath, T.M.; Khayet, M.; McLaughlin, M.J. Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil. 2014, 384, 7–20. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Kumar, R.; Shastri, B. Role of Phosphate-Solubilising Microorganisms in Sustainable Agricultural Development. In Agro-Environmental Sustainability; Springer: Cham, Switzerland, 2017; pp. 271–303. [Google Scholar] [CrossRef]
- Tamrakar, A.; Upadhyay, K.; Bajpai, S. Spatial variation of Physico-chemical parameters and water quality assessment of urban ponds at Raipur, Chhattisgarh, India. IOP Conf. Series: Earth Environ. Sci. 2022, 1032, 012034. [Google Scholar] [CrossRef]
- Bhunia, G.S.; Shit, P.K. Appraisal of groundwater contamination and spatial variation using geostatistical modeling in Surguja district of Chhattisgarh, India. Environ. Earth Sci. 2021, 80, 760. [Google Scholar] [CrossRef]
- Smyntek, P.M.; Lamagna, N.; Cravotta Iii, C.A.; Strosnider, W.H.J. Mine drainage precipitates attenuate and conceal wastewater-derived phosphate pollution in stream water. Sci. Total Environ. 2022, 815, 152672. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Padda, K.P.; Chanway, C.P. In vitro and in vivo analyses of plant-growth-promoting potential of bacteria naturally associated with spruce trees growing on nutrient-poor soils. Appl. Soil. Ecol. 2020, 149, 103538. [Google Scholar] [CrossRef]
- Rawat, P.; Shankhdhar, D.; Shankhdhar, S.C. Plant growth-promoting rhizobacteria: A booster for ameliorating soil health and agriculture production. In Soil Health; Springer International Publishing: Cham, Switzerland, 2020; pp. 47–68. [Google Scholar]
- Zaheer, A.; Malik, A.; Sher, A.; Qaisrani, M.M.; Mehmood, A.; Khan, S.U.; Ashraf, M.; Mirza, Z.; Karim, S.; Rasool, M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019, 26, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Das Mohapatra, M.; Sahoo, R.K.; Tuteja, N. Phosphate solubilizing bacteria, Pseudomonas aeruginosa, improve the growth and yield of groundnut (Arachis hypogaea L.). Physiol. Mol. Biol. Plants 2024, 30, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.; Klepa, M.S.; Olchanheski, L.R.; de Alencar Almeida, M.; Chicora, K.; Prestes, L.; Rodrigues, E.P.; Hungria, M.; da Silva Batista, J.S. Phenotypic and genomic characterization of phosphate-solubilizing rhizobia isolated from native Mimosa and Desmodium in Brazil. Braz. J. Microbiol. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Shi, J.; Li, M.; Chen, J.; Wang, T.; Zhang, Q.; Yang, L.; Zhu, N.; Wang, Y. Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil. Agronomy 2024, 14, 1535. [Google Scholar] [CrossRef]
- Alam, S.; Khalil, S.; Ayub, N.; Rashid, M. In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganisms (PSM) from maize rhizosphere. Int. J. Agric. Biol. 2002, 4, 454–458. [Google Scholar]
- Miglani, R.; Parveen, N.; Bisht, S.S.; Panda, A.K.; Bala, M.; Upadhyay, J.; Kumar, A.; De Mandal, S. Earthworm Gut Microbiome: The Uncharted Microbiome. In Metagenomics and Microbial Ecology; CRC Press: Boca Raton, FL, USA, 2021; pp. 91–104. [Google Scholar]
- Sims, R.W.; Gerard, B.M. Earthworms, Synopses of the British Fauna No. 31; The Linnean Society/EJ Brill: London, UK, 1985. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Bulletin Fisheries Research Board of Canada, Nr. 167 (2nd ed.); Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; 310p. [Google Scholar] [CrossRef]
- Paul, D.; Sinha, S. Phosphate solubilizing activity of some bacterial strains isolated from jute mill effluent exposed water of river Ganga. Indian. J. Fundam. Appl. Life Sci. 2013, 3, 39–45. [Google Scholar]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Dash, M.C.; Sarkar, S.K.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- Muleta, D.; Assefa, F.; Börjesson, E.; Granhall, U. Phosphate-solubilising rhizobacteria associated with Coffea arabica L. in natural coffee forests of southwestern Ethiopia. J. Saudi Soc. Agric. Sci. 2013, 12, 73–84. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Manupoori, S.; Kanth, S.V. Screening of multi-faceted phosphate-solubilising bacterium from seagrass meadow and their plant growth promotion under saline stress condition. Microbiol. Res. 2022, 261, 127080. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Palit, R.; Sengupta, C.; Standing, D. Stress induced phosphate solubilization by ‘Arthrobacter’ sp. and ‘Bacillus’ sp. isolated from tomato rhizosphere. Aust. J. Crop Sci. 2010, 4, 378–383. [Google Scholar]
- Paul, D.; Sinha, S.N. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrar. Sci. 2017, 15, 130–136. [Google Scholar] [CrossRef]
- Maheshwari, D.K.; Saraf, M.; Aeron, A. Bacteria in Agrobiology: Crop Productivity; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Swift, R.; Denton, M.D.; Melino, V.J. Plant probiotics for nutrient acquisition by agriculturally important grasses: A comprehensive review of the science and the application. Annu. Plant Rev. Online 2018, 2, 537–584. [Google Scholar]

| S. No. | Mean Colony Diameter (cm) | Mean Halo Zone Diameter (cm) | Phosphate Solubilization Index |
|---|---|---|---|
| 1 | 0.5 | 0.2 | 1.4 |
| 2 | 0.3 | 0.2 | 1.6 |
| 3 | 0.6 | 0.3 | 1.5 |
| 4 | 0.4 | 0.2 | 1.5 |
| 5 | 0.5 | 0.1 | 1.2 |
| 6 | 0.8 | 0.3 | 1.3 |
| 7 | 0.5 | 0.2 | 1.4 |
| 8 | 0.5 | 0.1 | 1.2 |
| S. No | Biochemical Tests | Results |
|---|---|---|
| 1 | Cell morphology | Rod-shaped |
| 2 | Gram staining reaction | −ve |
| 3 | Oxidase activity | +ve |
| 4 | Catalase activity | +ve |
| 5 | Indole production test | −ve |
| 6 | Methyl red test | −ve |
| 7 | Voges–Proskauer test | −ve |
| 8 | Citrate utilization test | +ve |
| 9 | H2S production test | −ve |
| 10 | Urease activity | −ve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerketta, V.; Panda, A.K.; Kerketta, A.; De Mandal, S.; Bisht, S.S. Molecular Characterization of Phosphate Solubilizing Bacteria Klebsiella variicola PSEG-1 Associated with Aporrectodea rosea Gastrointestinal Tract. Bacteria 2025, 4, 5. https://doi.org/10.3390/bacteria4010005
Kerketta V, Panda AK, Kerketta A, De Mandal S, Bisht SS. Molecular Characterization of Phosphate Solubilizing Bacteria Klebsiella variicola PSEG-1 Associated with Aporrectodea rosea Gastrointestinal Tract. Bacteria. 2025; 4(1):5. https://doi.org/10.3390/bacteria4010005
Chicago/Turabian StyleKerketta, Vikash, Amrita Kumari Panda, Aseem Kerketta, Surajit De Mandal, and Satpal Singh Bisht. 2025. "Molecular Characterization of Phosphate Solubilizing Bacteria Klebsiella variicola PSEG-1 Associated with Aporrectodea rosea Gastrointestinal Tract" Bacteria 4, no. 1: 5. https://doi.org/10.3390/bacteria4010005
APA StyleKerketta, V., Panda, A. K., Kerketta, A., De Mandal, S., & Bisht, S. S. (2025). Molecular Characterization of Phosphate Solubilizing Bacteria Klebsiella variicola PSEG-1 Associated with Aporrectodea rosea Gastrointestinal Tract. Bacteria, 4(1), 5. https://doi.org/10.3390/bacteria4010005







